Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants☆
Beijing Key Laboratory of Bioprocess,Beijing Advanced Innovation Center for Soft Matter Science and Engineering(BAIC-SM),Beijing University of Chemical Technology,Beijing 100029,China
Yilin Zhao#,Yaoqiang Wang#,Gang Xiao*,Haijia Su*
Keywords:Biomaterial TiO2 Composite photocatalysts Trace pollutants
A B S T R A C T Trace environmental pollutants have become a serious problem with special attention on the hazardous heavy metals,refractory organics,and pathogenic microorganisms.With coupling biosorption and photocatalysis to develop biomaterial/TiO2 composite photocatalysts is a promising method to remove these trace pollutants because of the synergistic effect.Biomaterials provide multiple function groups which can selectively and efficiently enrich trace pollutants onto the surface of the photocatalysts,thus facilitating the following transformation mediated by TiO2 photocatalysis.Biomaterials can also help the dispersion and recovery of TiO2,or even modify the band structure of TiO2.The fabrication of chitosan/TiO2,cellulose/TiO2,as well as other biomaterial/TiO2 composite photocatalysts is discussed in detail in this review. The application significance of these composite photocatalysts for the selective removal of trace pollutants is also addressed.Several problems should be solved before the realistic applications can be achieved as discussed in the final section.?2019 The Chemical Industry and Engineering Society of China,and Chemical Industry Press Co.,Ltd.All rights reserved.
1.Introduction
Environmental pollution has become a serious problem overall the world,especially in developing countries like China.Heavy metals,refractory organic pollutants, pathogenic microorganisms are three major pollutants [1-3]. These pollutants, even in trace amount, will cause serious threaten to human's health.Heavy metals (Hg, Pb,Cd,Ag,Cu,Ni,etc.)are discharged from the wastes of various industries such as mining,fertilizer and pesticide industry and application,metallurgy,electroplating,photography,and atomic energy installation[4].They are highly toxic,carcinogenic,non-biodegradable,and will accumulate through the food chain, thus imposing tremendous health risks towards humans [5,6]. For example, long-term exposure to Cd can cause serious bone and kidney damage,and Hg is related to neurological disorder,disablement,blindness,and genetic defects[7,8].Refractory organics,such as pharmaceuticals,dyes,and plasticizers,are characterized by non-biodegradability, easy bioaccumulation, and widespread distribution in the environment[9,10].These compounds can lead to severe health threats such as chronic toxicity,endocrine disruption,and the development of pathogen resistance[10].Other than these two kinds of pollutants,pathogenic microorganisms have long been recognized as a kind of severe health risk to humans by causing various diseases[11].
Photocatalysis can convert light energy to generate energetic charge carriers which will trigger redox reactions for the molecules adsorbed on the photocatalysts [12]. Among the various semiconductor photocatalysts, nano-sized TiO2has been widely accepted to be the most promising one because of its favorable characteristics of low cost,non-toxicity,and long-term stability[13].TiO2photocatalysis has been successfully utilized to treat various pollutants [14]. For heavy metals,the metal ions can be reduced by the photo-generated electrons and removed or recovered[15].Actually,the photocatalysis mediated deposition of metal on photocatalysts has been widely accepted to be a promising procedure falling into the concept of turning waste into treasure,and the recovered metals can find wide applications such as catalyzing fine chemical synthesis as efficient nanocatalysts[16].For refractory organics,these pollutants can be efficiently decomposed into smaller molecules and finally into inorganics due to the strong oxidizing ability of the photo-generated reactive oxygen species(ROSs)[17].For pathogenic microorganisms,the cell wall and membrane will be fully destroyed under the oxidative pressure,leading to the deactivation of the cell,then the released cellular compounds can be further degraded into inorganics[18].
However,for the treatment of trace pollutants,photocatalysis by bare TiO2is always not efficient because TiO2alone is not always a good adsorbent for the pollutants. The light driven redox reactions should happen on the surface of photocatalyst and the adsorption of the pollutant molecules onto TiO2is prerequisite[19].
Photocatalysts are usually confined in a substrate to help their dispersion and facilitate their subsequent recovery from the environmental compartment after remediation,resulting in portable and“green”photocatalytic platforms.If the substrate is well designed,it can retain target pollutants close to the surface of the photocatalysts thus increasing the photocatalytic efficiency, especially for trace pollutants. Biosorption using live or deactivated biomaterials such as Saccharomyces spp.,Sargassum seaweed,chitosan,and cellulose,can efficiently adsorb various pollutants, thus enrich the trace pollutants and facilitate the further treatment[4,20-22].Immobilization and modification of nano TiO2with these biomaterials are promising ways to achieve the efficient enrichment of trace pollutants as well as the recyclability of nano TiO2.[2,3,23,24]Therefore, developing biomaterial/TiO2composite photocatalysts is a promising method to selectively remove trace environmental pollutants,and the obtained organic-inorganic composites exhibit combined and super-additive characters from both organic and inorganic materials.
The aim of this review is to summarize the recent advances in the development of biomaterial/TiO2composite photocatalysts and their applications in the treatment of trace pollutants,including our own research,and to address the challenge and future directions of combining biosorption and TiO2photocatalysis for environmental remediation.
2.Fabrication of Biomaterial/TiO2 Composite Photocatalysts
2.1.Chitosan/TiO2 composite photocatalysts
Chitosan,which is produced by deacetylation of chitin,is one of the most abundant biopolymers in nature,with unique properties such as biodegradability,biocompatibility, non-toxicity and low-cost [25].It has been widely used for environmental remediation acting as biosorbent for various pollutants or natural amicrobial agent[25].Chitosan/TiO2is one of the most widely studied biomaterial/TiO2composite photocatalysts because chitosan is a good support for the dispersion of TiO2nanoparticles and the synergistic effects between them can be expected[26].
Using the well-developed synthesis procedures for chitosan based composites, multifunctional chitosan/TiO2composite photocatalysts can be easily obtained because of the miscibility between chitosan and hydrophilic TiO2[26]. Pincus et al. [27] reported a novel nano-TiO2-enabled chitosan beads cross-linked with copper which is capable of oxidation of arsenite to the less-toxic and more easily adsorbed arsenate under UV light and selective adsorption of As in the presence phosphate,a strong adsorptive competitor and inhibitor of arsenic removal.Selective adsorption and photocatalytic reduction of heavy metals ions were also achieved by chitosan/TiO2composite photocatalysts.Typically,trace Ag ions were anchored onto the imprinted sites of chitosan and further reduced to well-dispersed Ag nanoparticles with various morphologies and the obtained Ag nanoparticles possessed favorable antimicrobial and catalytic activities [16,28,29]. Other than heavy metals,organic pollutants and pathogenic microorganisms can be also efficiently removed by the multifunctional chitosan/TiO2composite photocatalysts [30,31]. However,the microparticles of chitosan/TiO2composite possess relatively low surface area,thus restricting the efficient utilization of the active sites for adsorption and photocatalysis.
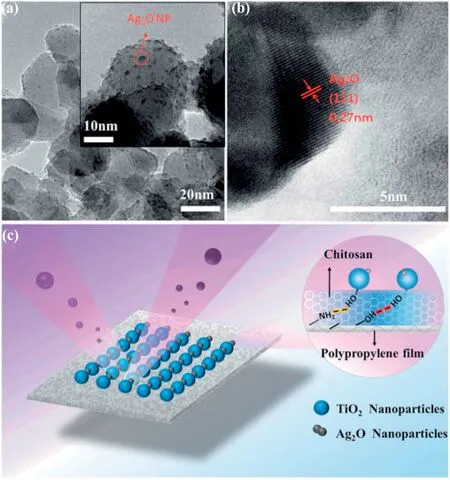
Fig.1.(a and b)HRTEM image and(c)schematic structure of the ATCPF[32].
Casting the chitosan/TiO2composite photocatalysts into films with or without substrate is an efficient way to enhance the accessibility of the active sites[31,32].For example,a novel sunlight active Ag2O/TiO2-modified chitosan-based photocatalytic film(ATCPF)was successfully prepared with high adsorption and photocatalytic activity,based upon the coupling of the synergistic catalytic technique of nano Ag2O/TiO2and membrane separation(Fig. 1) [32].It showed enhanced activity for the removal of both ampicillin and methyl orange based on adsorption and photocatalysis.Moreover,the photocatalytic film displayed excellent recycling properties for the degradation of methyl orange by being reused for 5 times without losing its photocatalytic activity.

Fig.2.Pictures of chitosan/TiO2 composite fiber treated with respective metal salt solutions[35].
As an abundant natural biomass,chitosan nanofibers are increasingly attracting more attention due to the structural advantages conveyed by the nanosized diameter of the constituent fibers [33].Decreasing fiber diameter caused many beneficial effects, such as increased specific surface to volume ratios[34].This provides a possible way to prepare chitosan/TiO2composite fibers with improved surface area and activities.Asiri et al.[35]report a simplistic approach to synthesis zero-valent metal nanoparticles modified chitosan/TiO2composite fibers which has good catalytic ability for all types of organic pollutants(Fig.2).The separation of the fibers was easily performed by simply pulling the fiber from the reaction matrix.
Compared with the disordered structures mentioned above,well designed nanostructures are preferred for the fabrication of chitosan/TiO2composite photocatalysts with special attention on core-shell nanostructures.A novel surface imprinted chitosan/TiO2core-shell hybrid material(SICT)is synthesized for the selective degradation of methyl orange in dual-dye systems(Fig.3)[2].TiO2nanoparticles were coated onto the surface of chitosan through hydrogen bond and the amount of TiO2used was obviously decreased.SICT could be reused directly without further desorption and regeneration for 10 cycles with preserving 60% of its activity, illustrating the favorable stability of the hybrid material.
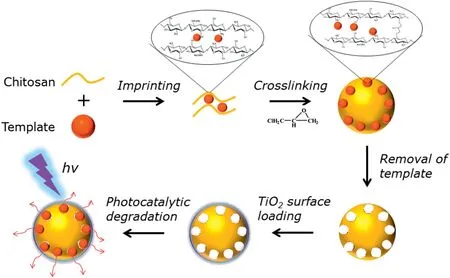
Fig.3.Schematic illustration of the preparation process of the surface imprinted chitosan/TiO2 composite[2].
2.2.Cellulose/TiO2 composites photocatalysts
Cellulose,an organic polysaccharide comprising a linear chain of several hundreds to many thousands of β(1 →4)links via D-glucose units,is mainly used to produce paper and paperboard.It has multiple hydroxyl groups on one long chain to form hydrogen bonds with other oxygen atoms on the neighboring chain[36].
2.2.1.Plant-derived cellulose
The method to synthesize cellulose/TiO2nanocomposite includes self-assembly, sol-gel method and polymerization. The method of self-assembly is the most commonly used. Cellulose nanocrystalsbased nanocomposites containing TiO2has been obtained through evaporation-induced self-assembly in aqueous solution.Ríos-Gómez et al. [37] discloses a simple modification of cellulose paper coated with polyamide-TiO2nanoparticles.The material offers a high sorption capacity(2.1 μg·cm-2)towards the selected model pollutant,methyl orange,allowing its isolation from a contaminated media.Additionally,a remarkable photocatalytic degradation both under UV and visible light (sunlight) was observed at short time of irradiation (typically 20-40 min),where the cellulosic substrate seems to play a crucial role in the process. The proposed simple approach could be in principle adapted to different target pollutants via selection of the appropriate polymer consequently paving the way to the utilization of novel environmentally sound,cheap and efficient nanocomposite materials in environmental remediation(wastewater purification).Pulp fibers were functionalized with polypyrrole in the presence of TiO2nanoparticles via in-situ chemical oxidative polymerization of pyrrole(Fig.4)[38].Increasing polypyrrole content in the composite enhanced the dielectric properties of cellulose,where dielectric properties such as AC conductivity and dielectric permittivity were increased with increasing polypyrrole content in the nano-composite.
Hierarchical nanostructures have been widely accepted as a promising way for the fabrication of cellulose/TiO2composite photocatalysts.Zhang et al.[39]have designed and constructed a hierarchically organized suprastructure comprising TiO2@Ag nanocomposite particles supported by cellulose microfiber matrices, which serves as both an efficient photocatalyst for the photodegradation of 4-chlorophenol into mineralized small molecules and a robust substrate for plasmonenhanced molecular spectroscopy(Fig.5).The current materials system and methodology may be seamlessly applied to the mechanistic investigations of other photocatalytic reactions because TiO2exhibits excellent photocatalytic performances towards a large variety of reactions and Ag possesses highly tunable plasmon resonances and intense local field enhancements exploitable for SERS.Zhang et al.[40]aimed to fabricate 3D free-standing hierarchical porous TiO2and SiO2with tunable porous structure and high specific surface area by using cellulose aerogel as a new biotemplate. Xing et al. [41] prepared a TiO2/Ag sponge-like nanostructure composites by the surface sol-gel method with the template of natural cellulose.The Ag nanoparticles are deposited on the TiO2nanosponges through UV irradiation photoreduction of silver nitrate solutions.The superior activities of TiO2/Ag nanosponge composite photocatalysts can be attributed to the unique nanosponge morphology,uniform dispersion of Ag nanoparticles,and strong interaction between Ag and TiO2nanosponges(Fig.6).
2.2.2.Bacterial cellulose
In comparison to the plant-derived cellulose, bacterial cellulose(BC),produced by acetic acid bacteria Gluconobacter xylinum,is obtainable in a pure form which requires no intensive processing to remove undesirable impurities and contaminants such as lignin, pectin and hemicellulose[39].BC excels with respect to its high purity,high hydrophilicity,biocompatibility,high mechanical properties,and ultrafine network structures[42].Bacterial cellulose,however,is economically unattractive due to excessive cost of carbon source[43].Nevertheless,its suitable application particularly in medicine has also urged the effort to produce the bacterial cellulose in the industrial scale.Typically,BC is produced in sphere-like particles,fibrils,and membrane form[44].
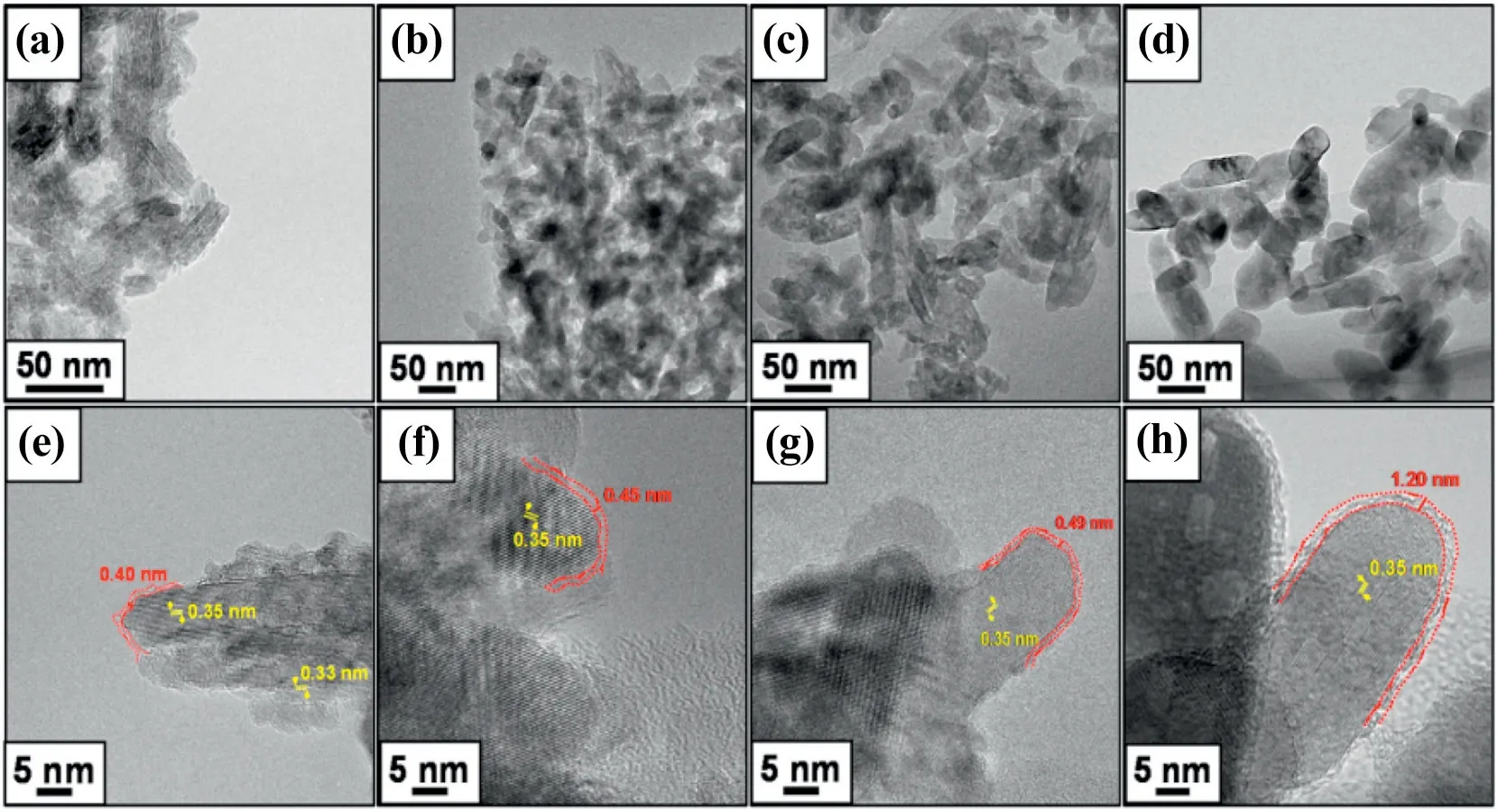
Fig.4.TEM and HRTEM images of(a)and(e)is CMT-75,(b)and(f)is CMT-300,(c)and(g)is CMT-400,and(d)and(h)is CMT-500,respectively[38].
Based on the greater interactions of bacterial cellulose with TiO2during multilayer deposition,Giovanna Machado et al.prepared TiO2/Au thin films supported on bacterial cellulose by layer-by-layer technique[45].The SiO2-(WO3)x·TiO2composite aerogels with higher pore volume and anatase TiO2crystallinity(Fig.7)were synthesized by ambient pressure drying [46]. In the process of synthesizing this composite aerogels, bacterial cellulose not only serve as the template inducing(WO3)x·TiO2nanoparticles to deposit, but also as the pore forming agent and structure director to construct the porous structure with SiO2gel together.The as-prepared composite aerogels exhibited higher specific surface area and pore volume, and showed much higher removing rate of Rhodamine B than P25 powder due to its enhanced adsorption/photocatalytic activity.
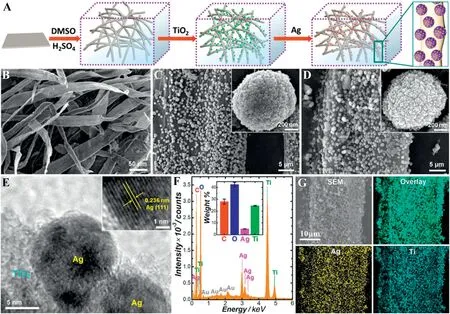
Fig. 5.(A)Scheme illustrating the assembly of TiO2@Ag/CMFs. SEM images of (B) swollen CMFs,(C)TiO2/CMFs,and(D) TiO2@Ag/CMFs. The insets in panels C and D are higher magnification SEM images of an individual TiO2 particle and TiO2@Ag particle grafted on the CMFs.(E)HRTEM image showing the structures of Ag nanoparticles grafted on the TiO2 surface.The inset is a HRTEM image showing the(111)lattice spacing of a Ag nanoparticle.(F)EDS spectrum of TiO2@Ag/CMFs.The inset shows the weight percentages of C,O,Ag,and Ti in the TiO2@Ag/CMFs.The error bars represent the standard deviations obtained from three samples synthesized following the same protocol. (G)SEM image and spatial distributions of Ag and Ti elements mapped by EDS[39].
Hierarchical nano-structuration has proven to be effective in optimizing the functional performance of nanomaterials.Bacterial cellulose could act as a template for TiO2nanoparticles generation.Nanoparticles can locate on the fibers/particles surface via H-bonding interactions.Using bacterial cellulose as a bioscaffold, a titania tube-in-tube scaffolding architecture with multilength-scale structural hierarchy(TITS TiO2)was accomplished by a precursor scaffolding-concurrent epitaxial growth approach. The BC@SiO2@TiO2precursor scaffold with a sandwiching SiO2layer played an essential role in the formation of the tube-in-tube geometry.This material showed superior photocatalytic activity and UV light absorption ability[47].TiO2/BC hybrid composites were prepared based on bacterial cellulose produced by gluconobacterxylinum,being the bacterial cellulose as a hydrophilic substrate for TiO2nanoparticles synthesized via sol-gel.Taken into account hydrophilic nature of the cellulose,TiO2nanoparticles were located on the surface of the fibers due to hydrogen bonding interactions[48].
2.3.Other biomaterial/TiO2 composite photocatalysts
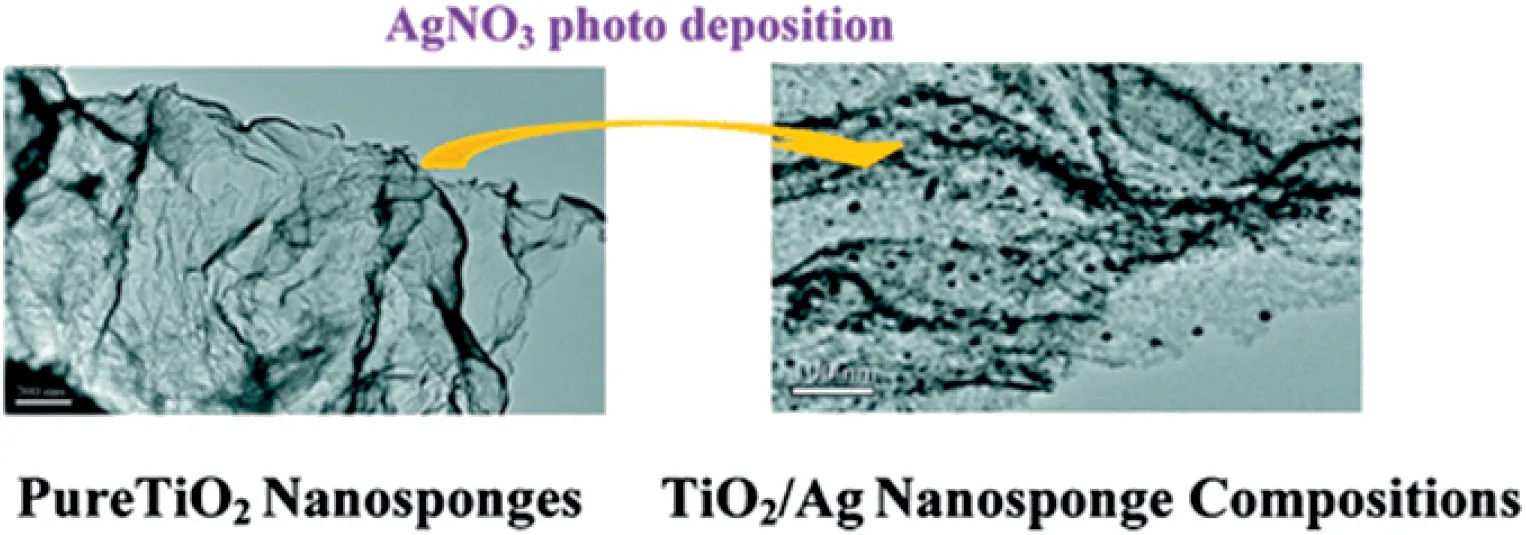
Fig.6.Cellulose nanocrystal-templated synthesis of mesoporous TiO2 with dominantly exposed(001)facets for efficient catalysis[41].
Other than the most studied chitosan and cellulose,some other biomaterials have also been used for the fabrication of biomaterial/TiO2composite photocatalysts.Li et al.[49]prepared composite films by extrusion technology using zein,poly propylene carbonate and nano TiO2.The zein and poly propylene carbonate could form a homogeneous network structure and uniform distribution of nano-TiO2in films. The major forces responsible for complex formation structural is ester groups. The photocatalytic removal rate of MO could achieve up to 85.06%. The antibacterial experiment indicated that zein/PPC/nano-TiO2film had good activity against the Escherichia coli,Staphylococcus aureus and Salmonella. Jackfruit filum polysaccharide (JFPS) was extracted and confirmed to contain neutral and acidic polysaccharides,largely composed of acidic polysaccharides[50].Biodegradable JFPStitanium dioxide (TiO2) nanocomposite films were fabricated using the solvent casting method combined with photocatalysis.The JFPS/TiO2composite films had stronger antimicrobial activity against E.coli than S.aureus.
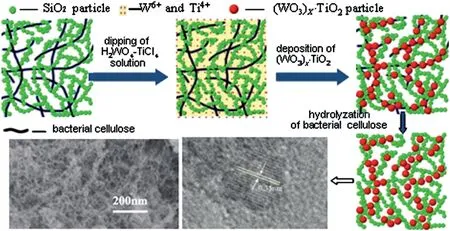
Fig.7.Synthesis of highly porous SiO2-(WO3)x·TiO2 composite aerogels using bacterial cellulose as template with solvothermal assisted crystallization[46].
Lignin,a three-dimensional network macromolecule,is electronegative and has a strong affinity for the positively charged metal ions;lignin with phenolic hydroxyl-,alcoholic hydroxyl-,and carboxylic groups can react chemically with other polymers to form hybrid composites[51].Native unmodified lignin has been used as a template for the formation of mesoporous TiO2nanoparticles via a hydrolysis precipitation method exploiting the electronegativity and the network skeleton of lignin(Fig. 8) [52]; as-synthesized composite had a BET surface area of 165.8 m2·g-1and a pore volume of 0.312 cm3·g-1.In view of the interactions between hydroxyl groups of lignin and the surface hydroxyl groups of the TiO2precursor,the calcined TiO2particles have a finer crystallite size, a mesoporous structure and are well separated, to avoid grain growth. However, titanium atom has a relatively low electronegativity and can react rapidly in a nucleophilic reaction medium in which lignin,a polycyclic and three dimensional mesh organic macromolecule with several electronegative OH groups,should have strong affinity for the positively charged metal ions;lignin with phenolic hydroxyl,alcoholic hydroxyl,and carboxyl groups can chemically react with other polymers to form hybrid composites. A sol-gel microwave technique was used to prepare lignin-based carbon TiO2composite photocatalyst.Here,lignin not only was used as a natural carbon source for modification of TiO2but also can be used as the biomass resource for green chemical production[53].
Waste biomass is a typical solid waste and can be valorized to synthesize low-cost biosorbents for various pollutants [1]. Employing waste biomass to prepare biomaterial/TiO2composite photocatalysts is attractive because this process will realize the valorization of waste at the same time.Mycelium is a major waste of fermentation industries with more than 8000 tons of output per year in China[1].Huo et al.[54]used mycelium of Penicillium chrysogenum as the core biomaterial to prepare a novel surface imprinted mycelium/chitosan/TiO2composite with core-shell structure.The obtained composite showed enhanced ability in removing trace Ag+ions in wastewater through the synergistic effect of selective adsorption and photocatalytic reduction.
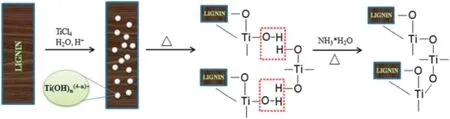
Fig.8.Formation mechanism of mesoporous TiO2 with lignin as a template[52].
Other than the above mentioned biomaterials which are not active for biocatalysis,enzymes or microbial cells can be also used for the fabrication of biomaterial/TiO2composite photocatalysts.This falls into the concept of photobiocatalysis,which has become a hot topic very recently[55].Wang et al.[24]fabricated an algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Fig. 9 showed the optical image of algae decorated nanofiber membrane and the detailed structure of algae cells dispersed nanofiber mats.Bacterial cellulose nanofibers produced by the fermentation of Komagataeibacter xylinus were oxidized to install aldehyde groups on the surface of BC,which were used as anchors for laccase immobilization via crosslinking[56].The method offers a simple technique that takes advantage of the biocatalytic property of laccase and the photocatalytic property of TiO2for dye degradation.The OBC/TiO2-Lac support system under UV irradiation demonstrated very promising 95%dye degradation efficiency within 3 h.

Fig.9.Optical image of algae-decorated nanofiber membrane(a)and SEM images of Chlorella vulgaris on the surface of TiO2/Ag NF at(b)high and(c)low magnification[24].
2.4.Synergistic effects based on material design
The original intention for the fabrication of biomaterial/TiO2composite photocatalysts is to utilize their synergistic effects.Firstly,multifunctional biomaterials can act as a good support for the well dispersion TiO2nanoparticles,enhancing the stability and recyclability of the nanoparticles.Secondly,the favorable adsorption ability of biomaterials and photocatalytic activity of TiO2can be integrated into one material which is capable of the removal of various pollutants in trace amount.Further studies reveal that,under specific conditions,the band structure of TiO2can be tuned to enhance its utilization of visible light and the separation of light induced charge carriers [31,56,57]. Metal doping is widely employed to further improve the photocatalytic activity of TiO2under visible light for biomaterial/TiO2composite photocatalysts [3,32,35,39].Other than the synergistic effects mentioned above,introducing other materials or synthesis procedures is possible to enhance the specific performance of biomaterial/TiO2composite photocatalysts.Two typical cases are enhancing the selectivity for target pollutants through molecular/ion imprinting and making the material easier to be separated and recovered by introducing magnetic Fe3O4[2,54,58].
The synergistic effects between biomaterials and TiO2depend on the formed structure and their interaction. Until now, most researchers employed facile synthesis procedures based on the simple mixing of biomaterials and TiO2.Although microparticles,supported and free-standing films can be synthesized using different post treatment methods,disorder nanostructures were always obtained,restricting the full utilization of the materials.Further studies should focus on the design of highly ordered nanostructures and hierarchically organized suprastructure.Fabrication of biomaterial/TiO2composite photocatalysts with core-shell nanostructures is a good try to exposure the active sites on the surface of the materials,thus enhancing the adsorption capacity as well as photocatalytic activity[2].Biomaterial/TiO2composite photocatalysts can be formed based on various interactions between them,such as hydrogen bond,electrostatic interaction,and O-Ti-C or Ti--O--C bonds[2,26,56].Many properties of the materials such as stability,band structure of TiO2,and exposure of the active sites highly depend on the interaction between biomaterials and TiO2.It is better to think twice when to decide strong or weak interaction, considering the nanostructures and the application purpose.
3. Applications in the Selective Removal of Trace Environmental Pollutants
3.1.Selective removal of trace heavy metals
In general,the surface of pure nano-TiO2without chemical modification has few reactive functional groups.So the adsorption capacity of nano-TiO2for heavy metals is quite low and the selectivity of nano-TiO2for heavy metals is unsatisfactory.Furthermore,the interaction between TiO2and heavy metal ions is weak,and heavy metal ions can be easily desorbed,thus causing secondary pollution[59,60].Biomaterials possess multiple functional groups, such as hydroxyl, carboxyl, and amino groups.These groups can selective recognize and capture various heavy metals through chemical interactions such as chelating and ion exchange [61-63]. Therefore, efficient and selective adsorbents for heavy metals can be fabricated combing biomaterials and nano-TiO2due to their synergistic effect(Fig.10).In order to further improving the selective adsorption performance,the recognition sites for the target metal ions can be obtained by the ion imprinting technology.Various biomaterials have been employed to prepare biomaterial/TiO2composites for the selective removal of trace heavy metals.For example,Wu et al.[63]immobilized TiO2nanoparticles into the polysaccharide network of carboxymethyl chitosan-hemicellulose to prepare the ternary complex of carboxymethyl chitosan, hemicellulose, and nano-TiO2(CHNT), which could selectively adsorb six heavy metal ions through the multiple coordination groups such as hydroxyl,carboxyl,and amino groups as characterized by FTIR.The highest adsorption capacity was observed for Ni(II), followed by Cd(II), Cu(II), Hg(II), Mn(VII),and Cr(VI).Li et al.[30]immobilized nano-TiO2on the molecularly imprinted chitosan matrix to prepare a novel chitosan/TiO2composite,which can not only degrade organic pollutants,but also recover Ni(II)ions through efficient adsorption.
As a physical phase transfer process,adsorption cannot thoroughly remove the heavy metals. Biomaterial/TiO2composites can not only selectively adsorb trace heavy metal ions, but also photocatalytic conversion several heavy metals to their less toxic species.For example,highly toxic Cr(VI) can be reduced to less toxic Cr(III) through TiO2photocatalysis.For some metals with higher redox potential,such as Ag,the photo-generated electrons can reduce them into metallic state,thus the recovery of metals can be achieved[54].Like other light induced processes,the photocatalytic redox process of TiO2for heavy metal ions is highly dependent on the life-time of the photo-generated charge carriers,as it is essential for the charge carriers to migrate to the surface to trigger the redox reactions.The existence of biomaterials can cause lattice defects in TiO2through carbon/nitrogen doping effect,thus weakening the recombination of the charge carriers and promoting the photocatalytic efficiency [64]. For example, Zhang et al. [61] used glutaraldehyde to crosslink nano-TiO2with chitosan to prepare titanium-chitosan composite(Ti-CTS),and applied the synthesized composite for the photocatalytic removal of Cr(VI).FT-IR and XPS analysis revealed the possible removal mechanism:(1)Cr(VI)was firstly adsorbed through electrostatic attraction(Ti4+and HCrO4-);(2)photo-generated electrons was transferred to Cr(VI)which was reduced to Cr(III);(3)the obtained Cr(III)was further adsorbed onto the Ti-CTS composite. Pincus et al.[65]combined nano-TiO2with chitosan and further bonded Cu to form chitosan-TiO2-Cu(CuTICB)composite photocatalyst by Lewis acid-base coordination between Cu(II)and chitosan amine groups.The composite photocatalyst can catalyze the light driven oxidation of As(III)to less toxic As(V)which can be more easily adsorbed.It can also selectively adsorb arsenite and arsenic in the presence of phosphates.Some typical examples of the removal of trace heavy metals by biomaterial/TiO2composite photocatalysts are given in Table 1.
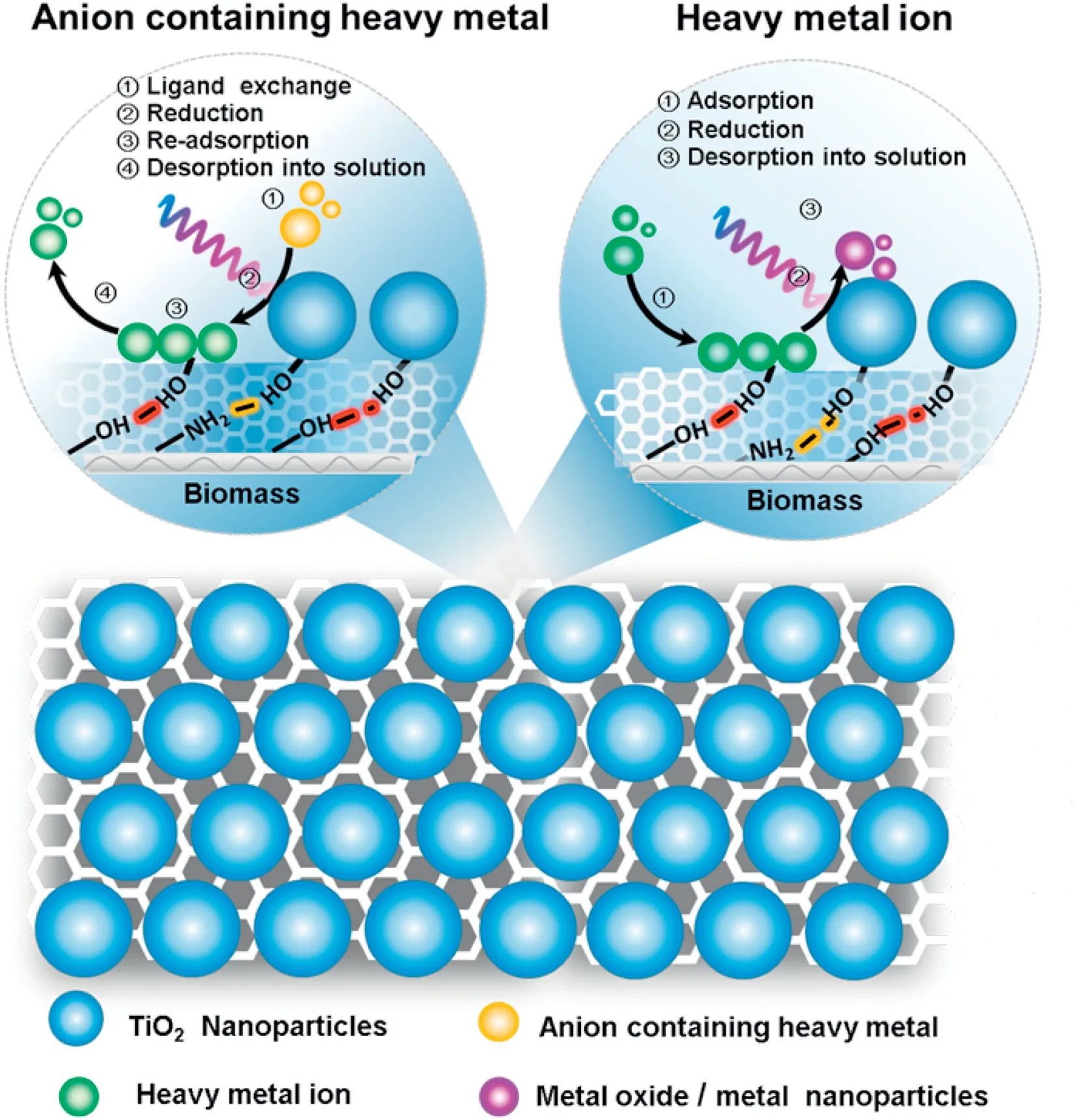
Fig.10.The mechanism of biomass/TiO2 nanocomposites for removing the heavy metals.
3.2.Selective removal of trace organic pollutants
In recent years,photocatalytic treatment of organic pollutants by biomaterial/TiO2composite photocatalysts has become a hot topic.The process is similar with that for the treatment of heavy metal contaminants(Fig. 11). Firstly, organic pollutants must be adsorbed on the surface functional groups of the biomaterials.Then the adsorbed pollutants can be further converted mediated by TiO2photocatalysis.Under illumination,TiO2is excited to generate hole/electron pairs.Holes possess strong oxidizing ability and will oxidize or even mineralize these organic pollutants into CO2and H2O.The photo-generated charge carriers can further interact with O2and H2O to form ROSs which enable more efficient photocatalytic oxidation of organic contaminants.In order to further enhance the visible light response and quantum efficiency,metal doping, non-metal doping, and construction of heterostructured photocatalysts with other semiconductors are always employed[67-69].For example,Zhu et al.[70]cross-linked nano-TiO2with chitosan to form a composite,and then deposited CdS nanocrystals on the surface.The decolonization effect of the composite photocatalyst for the azo dye methyl orange was evaluated and the results showed that the CdS/TiO2/CSC heterojunction possessed obvious enhanced activity towards methyl orange under simulated sunlight compared with the bare nano-TiO2.Zhu et al.[71]prepared zinc-doped titanium dioxide/zinc oxide/chitosan nanocomposite films(TiO2/ZnO/chitosan NTFs)by embedding zinc ions and nano-TiO2into chitosan films.The photocatalytic activity of NTFs on methyl orange results showed that NTFs possessed higher photocatalytic activity under simulated solar radiation with efficient oxidative decolonization of methyl orange dyes.
The synergistic effect of biomaterials and nano-TiO2in the degradation of organic pollutants using biomaterial/TiO2composite photocatalysts was studied by many researchers. Farzana et al. [72]prepared chitosan-nano-TiO2composite(CTC)photocatalysts,and investigated the activity towards the degradation of anionic dye red2(RR),cationic dye methylene blue(MB)and zwitterionic dye Rhodamine B(RB).The results show that CTC showed favorable photocatalytic activity for all the three organic dyes,but better selectivity for anionic dye RR.This phenomenon can be attributed to the superior adsorption capacity of chitosan for RR. Stan et al. [73] reported that the nanoscale TiO2-1%Fe-N particles prepared by hydrothermal method were coated on cotton fabric,and the obtained the composite photocatalyst showed obviously enhanced visible light photocatalytic activity for MB degradation compared with bare TiO2(P25).The selected results of the removal of trace organic pollutants by biomaterial/TiO2composite photocatalysts are given in Table 2.

Table 1Recent reported examples on the removal of trace heavy metals by biomaterial/TiO2 composite photocatalysts
3.3.Deactivation of pathogenic microorganisms
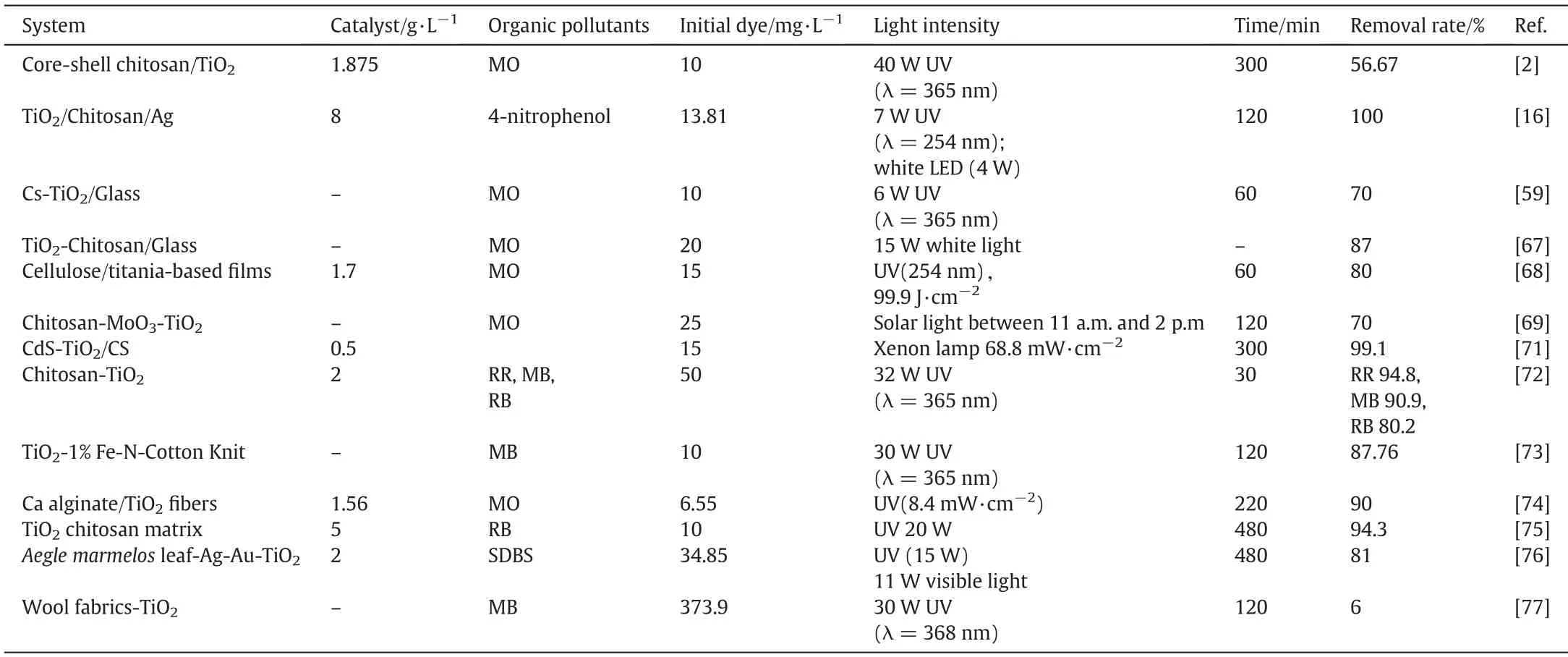
Table 2Comparison of the removal effects of bio-based titanium dioxide on organic pollutants recently reported

Fig.12.The mechanism of biomass/TiO2 nanocomposites for antimicrobial activity.
Biomaterial/TiO2composite photocatalysts have also been proven to be active for the deactivation of pathogenic microorganisms.This bactericidal activity is derived from the synergistic effect of the two counterparts(Fig.12).Biomaterials such as chitosan,proteins,lipids,etc.have long been recognized to be effective natural antibacterial agents[78-80].For example,the cationic chain of chitosan can interact with the exposed negatively charged macromolecular residues on microbial cells,affecting cell wall morphology and cell membrane permeability,and directly interfering the activity of enzymes responsible for microbial grow[80].Nano-TiO2is widely accepted to be a broad-spectrum semiconductor bactericidal material.The antibacterial activity of TiO2is derived from the production of a large amount of reactive oxygen species which will cause oxidative stress on various pathogenic microorganisms such as bacteria and fungi [3,81]. Furthermore, when the composite photocatalysts formed,the presence of biomaterials can capture the microbial cells onto the photocatalysts surface,thus facilitating the further deactivation by photocatalysis.The most commonly used biomaterials for this purpose is chitosan.The obtained chitosan-nano-TiO2composite showed favorable antibacterial activity against various bacteria such as E.coli and S.aureus as well as various fungi such as Candida albicans and Aspergillus niger under illumination[57,82,83].
The antimicrobial activity can be further enhanced by incorporating plasmonic metals,such as Ag and Cu,which have long been recognized to be efficient dopant to modify the band structure of semiconductors.Xiao et al.[3]prepared a chitosan-TiO2composite photocatalysts and further deposited Ag NPs onto the imprinted sites. The obtained Ag NPs@CTA composite photocatalysts showed excellent antimicrobial activity against C.albicans,S.aureus,and E.coli under visible light irradiation.Ag-NPs@CTA not only destroyed cell wall and cell membrane,but also caused metabolic dysfunction of bacteria.
Combining with other photocatalytic materials is another way to enhance the antimicrobial activity of the biomaterial/TiO2composite photocatalysts. Xu et al. [84] synthesized self-assembled films of graphene oxide(GO)and chitosan(CS)biopolymers,and embedded titanium dioxide(TiO2)nanoparticles on the surface to obtain TiO2@GO@CS composites.Antimicrobial experiments results showed that TiO2@GO@CS nanofilm exhibited excellent biocompatibility and antibacterial activity in animal or plant cell culture,and has favorable antibacterial activity against the test strain A. niger. When the ratio of graphene oxide, chitosan, and titania nanoparticles in the nanocomposite is 1:20:4,the nanofilm exhibits the highest antibacterial activity against A.niger.
3.4.Reuse of the composite photocatalysts
In order to use bio-based photocatalysts more effectively and reduce the cost,it is often necessary to investigate the reuse performance of the catalyst and to explore more efficient catalyst regeneration techniques.During the reuse of biomaterial/TiO2composite photocatalysts,contaminants,products,as well as intermediates may accumulate on the catalyst,occupying the active sites,and causing the decreasing in catalytic activity.Furthermore,some targets with high value need to be desorbed and recovered.Therefore,it is necessary to desorb and regenerate the catalyst to restore its active state depending on the properties of pollutants and the structural characteristics of the catalyst.
(1) When the binding of the contaminant on the catalyst is unstable,the desorption regeneration of the catalyst can be carried out by simple water washing or alcohol washing. Magesan et al.[69]washed the methyl orange-degraded chitosan-TiO2-MoO3composite directly with ethanol for three times and the catalyst can be recycled for four times.Xiao et al.[16]reported that after photocatalytic reduction of 4-nitrophenol, the chitosan-TiO2-Ag composite photocatalyst was directly washed for three times with deionized water,and retained over 95%of the activity for 4-nitrophenol reduction in five recycles.
(2) For heavy metal ions or organic dyes which are strongly bonded to the catalyst by electrostatic interaction or chemical coordination,it is necessary to adopt some strong desorption and regeneration procedures such as pickling alkaline washing,ion exchange and chelating agent washing,etc.Generally,the adsorption of cations by TiO2adsorbent under acidic conditions is weakened by the competition of H+.Similarly,the adsorption of anions under alkaline conditions is weakened by the competition of OH-.Therefore,the cations can be desorbed with a strong acid such as hydrochloric acid,and the anions can be desorbed with a strong base such as NaOH.Although it's desorption performance is quite good,strong base and acid will cause the destruction of the catalyst structure.For example,the chitosan of chitosan-TiO2composite will dissolve under acidic conditions.Therefore,better desorption effect should be achieved with the structure and activity of the catalyst unaffected.Generally,various coupling agents can be used for desorption and regeneration of the catalyst, and the most commonly used one is EDTA[30,62].Wu et al.[62]used 0.1 M EDTA solution to desorb the carboxymethyl chitosan nano-TiO2(CHNT)composite with the desorption rate of more than 80%and activity maintained over 75%after 5 cycles.
(3) Considering the cumbersome process of desorption and regeneration,and consuming a large amount of regent,it's better to fabricate stable nanoarchitecture which can be easily regenerated.For example,Xiao et al.[2]reported that the chitosan-TiO2composite does not require any desorption or regeneration operations and can be reused directly for 10 cycles with preserving over 60%of its activity.
4. Large-scale Preparation and Applications of Biomaterial/TiO2 Composite Photocatalysts
Sol-gel method is a simple,low-cost,and reproducible method for the synthesis of TiO2photocatalyst.It is believed to be one of the most promising procedure for scaling-up[85-87].Huang et al.[86]developed a facile modified sol-gel method which allows for the preparation of TiO2nanoparticles in large-scale.The obtained N-TiO2showed satisfactory photocatalysis activity under visible light and the photodegradation rate constant for the contaminants was about 6 times higher than that of the pure TiO2sample.Lopez et al.[87]developed a revised sol-gel formulation,combining two feasible and cost-effective surface immobilization processes,spray and doctor blade coating techniques,to prepare a large scale TiO2thin films(12 cm×22 cm)The large scale TiO2films produced showed a high photocatalytic activity which was evaluated by the degradation of methyl orange (MO) in aqueous solution.
Compared with the preparation of TiO2nanoparticles,the preparation of biomaterial/TiO2composites photocatalysts is more complicated,especially when the specific nanostructures are needed.The scale-up of the lab-scale procedures has not been achieved until now[88].There are several problems restricting the large-scale preparation of biomaterial/TiO2composite photocatalysts.(1)Systematical and in-depth study on the scale-up preparation of bio-nanocomposites has not been done and the guiding principles are lacking.(2)Industrial reactors and devices cannot be directly used because of the unique properties of biomaterial/TiO2composite photocatalysts.Modification of these reactors and devices should be done together with the change on many details in the well established procedures in the lab.(3)The cost of raw materials and the product yield should be taken into consideration based on technical and economic analysis.
For the application of biomaterial/TiO2composite photocatalysts in the removal of trace environmental pollutants in large-scale,the core issue is the development of proper photocatalytic reactor to realize the efficient utilization of solar energy and the well mix of the photocatalysts with the pollutants.Some pilot-scale solar-driven photocatalysis systems have been developed recently for various applications[89-91].For example,Salgado et al.[89]reported the efficiency of Au/TiO2photocatalysts for simultaneous hydrogen production and removal of organic pollutants from aqueous solution under direct solar radiation in pilot scale.However,the quantum efficiency for H2evolution is only 1.8%,which is still quite low and cannot meet the requirements for the realistic application of photocatalytic hydrogen production.Zhao[92]employed the sol-gel method to prepare nano-TiO2in large scale,and designed a 100 L photocatalytic system based on a batch-type slurry bed reactor for the treatment of dyeing wastewater. The treatment capacity is 1 m3·d-1, and the discharged water reached the first-class standard for water.However,the recovery of powdered TiO2in system remained a problem.Therefore,before the above mentioned problems are well addressed,these is still a long way to go to realize the industrial applications of biomaterial/TiO2composite photocatalysts.
5.Conclusions and Future Perspectives
In this review,we summarized the recent advances in the development of biomaterial/TiO2composite photocatalysts and their applications in the treatment of trace pollutants.Chitosan,cellulose,as well as other biomaterials can be utilized to support nano-TiO2to fabricate various biomaterial/TiO2composite photocatalysts.Biomaterials can help to enrich the trace pollutants and facilitate the light driven redox reactions on TiO2surface. They are also beneficial for the dispersion and the recovery of the nano-sized TiO2.Under certain interactions between biomaterials and TiO2,the band structure of the photocatalysts can also be tuned.Various trace pollutants containing heavy metals,refractory organics,and pathogenic microorganisms can be selectively and efficiently removed through the synergistic effect of the biomaterials based adsorption and the redox reactions mediated by the photogenerated charge carriers formed on the TiO2surface.The application significance was further discussed by comparing the recently reported results on the treatment of trace pollutants by biomaterial/TiO2composite photocatalysts.
Other than environmental remediation as discussed in this review,there are also many other applications are under rapid development with combining the selective recognition ability of biomaterials and the superior catalytic activity of TiO2.For example,in biomedical field,biomaterials can assist TiO2nanoparticles to reach the focus of infection,where TiO2will play its role in killing cancer cells without affecting others.Furthermore,in fine chemical synthesis field,TiO2can be used to catalyze several important reactions,such as oxidation and C--C coupling,and the selectivity can be further regulated by designing specific recognition sites in biomaterials.
Although obvious advances have been achieved in recent years in this area,there are still several problems waiting to be solved.Future work should be focused on the following aspects:1)rationally design the nanoarchitecture of the composite to achieve the ordered assembly of functional groups and nano-TiO2on the surface;2)construct chemical interaction between biomaterials and TiO2to modify the band structure of TiO2in order to enhance the visible light response and the transfer of the photo-generated charge carriers;3)scale up the lab procedures with preserving the activity for the treatment of trace pollutants. With these aspects achieved, wide applications of biomaterial/TiO2composite photocatalysts in environmental remediation can be expected.
 Chinese Journal of Chemical Engineering2019年6期
Chinese Journal of Chemical Engineering2019年6期
- Chinese Journal of Chemical Engineering的其它文章
- Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
- Amorphous and humidity caking:A review☆
- Intensification of chemical separation engineering by nanostructured channels and nanofluidics:From theories to applications☆
- Progress in molecular-simulation-based research on the effects of interface-induced fluid microstructures on flow resistance☆
- Recent developments on catalytic membrane for gas cleaning☆
- Advancement in separation materials for blood purification therapy☆
