Tolerance to Iron-Deficiency Stress of Three Apple Rootstock Species in Hydroponic System
JIA Xu-mei, ZHU Yan-fang, HU Ya, CHENG Li, ZHAO Tong, WANG Yan-xiu
College of Horticulture, Gansu Agricultural University, Lanzhou 730070, PRC
Abstract [Objective] Iron deficiency is one of the most important crop element deficiencies in the Loess Plateau of northwestern China. The selection for crop cultivars that are tolerant to low iron levels could be one of the approaches to solving the problem and improving crop production. [Method] Three major apple root stock species (Malus prunifolia, Malus sieversii and Malus baccata) were selected to evaluate their tolerance to iron deficiency in hydroponic system. A 3×2 factorial pot experiment was conducted with three replicates in a greenhouse at Gansu Agricultural University, Lanzhou, China. [Result] The SOD, POD and CAT activities in roots and stems of the 3 root stock species in Fe-deficient Hoagland solution decreased,however Malus sieversii got the less reduction and had better root architecture and growth than the other species. The aboveground biomass, plant height, chlorophyll content, total root length and lateral root number were correlated positively with irondeficiency stress tolerance. The species’ tolerance to iron-deficiency from high to low was M. sieversii’s>M. baccata’s>M. prunifolia’s. Moreover, the improvement of some morphological features such as root length, above-ground biomass, plant height and lateral root number in apple could be conducive to breeding cultivars with tolerance to iron-deficiency stress. [Conclusion] Malus sieversii had better tolerance to irondeficiency stress than the others in this study.
Key words Apple rootstock; Iron deficiency; Hydroponic system; Root architecture;Principal component analysis
1. Introduction
Micronutrient elements such as Cu, Zn, Mn, Fe, Ni and Mo are essential for growth and development in all plants[1]. Iron is one of the most indispensable elements in mineral nutrition of plants, especially in fruit trees[2-3]. The iron-deficiency usually occurs in crops grown in calcareous soils[4], and it is a common nutritional disorder in apples cultivated in northern China[5-6].
The horticultural industry of China generates significant revenue by producing the apples. At the end of 2013, approximately 227 million hectares of land was under apple cultivation in China (Ministry of Agriculture, 2013)[7]. The Northwest Loess Plateau is well known area for apple production in China[8]. However, due to the calcareous property of the soil and its high pH, the iron deficiency is a major problem which adversely affects fruit quality. High active lime concentration in soil makes it difficult for apples to absorb the iron[9], and inadequate iron leads to interveinal yellowing of leaves and negatively affects fruit yield and quality[10]. For instance, the soybeans(Glycine max(L.) Merr.) grown in high pH soil often suffer from iron deficiency which is characterized by chlorosis, thus resulting in substantial yield losses.The iron efficient-soybean cultivars maintain more efficient photosynthesis and produce higher yields than inefficient cultivars under iron-deficiency conditions[11]. Studies have already been conducted on the iron stress tolerance in some species such asMalus xiaojinensis,PrunusandCitrus lemon[12-14].
The iron could regulate several biochemical and physiological processes in plants, including photosynthesis, respiration and cell wall metabolism[15].The antioxidant enzymes such as catalase (CAT),peroxidase (POD) and superoxide dismutase (SOD)are considered as the bioindicators of active Fe in plant cells as iron is required to bind their active sites[16-17].Furthermore, iron deficiency can lead to changes in plant morphology and physiological abnormality such as malformation of root tips, proliferation of root hairs, decline in phenol synthesis[18]and significant improvement of root ability to secrete H+[19]. The bioaccumulation factor (BAF) and translocation factor(TF) are two indicators for evaluating Fe accumulation and translocation efficiency[20].
This study was to (i) identify and select root stocks that are tolerant to iron-deficiency stress to improve apple yield in the Loess Plateau of China and(ii) identify the morphological traits that can enhance the ability of the root stocks to tolerate iron deficiency stress and recommend the improvement of these traits for apple breeding programmes.
2. Materials and Methods
2.1. Plant material
The seeds ofMalus prunifoliawere obtained from the garden field of Ningxian County of Gansu and the seeds ofMalus sieversiiandMalus baccatawere obtained from Gaolan County of Gansu Province in China.
2.2. Stress treatment
The seeds were washed with distilled water and exposed to 4℃ in dark environment for germination.Then the pre-germinated seeds were planted in 40 cm×50 cm plastic pots containing substrate. The 90 d old seedlings were transplanted to Hoagland's nutrient solution with Fe3+-EDTA at either 40 μmol/L(Fe-sufficient) or 0 μmol/L (Fe-deficient) and the plants were cultivated with continuous aeration.The Hoagland’s nutrient solution was composed of Ca(NO3)2·4H2O (945 mg/L), KNO3(506 mg/L),NH4NO3(80 mg/L), KH2PO4(136 mg/L), MgSO4(493 mg/L), Ferris salt solution (2.5 mL/L) and trace element solution (5 mL/L), pH=6.0. Ten replicates per treatment and five plants per replicate were set for 16 h at 28℃ in the light and for 8 h at 25℃ in the darkness.
The experiment was 3×2 factorial in completely randomized design with three replicates, which consisted of 3 root stock species and 2 treatments with Hoagland solution, each experimental unit (replicate)contained 5 plants, so there were a total of 90 plants for the experiment. Data was collected 40 d after the treatment.
2.3. Measurement of indices
The stem diameter was measured with vernier calipers, the number of leaves was counted manually,the fresh shoots were measured with electronic balance and the plant height was measured with a plastic ruler from stem base to the terminal bud at the top of plant.The whole root architecture was determined with EPSON Expression 10 000XL A3 High-resolution Scanner (EPSON Expression 10 000XL A3, EPSON,Japan). The chlorophyll content was determined by the acetone method and root activity was determined by the triphenyl tetrazolium chloride (TTC) method represented by TTC reduction activity. The active iron that can be used by the plants was measured by atomic absorption spectrometer (TAS-990) (AAS). The CAT activity was determined by measuring the reduction of H2O2concentration at 240 nm[21]. The activity was calculated by using extinction coefficient of 40.0 mM/cm for H2O2at 240 nm. The SOD and POD activities were determined by the nitroblue tetrazolium staining method and the guaiac wood phenol method.
2.4. Statistical analysis
The experimental data collected were analyzed by Analysis of Variance (ANOVA) and the means were separated by Duncan’s Multiple Range Test atP≤0.05 using SPSS software version 22.0. The figures were created with Origin software version 8.0.
3. Results and Analysis
3.1. Effects of iron deficiency on morphological characteristics
The growth characteristics of the seedlings as shown in Table 1. In iron-deficient medium, the plant height, stem diameter, number of leaves and shoot fresh weight of the 3 species were relatively low and similar. These indices inM. prunifoliawere low compared with the other species, but there was no significant difference betweenM. sieversiiandM. baccata. The plant height ofM. sieversii,M.prunifoliaandM. baccatain the iron deficiency solution decreased by 14.35%, 14.23% and 7.28%,respectively. The decrease of stem diameter observed inM. sieversii,M. prunifoliaandM. baccatawere found to be 12.09%, 12.24% and 5.04%, respectively.The number of leaves inM. sieversii,M. prunifoliaandM. baccatadecreased by 15.15%, 24.98% and 28.12%, respectively. The shoot fresh weight inM.sieversii,M. prunifoliaandM. baccatadecreased by 55.37%, 63.56% and 38.83%, respectively.
3.2. Effects of iron deficiency on root architecture
Table 2 showed the change of root architecture in apple root stock seedlings with the 2 nutrient solution treatments. The results showed thatM. sieversiihad better root architecture with the iron-sufficient or the iron-deficient nutrient solutions than the other two species. TheM. sieversiihad greater total root length,lateral root number, root tips, surface area, projected area, root volume, number of forks and crossings with the iron-deficient nutrient solution. On the contrary,root architecture indices ofM. prunifoliaandM.baccatawith the iron-deficiency treatment were lower than with the iron-sufficiency treatment (Fig. 1).
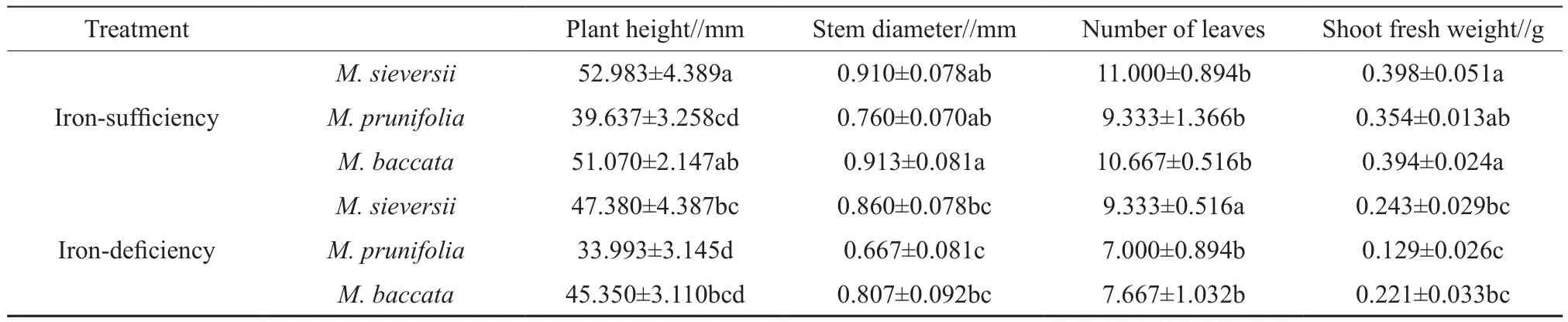
Table 1 Effects of iron-deficiency on growth characteristics of the 3 apple rootstocks
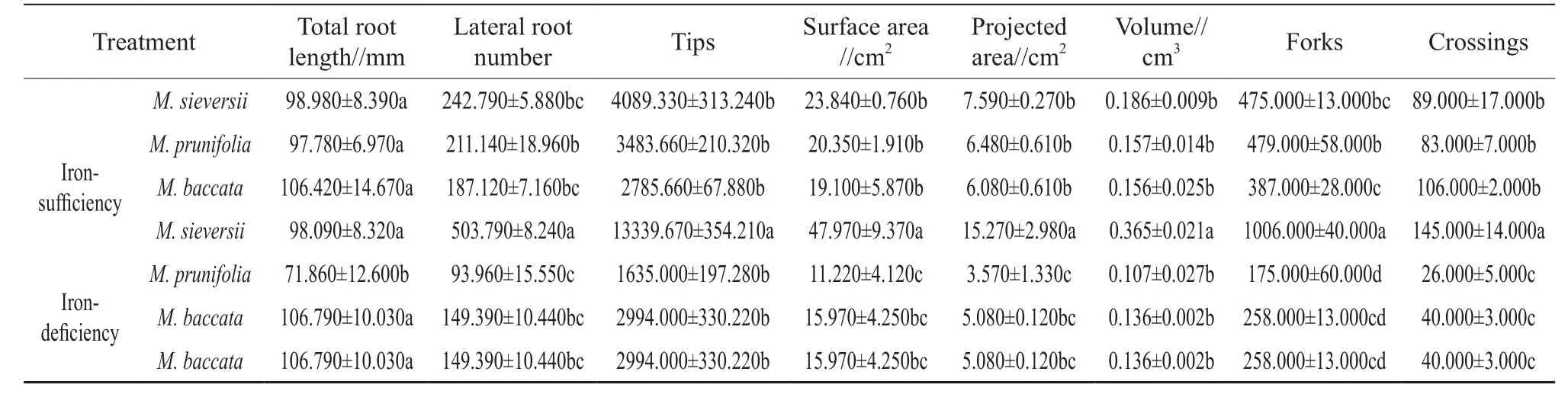
Table 2 Effects of iron-deficiency on the root architecture of 3 apple rootstocks
3.3. Effects of iron deficiency on physiological indices
The chlorophyll content ofM. prunifoliawas higher than that of the other two species in the ironsufficient media or the iron-deficient media; whileM.baccatahad the least chlorophyll content (Fig. 2A).As shown in Fig. 2B, root activity ofM. prunifoliawas the highest in the iron-sufficient medium, with 0.320 μg/h·g·FW, while the root activity was reduced by 2.7-fold in the iron-deficient medium. In the ironsufficient medium or the iron-deficient medium, the root activity ofM. baccatawas the least, with the values of 0.099 and 0.074 μg/h·g·FW respectively.
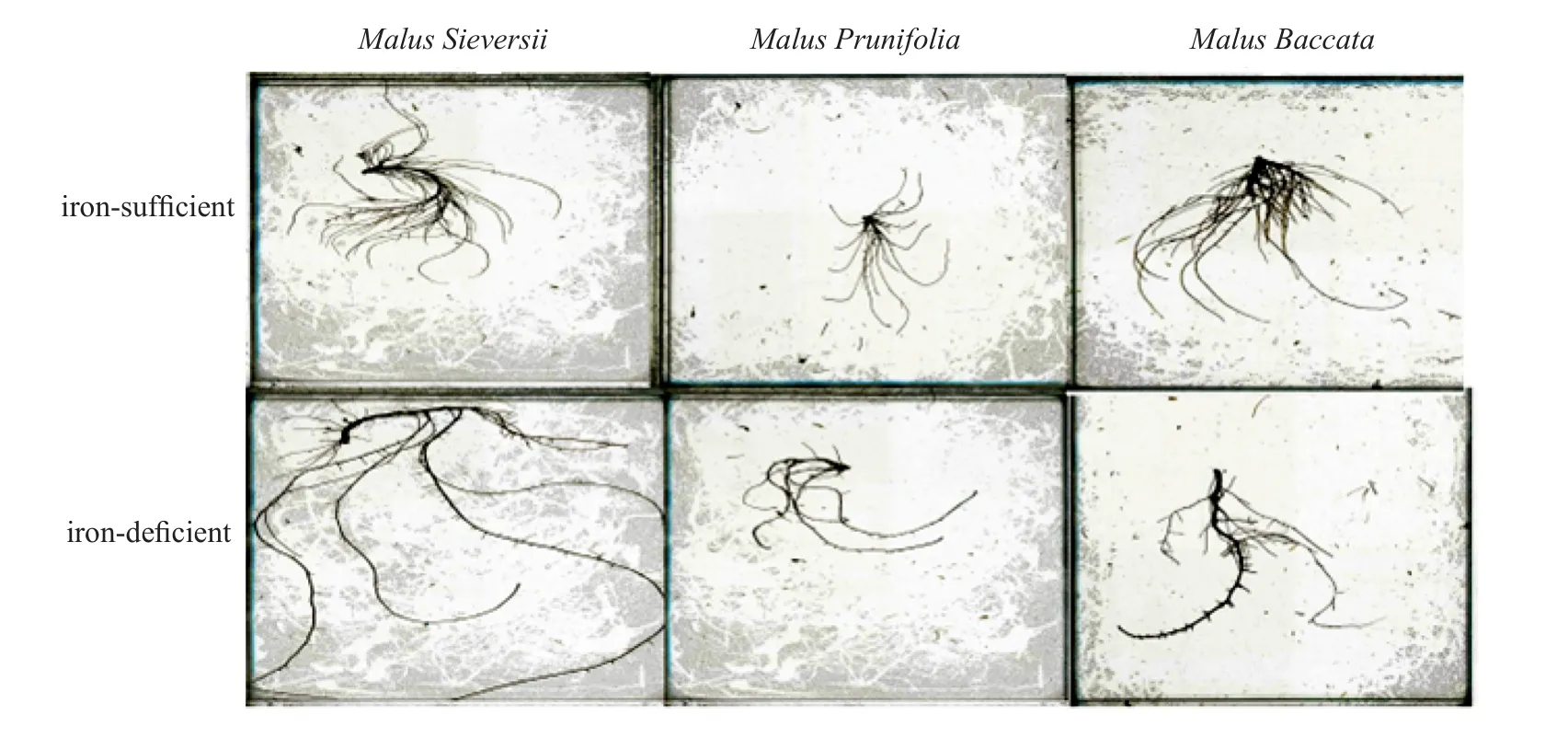
Fig. 1 Effects of Fe treatment on root scanning of 3 apple rootstocks species
As shown in Fig. 3, significant change occurred in antioxidant enzyme activities of the 3 apple rootstocks’ seedlings with the different treatments.The SOD, POD, CAT activities in the roots and leaves under the iron-deficiency stress condition decreased significantly. Besides, their activity in the roots was higher than in the leaves, and there was significant difference in these activities among the different species.
Under the iron-deficiency stress conditions, the SOD activity ofM. sieversii,M. prunifoliaandM.baccatain the leaves decreased by 20.00%, 17.20%and 40.70%, while that in the roots decreased by 31.10%, 72.60% and 33.60%, respectively. In the roots, the SOD activity ofM. prunifoliawas found to be highest, with 1 456.320 U/g·FW (Fig. 3A). The CAT activity ofM. sieversii,M. prunifoliaandM.baccatain the leaves under the iron-deficiency stress conditions decreased by 26.92%, 38.6% and 59.80%,while that in the roots decreased by 25.70%, 45.50%,and 74.40%, respectively (Fig. 3B). The POD activity ofM. sieversii,M. prunifoliaandM. baccatain the leaves under the iron-deficiency stress conditions reduced by 51.80%, 53.20% and 61.90%, while that in the roots decreased by 22.00%, 46.60% and 64.50%,respectively (Fig. 3C). In the leaves and roots, the reduction of 3 antioxidant enzyme activities of theM.sieversiiwas lower than that of theM. prunifoliaand theM. baccata, respectively (Fig. 3).
3.4. Effects of iron deficiency on Fe concentration
The variation of Fe concentrations in different organs of the 3 apple rootstock species was shown in Table 3. The Fe concentrations in the stems were higher than in the other organs. Moreover,the Fe concentrations of the 3 rootstock species in the iron-deficient medium were lower than in the iron-sufficient medium. Compared with the ironsufficient medium, the Fe concentrations in the roots ofM. sieversii,M. prunifoliaandM. baccataunder the iron-deficiency stress conditions decreased by 23.31%, 34.36% and 37.56%, by 17.21%, 18.41%and 24.39% in the stems, and by 25.60%, 22.05%and 35.57% in the leaves, respectively. As shown in Table 4, the bioaccumulation factor (BAF) in the roots, stems and leaves of the 3 species was 0.9~1.2,1.5~1.8, and 0.9~1.3, respectively. The BAF in the stems was higher than in the roots and in the leaves.The translocation factor (TF) in the stems and the leaves was 1.4~2.4 and 0.7~1.4, respectively. The TF in the stems was also higher than in the leaves. The TF of the 3 species in the iron-deficient mediun was significantly higher than in the iron-sufficient medium.
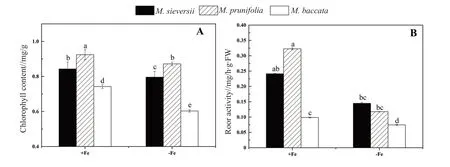
Fig. 2 Effects of Fe-deficient on the chlorophyll content and root activity of 3 apple rootstock species
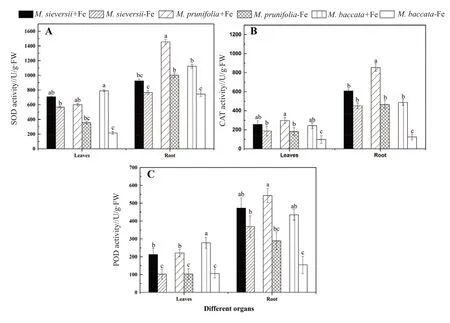
Fig. 3 Effects of Fe-deficient stress on SOD, POD, CAT activities in leaves and roots of 3 apple rootstock species
3.5. Correlation analysis
It is important to evaluate the correlation between Fe concentration in different organs and other indices in order to identify corresponding indices for improving the absorption of Fe. As shown in Table 5, the correlation analysis showed that there was a significant correlation between Fe concentration in the roots and shoot fresh weight (0.998) or total root length (0.994), and a negative correlation between Fe concentration in the roots and that in the leaves(-0.297). The Fe concentration in the roots also had a significant correlation with the plant height (1.000).Moreover, there was a negative correlation between Fe concentration in the stems and Fe concentration in the roots (-0.997) or plant height (-0.997), and a positive correlation between Fe concentration in the stems and SOD in the root. The Fe concentration in the leaves and POD in the leaves had a negative correlation (-0.993), and there was a significant correlation between Fe concentration in the leaves and root activity (0.999) or POD in the roots (1.000).
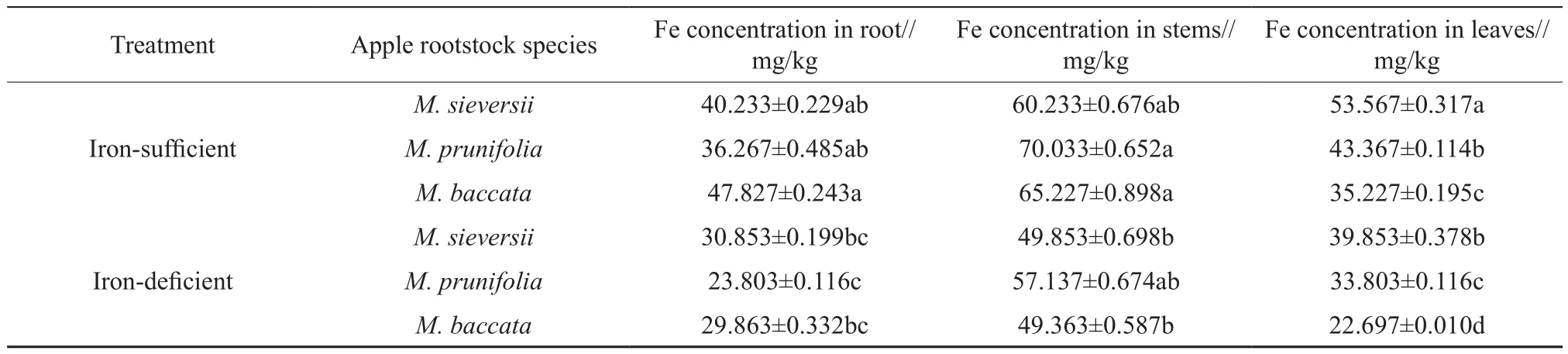
Table 3 Effects of iron deficiency on Fe concentration of 3 apple rootstocks
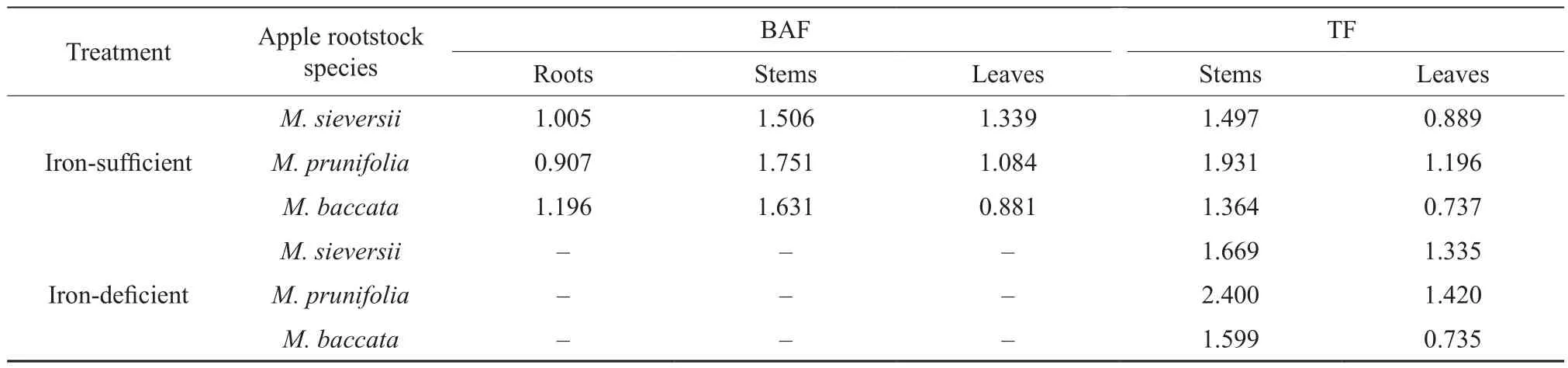
Table 4 Bioaccumulation factor (BAF) and Translocation factor (TF) of iron uptake in 3 apple rootstocks

Table 5 Pearson correlation coefficients between Fe concentration in different organs and other indices
3.6. Principal component analysis
In order to comprehensively evaluate the tolerance to iron deficiency of the 3 apple rootstocks,16 indexes under iron-deficiency stress conditions were analyzed by the principal component analysis.As seen from Table 6, two principal components with the eigenvalue >1 were extracted and the eigenvalues were 10.499 and 5.501, respectively. And the first and second principal component variance contribution rates reached 65.620% and 34.380%, respectively.Notably, the cumulative variance contribution rate of the second principal component was 100.000% (more than 85%) without missing variables. Therefore, the first two principal components could completely reflect the different information of the resistance to iron deficiency. Furthermore, Table 7 showed that the principal component scores which could directly evaluate the resistence of the 3 apple rootstocks under the irondeficiency stress condition. The final scores were derived from the two principal components together based on the formula: F=F1×65.620%+F2×34.380%.Accordingly, the scores from high to low wereM.sieversii’s>M.baccata’s>M.prunifolia’s.

Table 6 List of principle components, percentage of variance and cumulative percentage
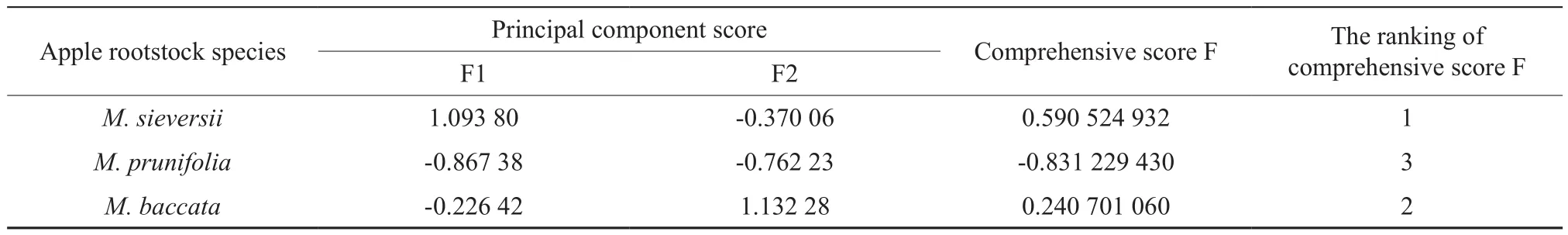
Table 7 The comprehensive score and ranking of 3 apple rootstocks
4. Discussions
A tolerant rootstock should be used to cope with abiotic stress[22]. Nutrition diagnosis is a common method for screening tolerant rootstock which is affected by plant species, environmental conditions and developmental stages[23]. It is essential to choose appropriate physiological parameters and experimental method in screening the rootstock tolerance to irondeficiency .
In previous studies, HAN Jet al.[24]monitored plant height, stem diameter, number of leaves,chlorophyll content and change of root architecture which were used as physiological and biochemical indices for screening resistance gene of apple under iron-deficient stress. In addition, HAN Z Het al.[25]screened the apple rootstock tolerance to irondeficiency by the combination of hydroponic culture,sand culture and field research, and took the iron content and the chlorophyll content of leaves as the bases for selection. LIAO Yet al.[26]tested the MDA content, SOD and POD activity, and other biochemical indices, so as to evaluate the Mn tolerance inCamellia oleifera.
Based on the previous research results, the growth indices, root architecture and biochemical indices were used for evaluating the resistance to irondeficiency of the 3 apple rootstocks in this study. These indices could comprehensively reveal the processes and characteristics of iron metabolism from different angles. The results indicated that the growth of the 3 apple rootstocks in the iron-deficient nutrient solution were retarded compared with that in the iron-sufficient nutrient solution. The 3 apple rootstocks may decrease in the biomass of shoot to adapt to the environmental stress. All the apple rootstocks decreased the number of leaves and shoot fresh weight in response to the stress, but the reduction varied from species (variety)to species (variety). Similar observations were made in other studies[27-28]. It is well documented that iron is an essential element of chlorophyll synthesis and plays an important role in the activation of chlorophyll syntheses[29]. Some studies showed that the chlorophyll content decreased in the iron-deficient nutrient solution, which could caused the chlorosis and thus decreased the photosynthesis and the accumulation of carbohydrate. Obviously, these phenomena showed that iron-deficiency could significantly affect aboveground biomass.
This study showed that the root architectures ofM. prunifoliaandM. baccataunder the irondeficiency stress conditions were lower than under the iron-sufficiency stress conditions. However, the root architecture ofM. sieversiiwas significantly higher than that of the other two species under the iron-deficiency or iron-sufficiency stress conditions.After the exposure to iron-deficiency stress for 40 d,the root activity ofM. sieversiiwas the highest. In the iron-deficient environment, theM. sieversiihad greater root surface area, projected area and volume,total root length, lateral root number, the number of tips, forks and crossings. The improvement of the root architecture probably enhanced the absorption of iron,which increased the iron content in the plant under the iron-deficiency stress condition. Similar improvement in the root architecture ofMalus xiaojinensisandMalus hupehensisunder the Zn-deficient stress conditions were reported[30].
Plants cope with oxidative stress by regulating antioxidant enzymes, which could scavenge different reactive oxygen species (ROSs)[31-32]. This study showed that the antioxidant capacity of roots was higher than that of leaves probably because the roots of seedlings in the hydroponic system have access to inadequate O2to cause oxidative stress.After the exposure to iron, the increases of SOD,POD and CAT activity could effectively enhance the adaptability and resistance of plants and effectively scavenge oxygen free radicals to reduce the damage caused by iron deficiency[33-34]. On the other hand,iron is a constituent or cofactor of many antioxidant enzymes[35]. The reduction of Fe concentration in plant may be caused by the decline of SOD, POD,CAT activities in different organs in the iron-deficient solution. Furthermore, the decrease of the 3 enzyme activities inM. sieversiiin the iron-sufficient solution or the iron-deficient solution was the least, suggesting that the anti-iron-deficient capacity ofM. sieversiiwas better than that of the other two species. These results were consistent with the principal component analysis,which showed that the tolerance to iron-deficiency of the 3 apple rootstocks wasM. sieversii’s>M.baccata’s>M. prunifolia’s.
The sensitivity of different species (varieties)to iron-deficiency depends on the ability of iron absorption in plants[36]. The BAF reflects the ability of agricultural products to absorb trace elements from the soil[37]. The TF is the ability of the plants to transfer metals from roots to above-ground parts and the greater TF was directly correlated with the tolerance to metals in plants[38-39]. After the exposure to iron-deficiency for 40 d, the Fe concentration of the stems under the two iron treatments was higher than that of the roots and of the leaves. Consistent with the Fe concentration, the BAF and TF of the stems in the 3 apple rootstocks were both greater than those of the roots and of the leaves. Some previous studies have showed that, under iron-sufficiency conditions,citric acid’s ability to transport Fe3+was increased in xylem, thus facilitating active iron to be transported to other organs[40]. Therefore, it can be inferred from the results that the stem of apple seedlings was the main iron storage and transport organ, which absorbed iron from the roots. Under the iron-deficiency conditions,the apple rootstocks probably make rapid response to the resupply of iron by making iron quick access via xylem to young leaves; meanwhile, more iron was ensured to be transported to the top of plant by transpiration pull. However, more studies should be conducted to confirm this mechanism.
5. Conclusions
The results of this study have shown that the different apple rootstock species (varieties) had different-level tolerance to iron-deficiency. The principal component analysis found that the species’tolerance to iron-deficiency from high to low wasM. sieversii’s>M. baccata’s>M. prunifolia’s, which suggested thatM. sieversiihad better tolerance to irondeficiency largely due to its better root architecture than the other two species. The correlation analysis showed that the SOD activity of the roots, the plant height and the POD activity of the leaves were negatively correlated with the Fe concentration in the plants. Some morphological features in apple tree,such as root length, fresh shoot weight, above-ground biomass, plant height and lateral root number, could be improved to enhance the tolerance to iron-deficient stress of the rootstocks.
 Agricultural Science & Technology2018年3期
Agricultural Science & Technology2018年3期
- Agricultural Science & Technology的其它文章
- Review of Factors Affecting the Yield and Quality of Corn Silage
- Effects of Biochar on Substrate Degradation and Ammonia Emission during Aerobic Composting of Chicken Manure
- Phthalate Esters Biodegradation by Fusarium Oxysporum in Vegetable Soil
- One-off Mechanized Fertilization for Maize in Semiarid Area of Jilin Province
- Multiple Shoots Propagation with Bioreactor Culture of Labisia pumila (Bl.) F. Vill.
- Genetic Similarity Analysis of Hunan Rice Landraces with Same or Similar Name in Household and Genebank Conservations
