Recent developments on catalytic membrane for gas cleaning☆
Jiahao Chen,Zhaoxiang Zhong,*,Yongsheng Xia,Xuebin Ke,Weihong Xing,*
1 State Key Laboratory of Materials-Oriented Chemical Engineering,National Engineering Research Center for Special Separation Membrane,Nanjing Tech University,Nanjing 210009,China
2 Faculty of Science and Engineering,School of Engineering and Computer Science,University of Hull,HU6 7RX,United Kingdom
Keywords:Catalytic membranes Gas cleaning Membrane Catalysts
A B S T R A C T Catalytic membrane,a novel membrane separation technology that combines catalysis and separation,exhibits significant potential in gas purification such as formaldehyde,toluene and nitrogen oxides(NOx).The catalytic membrane can remove solid particles through membrane separation and degrade gaseous pollutants to clean gas via a catalytic reaction to achieve green emissions.In this review,we discussed the recent developments of catalytic membranes from two aspects:preparation of catalytic membrane and its application in gas cleaning.Catalytic membranes are divided into organic catalytic membranes and inorganic catalytic membranes depending on the substrate materials.The organic catalytic membranes which are used for low temperature operation(less than 300°C)are prepared by modifying the polymers or doping catalytic components into the polymers through coating,grafting,or in situ growth of catalysts on polymeric membrane.Inorganic catalytic membranes are used at higher temperature(higher than 500°C).The catalyst and inorganic membrane can be integrated through conventional deposition methods,such as chemical(physical)vapor deposition and wet chemical deposition.The application progress of catalytic membrane is focused on purifying indoor air and industrial exhaust to remove formaldehyde,toluene,NOx and PM2.5,which are also summarized.Perspectives on the future developments of the catalytic membranes are provided in terms of material manufacturing and process optimization.? 2019 The Chemical Industry and Engineering Society of China, and Chemical Industry Press Co., Ltd.All rights reserved.
1.Introduction
Atmospheric pollutants including industrial fumes(e.g.nitrogen oxides,and particulate pollutants)and indoor harmful gases(e.g.formaldehyde and toluene)are the main cause of environmental problems[1,2].The current technologies for air pollution control are adsorption[3,4],absorption[5-8],catalysis[9,10]and membrane separation[11].Among these technologies,membrane separation is widely employed for its high separation efficiency,pollution-free characteristic and low energy consumption.Catalytic membrane,which not only possesses membrane separation but also endows the membrane with catalysis [12], has been widely studied such as biomass gas reforming[13-16],gas sensor detection[17]and H2production[18-20].Catalytic membrane that follows a cake filtration mechanism can simultaneously remove particulate matters and harmful gases during the air pollution control[21].This process can be described as follows:the air stream carrying harmful gas is transported to the catalytic membrane.The membrane intercepts the soot particles and forms a cake layer on the membrane surface,then the gas steam passes through the membrane,makes contact with the catalyst and degrades into clean gas.One of the typical schematic diagrams of catalytic membrane degradation towards exhaust gases is shown in Fig.1[22].
Several reviews have been reported on catalytic membrane in biomass gas production and liquid phase reactions[23-25].Review on catalytic membrane for air pollution control is not available.Review in this area is critical to understand the current research directions of catalytic membrane in the catalytic degradation of harmful gases. So we discussed the recent developments of catalytic membrane preparation and its application in gas cleaning.The preparation of catalytic membranes is divided into organic catalytic membrane and inorganic catalytic membrane fabrication. The preparation of organic catalytic membrane includes impregnation, vacuum suction, spray coating,electrospray and electrospinning,in situ growth and atomic layer deposition.The fabrication of inorganic membrane includes coating,sol-gel method, chemical vapor deposition and wet impregnation method.The advantages and disadvantages of these technologies used in fabricating catalytic membranes are analyzed.Catalytic membranes are applied in purifying indoor hazardous gases such as formaldehyde,toluene and industrial fumes like particulate matters and NOxand its catalytic performance and separation performance are highlighted.
2.Preparation of Catalytic Membranes
Generally, the catalytic membranes can be divided into organic membranes(polymer substrate)and inorganic membranes(ceramic and metal substrate).Organic materials such as polytetrafluoroethylene(PTFE),polyimide(PI)and Polyphenylenesulfide(PPS)have fine antipollution performance as well as excellent acid and alkali resistance and operate at relative low temperature(less than 300°C).However,inorganic ceramic or metal materials such as silicon carbide(SiC),alumina(Al2O3),and zirconia(ZrO2)are used at higher temperatures(800-1000°C)due to its high mechanical strength,excellent wear and corrosion resistance and superior thermal stability.
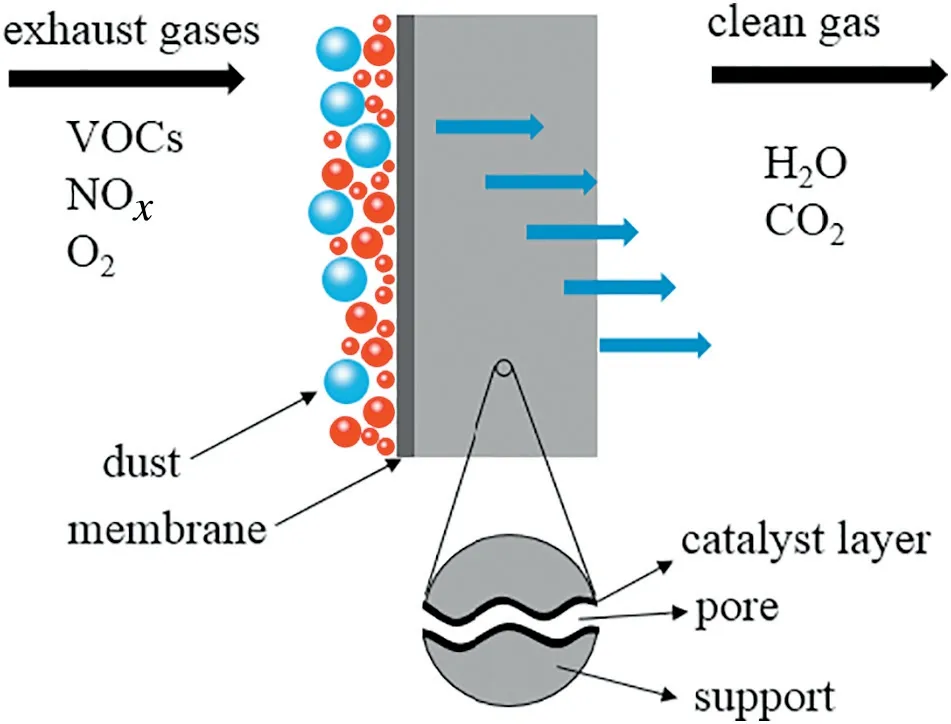
Fig.1.Schematic of catalytic membrane degradation towards exhaust gases[22].
2.1.Organic catalytic membranes
The organic catalytic membranes are prepared by modifying the polymers or doping catalytic components into the polymers [26,27].Several technologies have been employed such as coating,in situ growth of catalysts on polymeric membrane and grafting to endow the polymeric membrane with catalytic functions.
2.1.1.Catalysts coated on the surface of filter
The impregnation method is often used to coat the surface of the polymeric membrane with catalysts.Yang et al.[28]first synthesized a Mn-La-Ce-Ni-Oxcomposite solution and then infiltrated this suspension through a PPS filter via a vacuum suction method to obtain a Mn-La-Ce-Ni-Ox/PPS catalytic filter.A certain amount of PTFE emulsion with a mass percentage of 5%was used to immobilize the catalysts on the surface of the PPS filter.This obtained catalytic filter exhibits great resistance to chemical corrosion and high ash removal rate. On this basis,Yang et al.[29]used a novel method called functional foaming coating to prepare a Mn-Ce-Nb-Ox/P84 catalytic filter and applied this filter for removing nitrogen oxides and particulates.This Mn-Ce-Nb-Ox/P84 catalytic filter was achieved by immersing a P84 filter into the catalyst suspension with vigorous stirring and drying. Different amounts of catalyst coating on the Mn-Ce-Nb-Ox/P84 catalytic filter can be controlled through repeating this procedure.Han et al.[30]prepared a TiO2-polyester(PET)fiber filter using a spray coating method.Colloidal silica was added as a binder to the coating suspension.When the mass ratio of TiO2to binder (SiO2equivalent) is 1:1, the filter achieved the maximum removal efficiency.Compared with the traditional impregnation method,the spray-coating method has higher photocatalytic oxidation(PCO)efficiency and stability,making the catalyst distributed more evenly.Su et al.[31]fabricated a hierarchical structured TiO2/polyacrylonitrile(PAN)composite membrane via a simultaneous electrospray and electrospun process.Titanium dioxide(TiO2)suspension and PAN solution were selected and corresponding to electrospray and electrospun process, respectively. Fig. 2 shows the schematic of fabricating TiO2/PAN composite membrane. The TiO2nanoparticles were strongly combined with the PAN nanofibers under this process. Yang et al.[32]prepared TiO2/polyimide(PI)nanofiber composite membrane through electrospinning and annealing treatment.The results confirmed that the introduction of TiO2endows the TiO2/PI membranes with high photocatalytic activity and the PI nanofibers act as structural reinforcing material which can significantly improve the mechanical properties of the composite membrane.
The impregnation methods utilize the physical adhesion between the catalyst and the filter surface which results in a weak binding force between them.However,electrospinning can enhance the adhesion strength and provide high specific surface area and porosity but it is susceptible to solution properties and particle agglomeration.
2.1.2.In situ growth of catalysts
In situ growth of catalysts on filter is an effectively way to fabricate organic catalytic membrane which not only simplifies the preparation process but also enhances the bond strength between catalyst and the filter.
Zheng et al.[33]fabricated a MnO2-PPy/PPS catalytic filter via an in situ synthesis method and used it for selective catalytic reduction(SCR)of NO.This MnO2-PPy/PPS catalytic filter was prepared by uniformly decorating the surface of the filter with a manganese dioxide(MnO2)/polypyrrole(PPy)nanocoating.In detail, a PPS filter with a diameter of 40 mm was directly immersed in pyrrole acetone solution followed by dipping into an acidic KMnO4solution containing sulfuric acid.After washing with deionized water and drying,the MnO2-PPy/PPS catalytic filter was finally obtained.It was found that the pyrrole monomer is uniformly attached to the surface of the PPS filter due to the π-π conjugation effect between them.Then PPy was generated through in situ polymerization in acidic KMnO4solution while the KMnO4was reduced to nano-MnO2that can embed into the PPy matrix.Zheng et al.[34]also used the same way to prepare a Mn-CeOx/PPS catalytic filter with excellent denitration activity.They conclude that the PPS filter activated with nitric acid can generate oxygen functional groups which would promote the adsorption of Ce3+.The MnO2growth in situ on the PPS filter can be attributed to the redox reaction between KMnO4and HCl. Recently,Zheng et al.[35]obtained a nf-MnO2/PPS catalytic filter by first surface activation of PPS filter with surfactant sodium dodecyl sulfate(SDS)followed by potassium permanganate to generate nanoflower-like manganese dioxide growing evenly on the surface of PPS filter media.Fig.3 shows the schematic diagram of the nf-MnO2/PPS catalytic filter.Chen et al.[36]successfully fabricated a MnO2-Fe2O3/PPS catalytic filter by depositing MnO2-Fe2O3on the filter via a redox precipitation method and used this filter for low-temperature SCR of NO with NH3.Fig. 4 is the schematic of the MnO2-Fe2O3catalysts growing on the PPS nanofibers.The mechanism of MnO2-Fe2O3growing on PPS filter can be described as follows:The electrostatic force promoted the Fe3+ions to adsorb on the PPS filter and subsequently hydrolyzed to Fe(OH)3and HCl.Then,HCl was oxidized to Cl2due to the strong oxidizing property of KMnO4and in turn the KMnO4was reduced to MnO2.The MnO2-Fe2O3/PPS catalytic filter exhibits great NO degradation performance since the MnO2and Fe2O3strongly attached the PPS fibers.
2.1.3.Grafting of catalysts
Polymer modification is an effective way to prepare polymer catalytic membrane.Polymer modification includes blending and grafting.Among them,grafting is the most commonly used technology.Grafting is a process where the monomer is covalently bonded to the polymer chain to modify the surface of the polymer.There are two main grafting types[37]:grafting a single monomer and grafting a mixture of two(or more)monomers.Feng et al.[38]prepared poly(vinyl alcohol)(PVA)polymeric membrane and PVA/PAA blending membrane on the ceramic plate.Grafting the esterification catalyst Zr(SO4)2·4H2O on the active layer endows the membrane with catalytic property.Porous ceramic plates were used due to the poor chemical resistance and pressure resistance of porous polymeric membrane.It was found that detaching the catalyst active layer and the separation layer from each other during the fabrication of the catalytic membrane can prevent the catalytic component from diffusing into the separation layer as well as avoid direct contact between the catalyst and the active sites in the blending membrane.Feng et al.[39]first seeded a layer of ZnO nanocrystals on PTFE nanofibers through atomic layer deposition(ALD)and then the ZnO nanocrystals proceeded to form ZnO nanorods through hydrothermal reaction followed by depositing Ag nanoparticles on the surface of ZnO nanorods through a silver electroless deposition process to prepare hierarchical Ag/ZnO nanorods@PTFE catalytic filter,as is shown in Fig.5.Study has found that the vertical growth of dense uniform ZnO nanorods on PTFE fibers is beneficial to reduce the diameter of PTFE fiber so as to improve the gas permeation property and the specific surface area of the PTFE membrane.
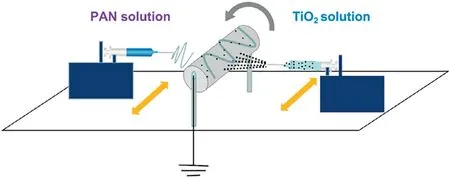
Fig.2.Schematic of fabricating a hierarchical structure TiO2/PAN composite membrane via a simultaneous electrospray and electrospun process[31].
Polymer grafting technologies such as atomic layer deposition not only endow the polymer with functions that are not available itself but also facilitate the contact between reactants and catalysts.In addition,functional materials can be grafted to polymer which depends on our requirement.
Several technologies such as impregnation,electrospinning and atomic layer deposition have been employed to fabricate organic catalytic membranes and their characteristics are summarized in Table 1.Conventional impregnation methods to fabricate organic catalytic membrane take advantage of the physical adsorption force. However,the electrospinning,in situ growth and atomic layer deposition endow the catalyst and the carrier with chemical bonding force which can improve the mechanical strength and the catalytic performance.
2.2.Inorganic catalytic membrane
The most common materials for the preparation of inorganic membranes are zeolite,metals and ceramics[40].Among them,ceramics are the predominant support of inorganic membranes due to its intrinsic thermal, chemical stability as well as superior mechanical strength.The commonly used ceramics include silicon carbide (SiC), alumina(Al2O3),zirconia(ZrO2)or a combination of these metal oxides.The inorganic catalytic membrane can be made from a catalytically active material or a catalytically active component embedded in the outer surface or inner pores of the membrane[41-43],as is shown in Fig.6.The catalyst and inorganic membrane can be integrated by conventional deposition methods, such as coating, sol-gel method and wet chemical deposition.
2.2.1.Catalysts act as a separation layer
Current views generally believed that the closer the distance between the catalyst and the membrane in catalytic membrane, the more effective the chemical reaction process will take place on catalyst since the mass transfer resistance of the product through the membrane is eliminated[44].This can be realized only when the catalysts act as separation layer.

Fig.3.Composite sketch of nf-MnO2/PPS catalytic filter[35].

Fig.4.Schematic of the MnO2-Fe2O3 catalysts growing on PPS nanofibers[36].
The TiO2membrane is a typical catalytic membrane which combined separation and catalysis.Membranes prepared by TiO2possess not only good separation performance but also excellent photocatalytic properties(PCO).Yuparat et al.[44]first coated TiO2suspension on the surface of the electrostatic air filter and then dried at room temperature to prepare a TiO2photocatalytic air filter for xylene removal.The study found that the substrate washed with 0.08 mol·L-1sodium dodecyl sulfate and 0.01 mol·L-1sodium fluoride prior to coating with TiO2suspension has the highest TiO2loading (285 g·m-2) on the filter. The TiO2particles agglomerated and adhered to the surface of the filter,resulting in less loss of TiO2loading.Ho[45]selected a ceramic filter with high surface area and three-dimensional(3D) porous structure to prepare a photocatalytic filter.This filter was coated with mesoporous TiO2membrane through the reverse micellar method for photocatalytic degradation of NO.Fig.7 shows the porous ceramic filter coated with TiO2membrane.The porous structure of the filter contributes to the enhanced flow turbulence and mixing in it.Also the high pore density of the TiO2membrane results in an enhanced photocatalytic activity.The TiO2membrane shows great potential in gas cleaning while its low response to ultraviolet makes it less efficient.
2.2.2.Catalysts loaded on the support
Zhao et al.[46]first deposited a layer of multi-walled carbon nanotubes (MWCNTs) on the Al2O3filter by chemical vapor deposition(CVD)and then a novel multifunctional Ag@MWCNTs/Al2O3catalytic filter with a hierarchical structure was obtained through embedding Ag nanoparticles on multi-walled carbon nanotubes.Fig.8 shows the schematic diagram of fabricating multifunctional Ag@MWCNTs/Al2O3catalytic filter with hierarchical structure.The introduction of MWCNTs significantly improved the BET surface area of Al2O3filter from(1.59±0.04) to(207.83±13.18)m2·g-1while the mean pore size was reduced from 3.24 μm(pristine filter)to 2.83 μm(catalytic filter).Leandro et al.[47]first synthesized the ceria-zinc mixed oxide catalysts via a solgel method and then deposited onto the cordierite substrates to prepare a catalytic filter.It was found that the Ce3+and Ce4+coexisting on the surface of the ceria-zinc mixed oxide catalyst further enhanced the catalytic ability.Gálvez et al.[48]prepared Al2O3-based catalytic filters by coating a catalyst suspension containing Cu,Co or V and K.Several suspensions were synthesized via a sol-gel method with Cu,Co,and V as catalytically active metals and K as promoter.Al2O3-based catalytic filter shows considerable activity both in NOxdegradation and soot removal.Liu et al.[49]prepared a catalytic filter by coating a mixed metal oxide catalyst Cu0.95K0.05Fe2O4on a diesel particulate filter(DPF).This filter can simultaneously remove NOxand particulate matter.
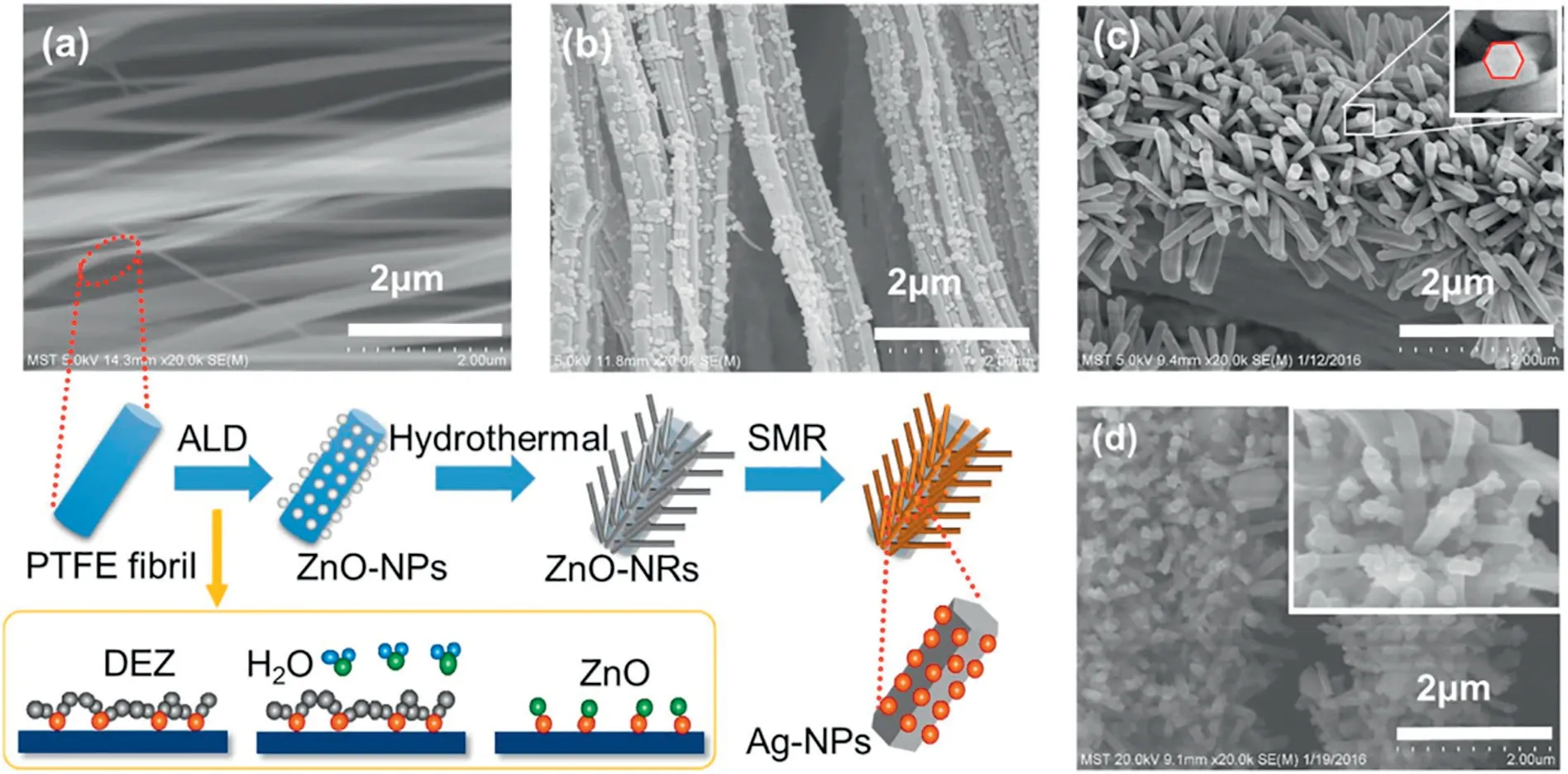
Fig.5.Schematic of the fabrication process of Ag/ZnO nanorods@PTFE filters and its corresponding SEM images at different stages of production:(a)Pristine PTFE,(b)ZnO-NPs@PTFE,(c)ZnO-NRs@PTFE,(d)Ag/ZnO-NRs@PTFE;inset image is a magnification micrograph of Ag/ZnO-NRs@PTFE[39].
Technologies such as chemical vapor deposition,sol-gel method and coating can promote the contact between reactants and catalyst which further improve the catalytic performance.However,the catalysts may drop from the support since coating relies on the physical adhesion.

Table 1Different fabrication technologies for organic catalytic membrane
2.2.3.Catalysts loaded inside support channels
Heidenreich et al.[22]obtained catalytic filter by depositing V2O5-TiO2-WO3into the pores of the SiC support through wet impregnation method. In this case, a dense mullite membrane (SiO2-Al2O3) was coated on SiC support to obtain a SiC filter.Then the filter was modified with TiO2solution and impregnated with NH4VO3and(NH4)6W12O39solution to obtain the catalytic filter.Choi et al.[50]prepared a catalytic filter with Pt-V2O5-WO3/TiO2catalyst supported on a SiC filter by vacuum aided dip coating.The Pt-V2O5-WO3/TiO2catalyst showed a synergistic effect for V2O5as the main catalytically active metal,WO3as promoter and TiO2as a coating layer to increase the BET surface area of the fresh SiC filter(0.06 to 0.36 g·m-2)for coating more catalysts.Pt was selected to provide elevated redox properties so as to enhance the photocatalytic reactions involving TiO2. Li et al. [51] prepared a new type of Pt/ZnO/SiC catalytic filter via a sol-gel method. Fig. 9 shows the morphologies of the bare SiC and the prepared Pt/ZnO/SiC catalytic filter.It was found that the introduction of ZnO layer increased the specific surface area of SiC by 8 times and the loading of Pt nanoparticle increased to 0.030 wt%(Pt/ZnO/SiC)from 0.017 wt%(Pt/SiC).However,the mean pore size of the catalytic filter was slightly reduced due to the introduction of ZnO coating layer but does not have a significant effect on the gas permeation rate.
Loading the catalysts into the support channel is an effective way to prevent the catalyst from being poisoned and wet impregnation method as well as sol-gel method are commonly employed during this process.Nevertheless,loading the catalysts into the support channel will inevitably result in flux decline since the catalysts occupied certain space of channel.
3.Catalytic Membrane in Gas Cleaning
3.1.Indoor gas purification
People spend most of their time indoors.Although the indoor air quality is less harmful to human health compared with industrial fumes,it may still result in other risks to human health[46].Indoor air pollution has become one of the primary environmental hazards to human health for these reasons [30]: fine particulate pollutants(PM2.5)enter human lung through breathing,affecting their respiratory system.For some volatile gas pollutants (such as formaldehyde and toluene),their concentrations indoor are always higher than outdoors[52].Therefore,the control of indoor air pollution is imminent.
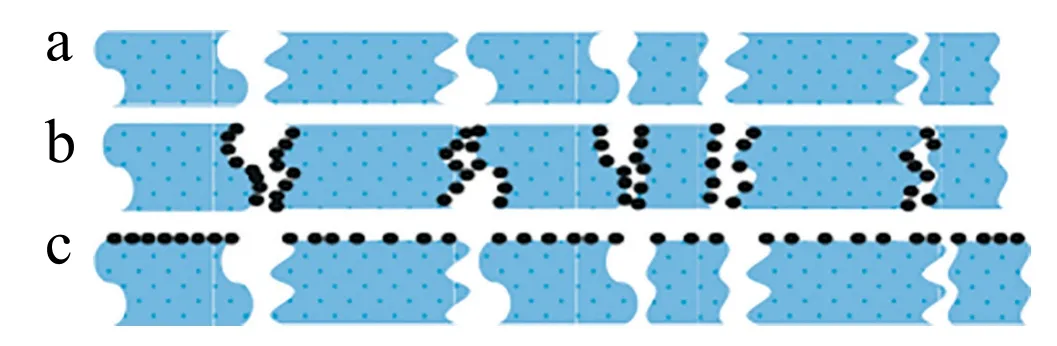
Fig.6.Different types of catalytic membranes with catalysts:(a)as a membrane layer;(b)inside pores;and(c)on the outer surface[43].
3.1.1.Formaldehyde treatment
Formaldehyde(HCHO)is a typical indoor pollutant.Formaldehyde with pungent odor causes allergy symptoms at relative low concentration and is a carcinogen.In the recent years,HCHO emitted from building decoration has been considered as one of the major causes of“Sick Building Syndrome”[53,54].In addition,HCHO is usually accompanied with small particles, which easily cover the active sites of catalyst resulting in a reduction on the degradation performance. Catalytic membranes can efficiently remove the small particles prior to the following catalytic process.
TiO2membrane has excellent photocatalytic activity under ultraviolet(UV)irradiation and can be applied to many occasions.Tetsuro et al.[53]prepared TiO2membrane on a soda lime glass by spin coating and placed it in a reaction vessel for photocatalytic reaction.The degradation rate of formaldehyde can reach up to 100%due to the high adsorption capacity of TiO2to formaldehyde.Photocatalytic degradation reactions can be described as follows[53]:TiO2generates protons and electrons under the irradiation of ultraviolet light.The formaldehyde then reacts with water and proton to produce formic acid and H+.Subsequently,formic acid reacts with proton to form CO2and H+.Finally,O2,electrons and hydrogen ions react to produce water. Below are the concrete

Fig.7.Image of the porous ceramic filter coated with TiO2 membrane.Insert image is high magnification of porous structure in ceramic filter[45].
photocatalytic reaction equations:

Oxidation:
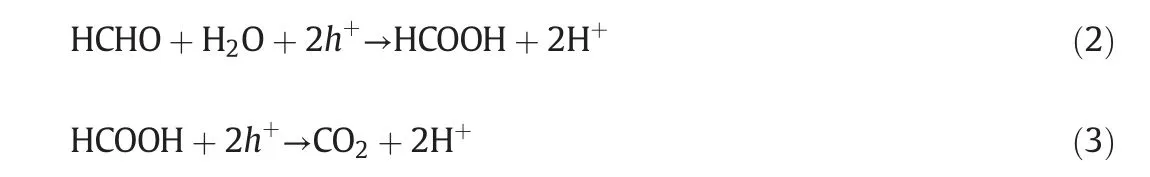

Wang et al. [55] prepared a TiO2 membrane through a surfactant assisted sol-gel process.The TiO2 membrane showed superior photocatalytic activity towards formaldehyde at room temperature with a 99% degradation rate can be realized. The mesoporous structure of TiO2 accounts for the high degradation rate since the mesoporous structure not only facilitates the mass transfer of the photochemical reaction,

Fig.9.SEM images of the morphology of(a)bare SiC,(b)ZnO coated in SiC pore,(c)Pt loaded in SiC,and(d)the prepared Pt/ZnO/SiC catalytic filter[51].
Reduction:but also increases the specific surface area of the membrane which further promotes the UV light absorbance.Chen et al.[56]prepared a Pt/ZnO/TiO2nanotube filter via modification of TiO2nanotube arrays and impregnation process.Formaldehyde and O2were oxidized to produce H2O and CO2under the catalysis of Pt and the conversion of formaldehyde is still 95% after 100 h of reaction. Han et al. [30] immobilized TiO2nanoparticles on the PET filter through spray coating and used it for photocatalytic degradation of formaldehyde.The results show that the degradation rates of formaldehyde before and after the stability test were 92.86%and 91.96%,respectively.
Feng et al.[39]prepared Ag/ZnO nanorods@PTFE catalytic filters by atomic layer deposition(ALD)and Ag electroless deposition.The results showed that the PTFE membrane modified with ZnO nanorods exhibiting an excellent dust retention effect with a 99.9999%dust rejection rate can be achieved at a relative low pressure drop.It was also found that the ZnO nanorods and Ag nanoparticles are beneficial to the antibacterial activity of the catalytic membrane,and the antibacterial activity against Escherichia coli and Staphylococcus aureus can reach up to 99.9%. The photocatalytic activity of Ag/ZnO nanorods@PTFE catalytic membrane towards formaldehyde reached 60%when irradiated with UV light.The Ag/ZnO nanorods@PTFE catalytic membrane possesses higher efficiency with a 7%improvement in removing formaldehyde compared with the Ag-free membrane.Zhao et al.[46]prepared a multifunctional Ag/MWCNTs@Al2O3hybrid filter via chemical vapor deposition (CVD). The FESEM images of particle retention by Ag/MWCNTs@Al2O3are shown in Fig.10.The simulated aerosol particles used in this work were SiO2particles with a diameter of 296 nm.From the FESEM images we can get that the SiO2nanoparticles were retained by the MWCNTs of the functionalized filter.The MWCNTs/Al2O3and Ag@MWCNTs/Al2O3hybrid filters could effectively intercept SiO2particles with a retention rate of 100%in 120 min,while the retention rate for pristine Al2O3filter was just 79.88%.In addition,the novel fabricated air filter exhibits excellent formaldehyde degradation rates of 82.24% at room temperature and 99.99%at 55°C.When comparing the hybrid filter with the pristine Al2O3filter,complete interception of indoor airborne microorganisms and particles can be realized at 35.60%pressure drop.This multifunctional filter has the highest quality factor(QF,particle rejection with a given particle size and pressure drop)and exhibits excellent hydrophobicity which contributed to the prolonged operating life as well as improved air purification efficiency,showing great promise to replace a single filter.

Fig.10.FESEM images of the SiO2 nanoparticles intercepted by the Ag@MWCNTs/Al2O3 hybrid filter.SiO2 particles retained by the Ag@MWCNT networks of the Al2O3 filter(a,b),retained in the inner pores of the Al2O3 filter(c),retained on the surface of the Ag@MWCNTs/Al2O3 hybrid filter(d)[46].
The TiO2membrane, as well as Ag/ZnO nanorods@PTFE and Ag/MWCNTs@Al2O3catalytic filters,shows superior performance towards degrading formaldehyde and intercepting dust. However, Ag/ZnO nanorods@PTFE catalytic filter owns excellent antibacterial activity and Ag/MWCNTs@Al2O3hybrid filter possesses excellent hydrophobicity.All of these are not available in TiO2membrane which proves the superiority of Ag/ZnO nanorods@PTFE and Ag/MWCNTs@Al2O3catalytic filters.
3.1.2.Treatment of gaseous benzene
Toluene is a typical indoor aromatic contaminant.Paint used in decoration contains large amounts of toluene,and excessive inhalation not only leads to aplastic anemia,but also increases the risk of leukemia.Catalytic membrane can be used to decompose and separate gaseous benzene.
Li et al.[51]prepared a new type of Pt/ZnO/SiC catalytic filter via a sol-gel method.The deposition of a ZnO layer significantly improves the loading of Pt nanoparticles from 0.017 wt%for Pt/SiC to 0.030 wt%for Pt/ZnO/SiC which further promotes the oxidation of toluene from 50.4%to 100%at 210°C.In addition,Pt/ZnO/SiC catalytic filter exhibited excellent toluene conversion performance(100%)at 210°C for 240 h when used in the long term tests.Li et al.[57]used the same method to fabricate a SiC@TiO2/Pt catalytic filter for simultaneously removal of mesitylene and particles and the concrete diagram is shown in Fig.11.This fabricated catalytic membrane exhibits excellent performance with completely degradation of mesitylene and 99.98%removal rate of Al2O3simulated dust (mean size 300 nm), which can be realized under the gas speed of 1.0 m·min-1and 262°C.The initial pressure drop of the SiC@TiO2/Pt catalytic filter was just 171 Pa.After 20 cycles of filtration and back pulsing, the pressure drop only increased to 185 Pa(Fig.12).Zou et al.[58]synthesized porous TiO2/SiC catalytic membrane on Al2O3substrate by ball milling and screen printing techniques.Compared with the pure TiO2membrane,the conversion of toluene for TiO2/SiC membrane increased by 1.61 times when toluene is mixed with dry air(relative humidity 6.5%).Su et al.[31]successfully prepared TiO2/PAN nanofiber membrane with hierarchical structure.Fig. 13 shows the mechanism of TiO2/PAN nanofiber membrane towards PM retention as well as toluene degradation.The constructed hierarchical structure can not only enhance the mechanical strength of the membrane but also increase the valid membrane surface area which is favorable to enhance the particle and toluene removal efficiency.This not only endows the composite membrane with enhanced photocatalytic activity but also improves its filtration performance.By calculating the quality factor QF, the composite membrane has the most balanced photocatalytic activity and filtration efficiency when the mass ratio of TiO2/PAN was 3:1.At this ratio,the conversion of toluene was 78%and the particle rejection was 89%.
Noble metal catalyst is effective for toluene oxidation while the high cost restricts its industrial application.Non-precious metal due to its low cost has been widely researched even the catalytic performance is not as well as noble metal catalyst.The efficient methods for improving the catalytic activation of non-precious metal are usually by improving the loading amounts and constructing hierarchical structure of catalyst membrane.
3.2.Catalytic membrane applied in industrial exhausts
Global warming,acid rain and other environmental problems such as the destruction of the ozone layer caused by air pollutants are drawing major attention.These air pollutants are mainly particulate matters(PM) and nitrogen oxides (NOx). Therefore, intensive treatment is needed for these pollutants before released to the atmosphere.
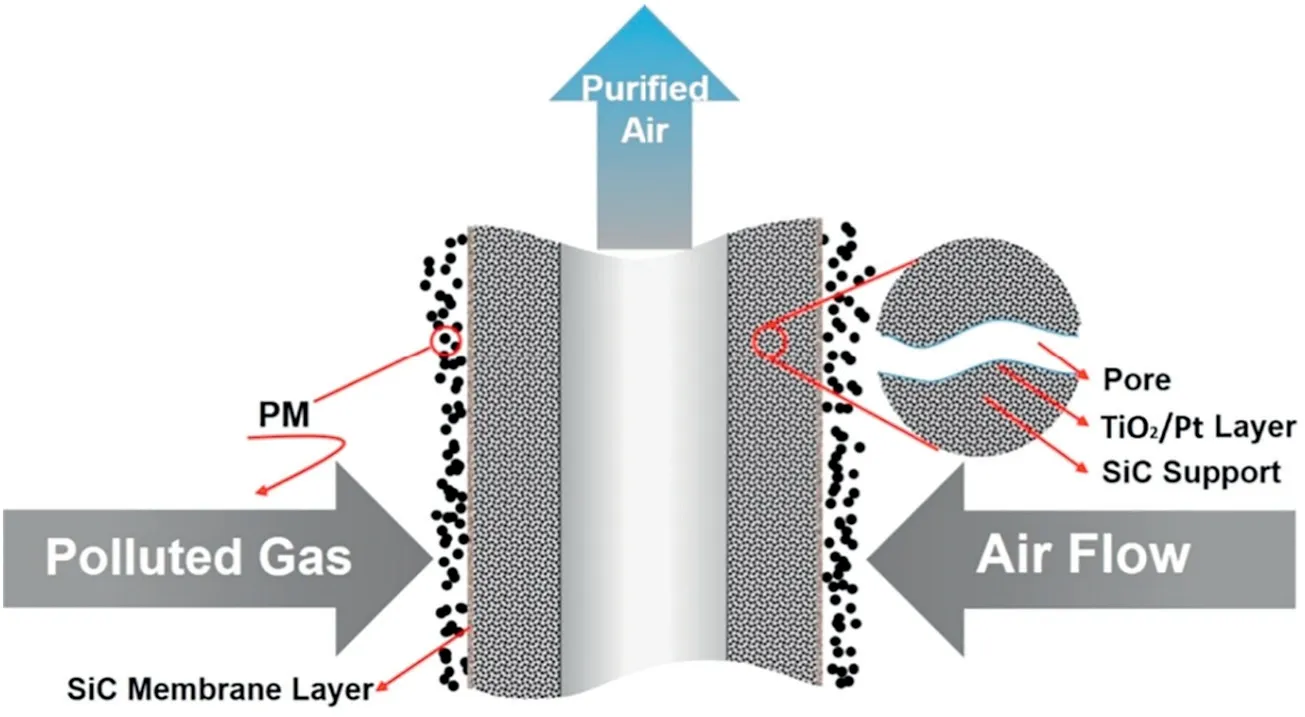
Fig.11.Schematic diagram of the mechanism of SiC@TiO2/Pt filter catalytic oxidation towards mesitylene[57].
3.2.1.Particulate pollution control
Incomplete combustion of fuel or engine produces many fine particles.Their emission to the atmosphere causes serious environmental damage.Fine particles are harmful substances and potential carriers or reactants for toxic metals,bacteria and viruses because of their large surface area[59].Prolonged exposure to PM2.5 may cause stroke,lung disease and cancer [31]. In addition,PM2.5 can reduce the visibility,which is the main cause of smog.Therefore,the treatment of particulate pollutants is urgent.
Ceramic filter loaded with catalysts can simultaneously filter and oxidize particles so as to maintain a low pressure drop and avoid the block of channel.Guido et al.[60]studied the catalytic filter to remove fuel particles by performing experimental and mathematical models.The results showed that the soot particles can be completely oxidized and the overall filtration efficiency is 80%.In this process,a low pressure drop can be maintained.Ciambelli et al.[61]prepared a soot particulate catalytic filter on a laboratory scale through depositing a Cu-V-K-Cl catalyst in Al2O3filter.The results showed that the filtration efficiency is greatly affected by α(feed mass flow ratio of air/fuel).The filtration efficiency decreased from 80%at α=40 to 60%at α=25 and finally to 40%at α=22.When α is 25,catalytic filter with 0.34 dm3was capable of treating about 450 dm3·min-1of exhaust gas.Ciambelli et al.[62]explored the effect of different volumes on the removal of PM from exhaust gases. They studied the catalytic oxidation of soot particles under the largest volume.The results showed that the catalytic filter displays an efficiency of 70%in removing soot particles and can simultaneously filter and burn the captured soot and partially accumulated soot at a high temperature of 650°C.Finally,Ciambelli et al.[63]compared the degradation of diesel engine exhaust by Cu-V-K-Cl supported on commercial alumina (92% Al2O3+ SiO2) filter and on pure Al2O3(99.9%)ceramic filter.They found that the particle filtration efficiencies of pure Al2O3and commercial Al2O3ceramic filters are 50%and 70%,respectively.

Fig.12.(a)Conversion rate of mesitylene as well as particle rejection as a function of the reaction temperature at the filtration velocity of 1.0 m·min-1, (b) dust rejection performance of the prepared SiC catalytic membrane and its corresponding pressure drop after 20 cycles of back pulsing[57].
3.2.2.Nitrogen oxide(NOx)degradation
Mixtures of nitric oxide(NO),nitrogen dioxide(NO2)and nitrous oxide (N2O) emitted from engines and industrial processes, often expressed as“nitrogen oxides”or“NOx”,result in different air pollution such as photochemical smog and acid rain[64,65].NO is a colorless,toxic gas that can rapidly react with atmospheric oxygen(O2)to form NO2. NO and NO2coexisting in atmosphere can produce harmful ozone at low atmospheric pressure. In addition, NO2can react with water to generate nitrous acid(HNO2)and nitric acid(HNO3)which endanger the atmosphere and ecological health[65].Therefore,it is necessary to degrade nitrogen oxides to N2, O2or other non-toxic and harmless gas.
Selective catalytic reduction(SCR)of NOx(x=1,2)with ammonia is an effective way to reduce nitrogen oxide emissions[66-68].Normally the harmful nitric oxide and nitric dioxide can react with ammonia to produce nitrogen and water which is a clean way to degrade nitrogen oxides.The concrete reactions of NOxand ammonia are as follows[65]:

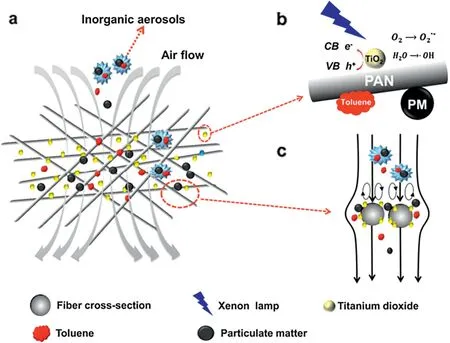
Fig.13.Mechanism of TiO2/PAN nanofiber membrane towards PM retention as well as toluene degradation[31].
Vanadium-based catalytic membranes are most commonly used in degrading NOx[69,70].Nacken et al.[71]coated TiO2-V2O5-WO3on a SiC-Al2O3porous ceramic filter.This filter can effectively degrade NOx.When the filtration rate of 2 cm·s-1is achieved,the maximum NO conversion of 96%can be obtained at 300°C.Kim et al.[72]studied the effect of adding MnOxto the TiO2-V2O5-WO3catalytic filter.The catalytic filter had a very wide temperature range(150-340°C)after addition of MnOxand achieved a NO conversion over 99.5% compared with the case without MnOxaddition.Fino et al.[73]prepared a MnOx·CeO2-V2O5·WO3·TiO2/PTFE catalytic filter by impregnation and microwave drying.The conversion of NO and benzene can reach up to 80%when operating at 30 N·m-3·m-2·h-1.The SO2poisoned catalyst can be recovered through dry-cleaning. Kim et al. [74] fabricated porous Pt-V2O5-WO3-TiO2/SiC catalytic filter for degrading NO. The results showed that NO is completely removed and the addition of Pt to the V2O5-WO3-TiO2/SiC catalytic filter significantly shifted the operating temperature from 260 to 340°C(V2O5-WO3-TiO2/SiC catalytic filter)to 160-240°C.

Park et al.[75]coated the CuMnOxcatalyst into the polysulfonamide(PSA)filter through vacuum suction in order to remove NOx.The catalytic filter had the highest NO removal efficiency of 94%at 200°C with a catalyst loading of 550 g·m-2.Yang et al.[28]prepared the Mn-La-Ce-Ni-Ox/PPS catalytic filter to simultaneously remove the particulate matter and degrade nitrogen oxides.The Mn-La-Ce-Ni-Ox/PPS catalytic filter exhibited the maximum conversion rate of 98.3%for NOxat 200°C and still maintained at 85%even with the presence of SO2,showing a great resistance to SO2poisoning.Chen et al.[36]successfully deposited MnO2-Fe2O3catalysts on PPS filter and used it for NO degradation.The results showed that a NO conversion range of 79.8%-99.2% can be achieved at a range of 140-180 °C with the gas hourly space velocity(GHSV)of 120000 cm3·g-1·h-1.Zheng et al.[33]prepared a MnO2-PPy/PPS catalytic filter through an in situ synthesis method for degrading NO.The results exhibit more than 70%conversion rate of NO at 160-180°C with the GHSV of 38000 h-1.
Although the efficiency of selective catalytic reduction of NOxwith NH3is very high,the introduction of a reducing agent such as ammonia during the reaction easily leads to deactivation or poisoning of the catalyst.Also the separation between ammonia and reducing gas is difficult which requires subsequent separation.Catalytic oxidation can treat NOxwithout introducing any reducing agents.Agus et al.[76]observed that NO can be endowed with a catalytic action when catalytic filter is used to remove soot particles and NOxat the same time,so that the reaction could be carried out continuously.The reaction equations were as follows:

Despite the production of CO as secondary product during this process,the CO emission concentration was 2-3 times lower than that of the commercially available continuous regeneration filters.Gálvez et al.[77]chose different highly dispersed boehmite as alumina precursors to prepare catalytic filters with Cu,Co and K as the active phase and used these filters for catalytic oxidation of NOx.Study has found that Co-containing catalysts are more active than Cu-containing catalysts in removing NOxand the maximum degradation of 62%for NO at temperatures between 475 and 575 °C. Liu et al. [49] coated a Cu0.95K0.05Fe2O4mixed metal oxide catalyst on a γ-Al2O3filter for simultaneous catalytic degradation of NOxand particulate matters.It was found that the oxidation temperature of NOxis the same as that of CO2,which means that NOxand PM can be simultaneously removed in the oxidizing atmosphere and the oxidation of PM is enhanced in the presence of NO and O2.However,this filter showed only 18%NOxdegradation.
There are also several researches concentrated on both degradation of NO and the particle rejection.Lee et al.[78]prepared a cordierite catalytic filter with V2O5loading.The removal rates of catalytic filter for soot and NOxwere 99.6%and 80%,respectively.The NOxremoval rate could increase to 90%by increasing the surface area of the filter components via an acid treatment.Kang et al.[79]infiltrated MnOxinto a bag filter using a vacuum suction method and placed between a polytetrafluoroethylene (PTFE) membrane and a polytetrafluoroethylene foam.The schematic diagram of the NOxdegradation as well as particle retention is shown in Fig.14.Dusts were well intercepted and collected by the membrane.The residual NOxwas degraded to the clean N2by the MnOxcatalyst which was protected by the filter from being plugged or poisoned. Catalytic bag filter with a 480 g·m-2MnOxloading has a 92.6%NOxconversion and a 99.9%particle removal efficiency at 423 K.Yang et al.[29]fabricated a Mn-Ce-Nb-Ox/P84 catalytic filter via a functional foaming coating method for simultaneous removal of particulates and nitrogen oxides. It was found that the prepared catalytic filter with a catalyst loading of 450 g·m-2can achieve a removal efficiency of 95.3% for NO and 99.98%for PM2.5 at 200°C.In addition,the NO removal efficiency can still maintain at 85.6%when SO2is introduced.
Selective catalytic reduction is a relative mature method used in degrading NOx.However,introduction of reduction agent like NH3will oxidize with the temperature rise which further reduces the validity of NO degradation.Also,NH3is corrosive to the pipeline which increases the maintenance costs.Catalytic oxidation,not needing any reducing agents,shows great flexibility in degrading NOx.However,its conversion rate is low compared with selective catalytic reduction method.Table 2 compares the performance of different catalytic membranes used in degrading NO.
It can be clearly seen that all MnOxcatalysts have high NO conversion in these catalytic membranes with a relative low reaction temperature at the range of 140-210°C.However,MnOxis susceptible to be poisoned by SO2and the narrow operation temperature window limits its industrial application.The TiO2-V2O5-WO3catalyst normally takes effect over 300 °C and owns a high degradation rate towards NO which has been widely applied in industry.Unfortunately,the comparative high operation temperature window always represents high energy-consumption for vanadium related catalysts. Further, the inherent toxicity to humans and environment calls for clean and efficient catalyst. In summary, addition of rare earth metals like Ce, Nb and transition metals can enhance the catalyst activity as well as the anti-sulfur dioxide poisoning.
4.Outlook
Catalytic membrane,made up of catalyst and membrane in various forms,is a type of membrane that simultaneously performs membrane separation and catalysis,in which the membrane traps particulate matters and the catalyst degrades harmful gas into non-toxic and harmless gas.Catalytic membranes are either based on organic and inorganic substrates.Several technologies have been applied to fabricate organic catalytic membranes such as electrospinning,in situ growth,and atomic layer deposition.In addition,sol-gel method,impregnation and chemical vapor deposition are commonly employed to fabricate inorganic catalytic membranes.Among these technologies, in situ growth is most effective as it not only enhances the bond strength between catalysts and the filter but also prolongs the service life of the catalytic membrane.Catalytic membranes have many applications in indoor harmful gases and industrial exhaust. The catalytic membrane can degrade formaldehyde and toluene, which are harmful to human health, to CO2and H2O without causing other pollution under normal conditions.Improving catalyst loading and constructing a hierarchical structure are effective ways to enhance the catalytic performance which are suitable not only for toluene and formaldehyde but also equal validity for other harmful gases.Ceramic filter loaded with catalysts can simultaneously filter and oxidize particles at a relative low pressure drop and avoid the block of channel.Selective catalytic reduction and catalytic oxidation are often employed when treating with NOx.Among the two technologies, catalytic reduction of NOxwith NH3is mostly used as the degradation efficiency is high and the final products are only N2and H2O.However,catalytic oxidation is preferred compared with selective catalytic reduction with NH3in the treatment of NOxsince the NH3will oxidize with the temperature rise which will reduce the validity of NO degradation.
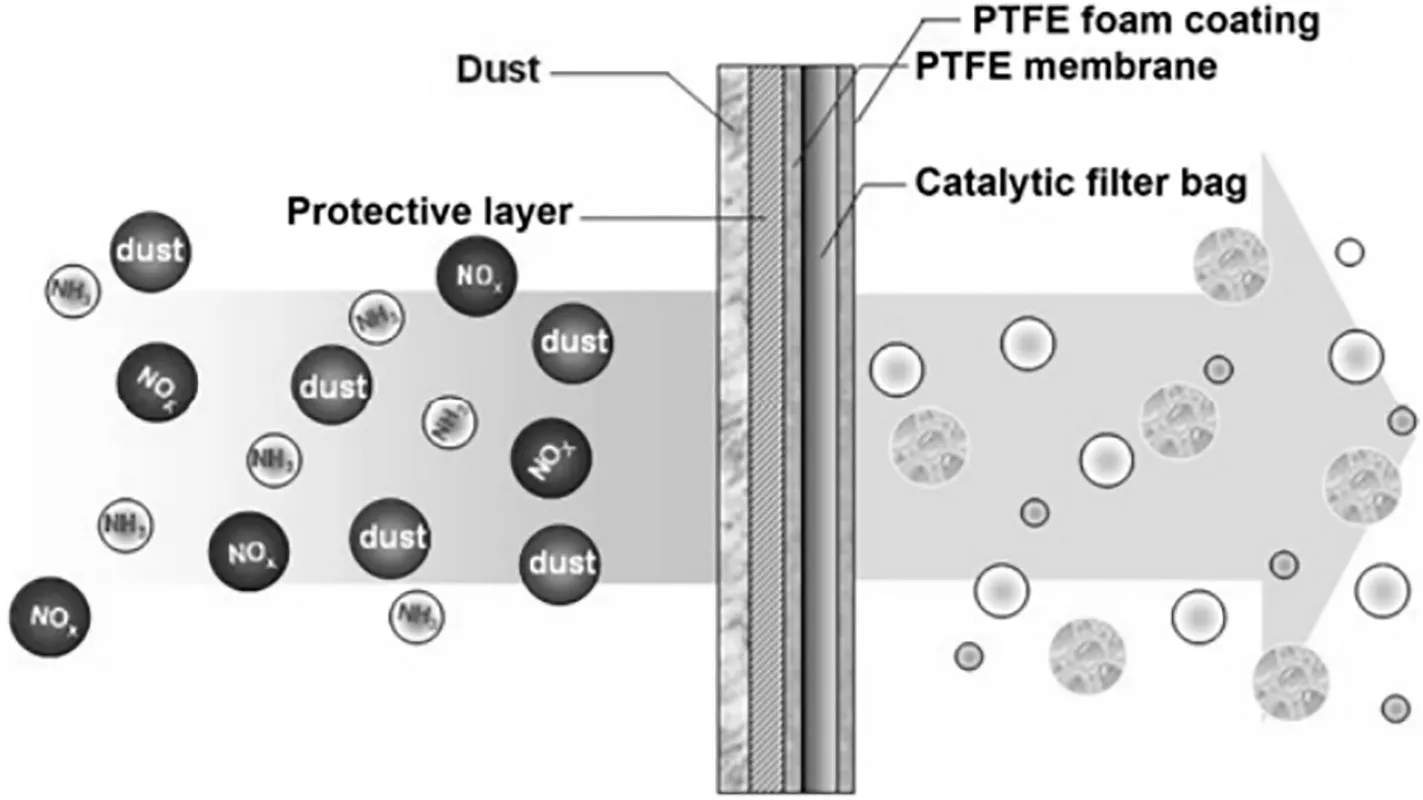
Fig.14.Schematic diagram of the NOx degradation as well as particle retention process through the MnOx-coated filter[79].
In the future,new approaches should be developed to overcome the problems of catalytic membrane flux since the flux will inevitably drop when the catalysts bind to the support.For example,efforts can be made to fabricate monoatomic catalysts in the support which will maximize the efficiency of catalyst and reduce the loss of flux.Also,SO2poisoning is a troublesome question when treating with NOxand developing novel strategies to enhance the resistance of catalyst poisoning is of great significance.Considering that the catalysis and separation are combined,the no longer needed separation unit would reduce the related equipment cost.In addition,solid matters retained by the membrane can protect the catalyst from being inactivated,which can prolong the duration of the catalyst.Membrane with catalytic activity will be an advantageous way to maximize economic efficiency(conversion and productivity of the target products, enhanced thermal stability, reduction of operating temperature,etc.)and the leading development trend of catalytic membrane technology.Atomic layer deposition(ALD)is a novel technology which can endow the membrane material with functions that are not available itself.In addition,electrospinning can be widely used to manufacture multi-functional membrane material.Fabrication of functional catalytic membrane with low cost and high efficiency can be a research hotspot in the future.

Table 2Comparison of NOx degradation performance using different catalytic membranes
 Chinese Journal of Chemical Engineering2019年6期
Chinese Journal of Chemical Engineering2019年6期
- Chinese Journal of Chemical Engineering的其它文章
- Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
- Amorphous and humidity caking:A review☆
- Intensification of chemical separation engineering by nanostructured channels and nanofluidics:From theories to applications☆
- Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants☆
- Progress in molecular-simulation-based research on the effects of interface-induced fluid microstructures on flow resistance☆
- Advancement in separation materials for blood purification therapy☆
