Amorphous and humidity caking:A review☆
Mingyang Chen,Dejiang Zhang,Weibing Dong,,Zhilong Luo,Chao Kang,Haichao Li,Gang Wang,Junbo Gong,4,5,*
1 School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 College of Chemistry and Chemical Engineering,QingHai Nationalities University,Xining 810007,China
3 Xi'an Modern Chemistry Research Institute,Xi'an 710000,China
4 Key Laboratory Modern Drug Delivery and High Efficiency in Tianjin University,Tianjin 300072,China
5 The Co-Innovation Center of Chemistry and Chemical Engineering of Tianjin,Tianjin 300072,China
Keywords:Agglomeration Particle Powder technology Amorphous caking Humidity caking
A B S T R A C T Caking of products is a common and undesired phenomenon in food,chemical,pharmaceutical,and fertilizer industries which leads to extra cost and irregular quality.In general,caking processes could be identified as amorphous caking or humidity caking.In this review,history of studying caking,formation,methods,and prospects of these two caking processes are summarized and discussed.The relevant studies from the 1920s to today are mentioned briefly.According to the different properties(i.e.hygrocapacity,hygrosensitivity,mechanical properties,and diffusion behavior)of amorphous powders and crystals,the conditions and mechanisms of amorphous and humidity caking are discussed.It is summarized that glass transition,moisture sorption,quantitative methods characterizing caking,accelerated caking tests,and simulation of caking behaviors are the main aspects that should be studied for a caking process.The methods for these five aspects are reviewed.Potential research points are proposed including caking of mixed particles,caking with phase transition or polymorph transition,non-homogenous caking,and simulation of caking.
1.Introduction
Bulk solids handling is the most common industrial activity in the world.Most materials in food,chemical,pharmaceutical,and fertilizer industries are preferred to be free-flowing,so that would be suitable for manufacturing processes and final application[1].However,under the reasonable conditions,e.g.transport,storage and drying processes,some materials tend to be lumps for extended periods.This is referred to as“caking”. Caking is first defined as the agglomeration of a freeflowing powder into lumps[2,3].Now it is commonly described as a phenomenon by which a low moisture, free-flowing powder is first transformed into lumps, then into an agglomerated solid, resulting in loss of functionality and lowered quality [4]. Formation of caking is complex. The mechanisms depend on substances and conditions.For instance,amorphous powders facilitate amorphous caking,while crystals facilitate humidity caking.Furthermore,different mechanisms occur with a same substance due to proper conditions. Besides, the mechanisms of humidity caking of amorphous powders differ significantly from the one of crystals[5].Nonetheless,a caking process could be identified macroscopically.Fig.1 shows the changes of two morphological indices of key states in a typical caking process: the ratio of instant system porosity to initial system porosity,p/p0;and the ratio of interparticle bridge diameter to particle diameter, Db/Dp. At the start,the individual particles contact.The p/p0is the highest and Db/Dpis the lowest due to no liquid/solid bridges or deformation. Then bridging occurs as a result of liquid bridge between particles,or surface deformation and sticking,etc.It generates forces among particles and leads to adhesion.As a result,the p/p0begins to decrease while Db/Dpincreases significantly. Eventually the solid bridges are generated based on the bridging situation.Particles are transformed into lumps.Hence the p/p0and Db/Dpremain fairly unchanged.Based on the mentioned above,caking is an agglomeration process.Agglomeration can be either desirable or undesirable[6].It is defined as a process to gather into a mass or cluster;to collect or come together in a mass;to collect into a ball,heap,or mass,specifically: clustered or growing together but not coherent [7]. For the desired processes, e.g. agglomeration technique in fluidized bed, spray dryer, rotating drum granulation,and spherical agglomeration in crystallization[8-10],size enlargement by agglomeration is the generic term for that unit operation of mechanical process engineering which is characterized by combination with change in particle size[7].Caking is one of the undesired agglomeration processes.It is only utilized to describe the agglomeration after production,e.g.drying,package,storage,transportation,and sale.
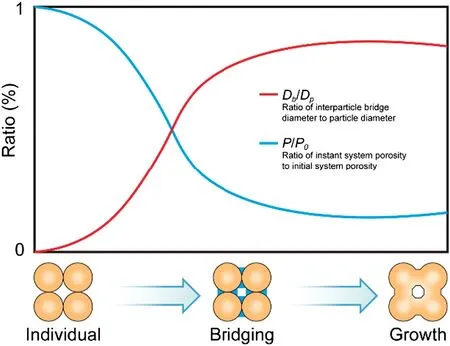
Fig.1.Typical stages in a caking process,indicating the changes in system porosity p/p0 and the ratio of interparticle bridge diameter to particle diameter Db/Dp at each stage[4,11].
Caking results in loss of product and in process interruption[12].De-caking manufacture is necessary, which costs extra time, man power and additional equipment.Nonetheless,2%-10%products are lost and the de-caked products present irregular quality[4,13].Caked materials cause a drop in the production rate,and in the worst complete stop of production [14]. In China, some typical undesired caking phenomena have also been reported.Fertilizers cake badly after storage,package, transportation, and sale due to their tendency of moisture sorption. The caked fertilizers could not meet the requirements of mechanized manufacture,even could not be used[15].The ideal product ratio of urea of a company form Shanxi is 98%.However,up to 50%caking ratio limited the actual qualified product ratio to be 80%-95%.Caking of NaCl and sugar is a serious problem during the transportation between north and south of China due to the difference of temperature and humidity,which has limited the business seriously[16].
Due to the severely negative impact on the quality of products and the complex mechanisms,researchers have focused on caking issues since 1920s.The research has involved fertilizer,food,pharmaceutical fields.Mechanisms have been investigated from macroscopic measurements,microscopic forces calculation,mathematical models to simulation. This review focuses on the most typical caking processes:amorphous caking and humidity caking.Relevant studies in the past one hundred years are summarized.Based on these studies,formations of the two caking processes are organized depending on amorphous and crystalline powders.The key research methods of caking are summarized.The potential research aspects are discussed.
It must be noted that,aggregation processes are not involved in this review. Aggregation describes a process that particles collide and adhere steadily via the Van der Waals attraction[11,17-19].There are no solid bridges among particles. Aggregates can withstand small shear stresses,but are frequently broken down by larger stresses[20],which significantly differ from agglomerates.
2.History
In the past one hundred years,caking was studied from macroscopic scale to microscopic views. Methods, apparatus and testers, mechanisms,and models were gradually established.The history was summarized in Table 1.
3.Formation of Caking
3.1.Properties of amorphous powder and crystal
Substances could be distinguished by two supra-molecular structures: (1) Amorphous. Molecules are arranged statistically with an energetically high level.It is a liquid-like state including a molecular disorder. The molecules are randomly distributed within a more or less rigid molecular matrix.An amorphous matrix is called a glass if it has a high viscosity.While heating such a glass,it gradually transforms into a rubbery structure and finally it liquefies.(2)Crystalline.Ions or molecules are highly tri-dimensional ordered with an energetically lower level.It might consist of polymer chains orientated in parallel,or be built of atoms and molecules arranged in form of a repeating three-dimensional geometrical pattern[94].Molecular mobility determines the supra-molecular structures(Fig.2).Free volume,the nonoccupied space in a molecular matrix,changes differently with temperature,which reflects the crystalline or amorphous structure.It increases continuously and steadily by heating an amorphous glass.A relatively obvious temperature interval could be observed after which the free volume increases more significantly.The lower border or the mean of this interval is glass transition temperature Tg. During or above this temperature,molecule clusters are set free and start to rotate and slip past each other.This state of amorphous powder is defined as glass transition.In contrast,discontinuous and critical temperature point which is called melting temperature Tmcould be observed by heating a crystal.Blow this melting point,the vibration of molecules about their position in lattice increases,while above that,the crystalline structure breaks down totally.Due to the difference of amorphous powder and crystal,substance properties, i.e.hygrocapacity, hygrosensitivity,mechanical properties, and diffusion behavior, differ significantly, which determines various caking mechanisms.
Hygrocapacity is the ability to bind water by absorption on the surface and into the molecular matrix, which describes an extent that the thermoplastic properties change as a result of absorption of water [94]. Water content of the product is usually considered to be the most important factor in promoting caking. In some caking situations, the presence of moisture is essential [33]. Hygrocapacity behavior therefore determines activation,extent,rate of some caking processes.In general,a substance in amorphous state presents higher hygrocapacity than that in crystalline state(Fig.3).Due to the molecular mobility(Fig.2),an amorphous moisture sorption curve is continuous and steady while a crystalline one increases significantly above a critical relative humidity that is generally called deliquesces point[95].
Hygrosensitivity is the changes in physico-chemical properties of substance linked to changing water content.For amorphous powders,water migrates into the molecular matrix and changes the free volume,which presents a decrease of glass transition temperature[94].With the increasing water content,these powders absorb the water molecules and the viscosity decreases steadily until they disintegrate into single molecules. In contrast, crystals maintain the mechanical properties with increasing humidity until they dissolve at the critical relative humidity. Crystals dissolve layer by layer from the outside without water migration into the supra-molecular structure.
Mechanical properties refer to viscous and elastic elements.Amorphous powders in glassy state behave more like elastic solids,while in rubbery or viscous state they exhibit liquid-like properties.As a result of these mechanical properties, amorphous powders tend to present sticky surface and plastification.Powders therefore are preferable to contact,adhere,and cake.For the crystals,the mechanical properties are comparably much more constant against temperature and humidity.In general,crystals present low viscosity and elasticity.
Diffusion behavior is the permeability of water or solution into molecules matrices.Due to the high free volume and macroscopic porosity,diffusion of amorphous powders is fast and easy to form a liquid film on the surface,whereas crystals tend to form liquid bridge on the contact points of particles instead of whole surface.

Table 1History of caking study
Table 1(continued)
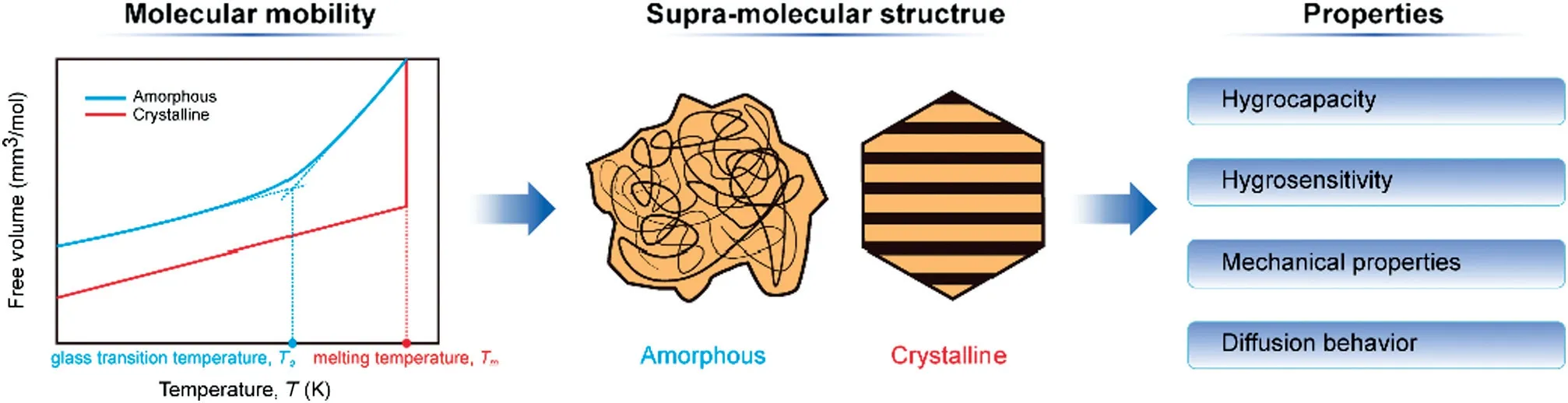
Fig.2.Illustration of relationship of molecular mobility,supra-molecular,and substance properties.
3.2.Amorphous caking
Amorphous caking is a unique caking mechanism of amorphous powders,which is common in food industry.The studied substances in literature include amorphous lactose,diary milk powder,onion powder,fish protein hydrolyzates,juice powder,meat,fruit and vegetable extract powders,etc.[52,62,84,96].As a result of molecular mobility,glass transition occurs and causes the change from glassy to rubbery state in the Tgrange, which is a potential motivation of amorphous caking.Fig.4 illustrates a typical amorphous caking process.Individual powders in glassy state contact at first.With temperature increasing and exceeding the Tg,molecule clusters start to set free and slip past each other,which caused the change of free volume.The visco-elastic properties of amorphous matrices therefore vary greatly.The contact points become flatten due to elastic property,which leads to contact area increasing.In additional the powders stick together as a result of viscous property.Based on the changes of the powder system and an effective contact time,a bonding between individual powders grows called sintering. Eventually a decrease of temperature facilitates the powder system back into glassy state and causes caking.

Fig.3.Illustration of moisture sorption behavior of amorphous powder and crystal.
It should be noted that amorphous powder crystallization may occur in an amorphous caking process.The change of compositions significantly affects sintering bridge,e.g.kinetics of sintering,radius of bridge,and mechanical strength of bridge.Yoshioka et al.reported crystallization of indomethacin from the amorphous state in the temperature range of 20°C above and below its Tg.Samples'significant crystallization to the most stable polymorphic form occurred over several days when stored below Tg. At storage temperatures near to and above Tgthe rates of crystallization increased as expected but a second less thermodynamically stable polymorph also appeared with the more stable crystal form [97]. Schmitt et al. reported that amorphous lactose appeared to give a mixed product consisting different crystal forms due to nucleation and growth above Tg[98].According to these reports,crystallization of amorphous powders is a non-negligible issue.However,it would be much more complicated to study amorphous caking considering this issue.In spite of it,it may be a necessary and challenging topic in further study.
3.3.Humidity caking
Humidity caking is facilitated by a liquid bridge caused by moisture sorption.It is regarded as the one of most important caking processes in the literatures[99].Generally,liquid bridge is easier to be facilitated due to the changes of ambient conditions,i.e.relative humidity,temperature,and stress.Besides,the capillary force caused by liquid bridges is much higher than the van der Waals force between particles.Consequently,particles show stronger tendency to bond each other.Hence,the extent of caking caused by a humidity caking process is commonly significant.
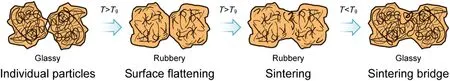
Fig.4.Illustration of an amorphous caking process.
The typical humidity caking processes of amorphous powders and crystals are summarized in Fig. 5. For amorphous humidity caking(Fig.5(a)),due to the continuous moisture sorption behavior,molecular free volume,and macroscopic porosity,amorphous powders contain more water content than crystalline ones.The condensed water forms both liquid bridges between neighboring powders and diffusion into molecular structures. It causes a decrease of viscosity and increase of elastic property,which leads to a plastification and sintering on the contact point.With the RH decreasing,a sintering bridge is generated due to the glassy state.As to the crystals,two main caking processes are presented based on their discontinuous moisture sorption behavior(Fig.3).Capillary condensation may occur when the RH is below the deliquescence point.Water condenses on the contact point of crystals only and forms a liquid bridge. It causes dissolution and capillary force.Recrystallization facilitated by RH decreasing generates a crystal bridge, which eventually causes caking (Fig. 5(b)). Fig. 5(c) shows that large amount of water would condense on the crystal surfaces if the RH exceeds the deliquescence point.A liquid bridge with a larger diameter or even a liquid film around the crystals forms.The surfaces of caked crystals may deform due to the severe recrystallization.
3.4.Factors of amorphous and humidity caking
It is of great importance to investigate the factors of caking processes for the mechanism study and industrial control. The summary in Section 2 has shown that most researchers focused on the factors of caking and tried to find the key factor of given substance.Theoretically,caking phenomena are triggered by the changes of substance properties caused by molecular mobility. Furthermore, the molecular mobility depends on temperature and pressure[94].It therefore could be concluded that temperature and humidity are the key factors of caking processes. In addition, according to the previous studies showed in Section 2, the subsidiary factors include particle size distribution,particle shape,consolidation time,stress,etc.(Fig.6).
For amorphous powders,the ambient temperature and glass transition temperature determine the caking behavior.Amorphous caking occurs when ambient temperature exceeds Tg. While a temperature lower than Tgmay facilitate humidity caking.RH has a great impact on Tg[4,5,94]. The work of Aguilera et al. shows that an increase in the water activity from 0 to 0.64 reduced the Tgof samples from 79.1 to-42.8 °C [96]. In an amorphous humidity caking process, humidity determines the water content and consequently the sintering,which plays a key role in sintering bridge formation. As to the subsidiary factors,consolidation time and stress have relatively more impacts on these two caking process.Because of the viscous and elastic properties,necessary consolidation time should be provided for sintering and higher stress leading to significant plastification and increase of contact area which enhanced the caking processes.

Fig.5.Illustrations for humidity caking processes of amorphous powders and crystals.a)Amorphous humidity caking.b)Crystalline humidity caking caused by capillary condensation.C)Crystalline humidity caking caused by deliquescence.
For crystals, temperature and humidity determine two key parameters,i.e. RH of capillary consideration and deliquescence,and consequently two humidity caking processes. Although both caking mechanisms are moisture sorption-dissolution-recrystallization,the extents of caking and morphology of final agglomerates are significantly different. In general, crystals present smooth surfaces and hardness structure against the stress and diffusion.As a result,the particle size and distribution,and particle shape are relatively important factors.Needle-like or plate-like fine crystals are preferable to cake.The reason is the increase of contact area and moisture sorption due to radius of meniscus.
4.Methods
Based on the discussion about factors of caking and history,glass transition and moisture sorption should be focused on in the caking study.In the meantime,characterization of caking and caking tests are the important technique that should be developed. With the aid of these methods,the caking behavior may be simulated and predicted via simulation software.These five aspects for studying caking were summarized in this section.
4.1.Glass transition
The measured glass transition temperature Tgstrongly depends on the method and the measurement parameters applied.As already mentioned,glass transition occurs in a relative temperature interval.As a result,the values also depend on the definition.Due to the influences of ambient conditions,the humidity should be controlled during measurement.
In general,Tgis determined by DSC(differential scanning calorimeter)with a heating and cooling process[51,60,96,100,101].It should be noted that the rate of heating and cooling has a non-negligible impact on the result. A slower DSC temperature scanning determined a relatively lower Tg.Hence,the rate is a key factor that should be the same during measurement. Tgcould also be determined via NMR. Free volume of amorphous powders changes during glass transition,which leads to an increase of average internuclear distance.The changes could be directly measured with proton spin-lattice relaxation time analysis[34].
According to the Fox and Flory equation below,the glass transition temperature of linear monodisperse homopolymers is proportional to the inverse of their molecular weight M[102]:

The relationship between Tgand moisture was described by Eq.(2)[94]:

The contact time between amorphous particles required to develop sufficient adhesion force to lead to caking could be calculated by Eq.(3)[6]:is assumed to be 0.1.η(T)could be calculated by Eq.(4)[103]:

The sufficient inter-particle adhesion force for

4.2.Water vapor sorption
Moisture sorption is the prerequisite of humidity caking, which plays a key role in the caking process. In Fig. 3, the critical value of RH that causes the significant increase of moisture sorption is called deliquescence point.Below the point,capillary condensation occurs at the contact points of crystals.The amount of water caused by capillary bridges could be calculated by the Kelvin equation[48,104]:

Deliquescence would dominate the moisture sorption when the RH exceeds the deliquescence point.The thermodynamics of deliquescence can be considered by Eq.(6)[64,105]:

The kinetics of deliquescence equation can be simplified as follows[105,106]:

Dynamic vapor sorption(DVS)is commonly utilized for the measurement.According to the water sorption curve,the amorphous powders and crystals could be distinguished. For the crystals, the RH of capillary condensation and deliquescence could be determined.Based on the water sorption behavior,the kinetics of crystals caking could be calculated with the Knudsen formula[73].Compared with the curves of pure samples,samples incorporated anti-caking agent,mixed samples,the effectiveness of anti-caking agent,phenomenon of deliquescence lowing,and proportion of amorphous and crystalline substances could be analyzed[64,73,107].

Fig.6.Factors of amorphous and humidity caking.RHc is the RH of capillary condensation,RHD the RH of deliquescence.Factors connected by solid line are considered to be relatively more important than the ones connected by dotted line.
4.3.Quantitative methods characterizing caking
Caking phenomenon is commonly characterized by flowability,microscopic attributes and extent of caking.A bin or funnel is utilized to characterize flowability by discharging mass flow rate. Angle of repose is also a method to reflect the flowability [4]. Microscopic attributes are ratio of system porosity to initial system porosity,ratio of interparticle bridge diameter to particle diameter,aspect ratio,circularity,convexity,sphericity,bluntness,etc.They could be measured via image analysis.Extent of caking could be described as caking intensity,caking strength,caking ratio,compressibility,etc.Caking intensity is used to characterize the force breaking the cake [53]. Apparatuses showed in Fig.7(a),(b),(c),and(e)are typical for characterization of caking intensity. Shear testers (Fig. 7(a) and (b)) are preferable for the research due to the easy operation and low investment[44,108-110].Micromanipulation particle tester(MPT,Fig.7(c))provides controllable conditions of pressure,temperature,humidity,and compression forces, which was utilized to study influence of environmental conditions on caking intensity [60]. The advantage of the device to apply pressure on particles (DAPP,Fig. 7(e)) is the ability to control two individual particles contact and apart.The details of caking processes and caking intensity could be observed,measured,and analyzed[92].Compressibility describes the shrinkage of the specimen during caking, and the pressure is related to the extent of powder caking[111].A penetration tester(Fig.7(d))could determine the value.Cake strength is the force holding the particles together[112].It could be determined by the blow tester(Fig.7(f)).Caking ratio is the percentage of detached particle[113],which characterizes the degree of caking.It is generally measured by sieving[12,78,79].
4.4.Accelerated caking tests
Caking experiments were first carried out on plant scale,which is called bag-storage test[22].In storage tests,powders were bagged in multiwall paper bags with asphalt-laminated moisture barriers.They were stored in the lower positions of several bag stacks which provide stress in an unheated building. Bags were inspected after several months of storage. They were removed from the stacks and were dropped four times from a height of 3 ft,once on each side and face.This experiment is time-consuming with unstable ambient conditions and large amount of samples.Accelerated caking tests are the experiments carried out in lab scale that simulate the caking process with a given setting of ambient conditions and stress[26].An accelerated caking tester usually contains a climate chamber to control the temperature and humidity with constant values or desired recycles.A controllable loading force at bottom simulates the bag stacks. These tests therefore accelerate the caking process and could also obtain relatively more accurate results[12,26,28,35,93].Recently,devices with camera and force applying system that control the contact of two individual particles were developed.Thus the double-particle system under specific temperature and humidity could be observed in situ[47,60,92].In addition,DVS could also be regarded as an accelerated caking tester due to the climate chamber of humidity and temperature[73,77].However,it should be noted that the stress could not be controlled in this device.
4.5.Simulation of caking behaviors
The ultimate goal of caking study is to predict the caking behavior under given conditions,e.g.temperature,humidity,stress,impurities,particle size and distribution, particle shape, and amount of anticaking agent. The industrial production routine therefore could be designed and the process control could be achieved. However, as already mentioned,caking processes are affected by a number of factors.It is complex and time-consuming to investigate via experiments.Thus the simulation of caking behavior with common property data of substances is of great importance.It could be achieved with two aspects of investigation:(1)particle packing simulation,and(2)basic thermodynamics and kinetics of substances.For the aspect of simulation,a twodimensional simulation with a contact-dynamics model and a DEM simulation with a three-dimensional elasto-plastic adhesive-frictional contact mode have been reported[60,92].As to the aspect of basic thermodynamics and kinetics of substances,heat&mass transfer model,relationship between moisture sorption with temperature,relationship between glass transition and water content,model for predicting the onset of caking and caking extent are required for amorphous powders[51],while heat&mass transfer model,relationship between moisture sorption with temperature,solubility and metastable zone,nucleation and growth models,model for predicting the onset of caking and caking extent are required for crystals.
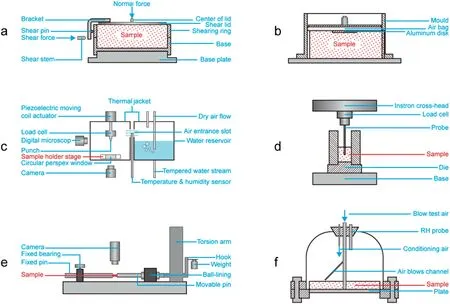
Fig.7.Apparatuses for characterizing extent of caking.a)Jenike shear cell.b)Ring shear tester.c)Micromanipulation particle tester.d)Penetration tester.e)The device to apply pressure on particles.f)Blow tester.
5.Prospects
The work in the past century has provided relatively a large amount of study that shows macroscopic caking mechanisms and some microscopic change of properties during caking,especially after the 2000s.Based on the present mechanisms and models,some researchers have started to simulate and predict caking behavior. Several suggestions are proposed below for the further study:
(1) Caking of mixed particles. Caking of crystalline-crystalline,crystalline-amorphous and amorphous-amorphous particle systems should be studied.Both amorphous caking and humidity caking may occur in a mixture system.In additional deliquescence lowing phenomenon may also be facilitated.The caking behaviors would be more complex and interesting.
(2) Caking with phase transition or polymorph transition.A caking process with these phenomena would change the moisture sorption and component of solid bridge.For amorphous caking,crystallization of amorphous powders is non-negligible.Characterizing the crystallinity of sintering bridge is the key that should be focused on.For humidity caking,different polymorph forms may be generated in solid bridge. Crystallographic theories should be adopted for this study.
(3) Non-homogenous caking.In most instances,studies assume that caking is a steady state process whereby all the powder subjected to atmospheric moisture over time will gain strength homogeneously, or have used testing methodologies which cannot differentiate the effects of non-homogeneity[1].However,nonhomogenous caking is common in industrial production or plant scale experiments. This phenomenon should be studied via relationship of stress distribution and particle packing structure, kinetics of moisture sorption and migration, kinetics of solid bridge formation,etc.
(4) Simulation of caking.Particle packing simulation should consider the factor of particle shape[114,115].Compared with models for predicting the onset of caking and caking extent of amorphous caking,the ones of humidity caking still need to be studied,especially the models for predicting the caking intensity via crystal bridge.
6.Conclusions
History of caking research in the past century is summarized.The research of caking started from the caking problems of salts,especially fertilizers.The original caking experiments and characterizations were bag-storage tests and photograph analysis on plant scale.Nowadays,accelerated caking tests with climate chamber and stress applying system or individual particle controlling system on lab scale could simulate the caking processes precisely.The techniques,e.g.DSC,NMR,DVS,and AFM have been adopted for studying the macroscopic and microscopic changes of powder surfaces and structure during caking.The thermodynamic and kinetic models of liquid/solid bridge formation and moisture sorption are proposed. Some simulations reported the prediction of caking behavior in recent years.
Properties of amorphous powder and crystal, i.e. hygrocapacity,hygrosensitivity,mechanical properties,and diffusion behavior,depend on the molecular mobility.Based on the significant difference from these properties,four basic formations of caking processes are summarized:amorphous caking caused by glass transition,humidity caking caused by sintering of amorphous powders, humidity caking caused by capillary condensation of crystals,humidity caking caused by deliquescence of crystals.The key factors of these processes are temperature and humidity which determine the formation of glass transition,capillary condensation and deliquescence. Other factors are particle size and distribution,particle shape,stress,consolidation time,etc.
The thermodynamic and kinetic models of glass transition and moisture sorption,characterization of caking(e.g.flowability,microscopic attributes,and extent of caking),accelerated caking tests,simulation of caking with two- and three-dimensional models are summarized for the design of industrial production routine and the process control.
Caking of mixed particles, caking with phase transition or polymorph transition,non-homogenous caking,and simulation of caking are considered to be the most potential aspects for further study.
Nomenclature
afwa coefficient describing temperature dependency of the free volume of the solid
af a coefficient
B constant
C constant
D contribute to heat transport
F contribute to heat transport
Ft force applied,N
fsthe fractional volume of the solid
fwarithmetic mean of the fractional volume of water
l azimuthal radius of a meniscus,m
M molar mass
NAAvogadro number,number of molecules per mol
p0the vapor pressure of pure water,Pa
R Kelvin radius,m
RH relative humidity
RHDRH of deliquescence
RHcRH of capillary condensation
r diameter of the sinter bridge,m
s radius of meniscus,m
T temperature,K
Tgglass transition temperature,K
Tg,sglass transition temperature of the solid,K
Tg,wglass transition temperature of water,K
Tg,∞glass transition temperature of a polymer with an infinite molecular weight,K
t time,s
Vmmolar volume of the liquid,m3·mol-1
W the sorption rate
w water content(wet-based)
X the diameter of the particle,m
γ surface tension,N·m-1
ηgthe viscosity in the glassy state,mPa?s
η(T) the viscosity of the material at temperature T,mPa?s
θ the additional free volume delivered by each chain end,mm3·mol-1
μ Chemical potential,J
μ0the standard chemical potential,J
ρ density,kg·m-3
 Chinese Journal of Chemical Engineering2019年6期
Chinese Journal of Chemical Engineering2019年6期
- Chinese Journal of Chemical Engineering的其它文章
- Recent advances in acid-resistant zeolite T membranes for dehydration of organics☆
- Intensification of chemical separation engineering by nanostructured channels and nanofluidics:From theories to applications☆
- Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants☆
- Progress in molecular-simulation-based research on the effects of interface-induced fluid microstructures on flow resistance☆
- Recent developments on catalytic membrane for gas cleaning☆
- Advancement in separation materials for blood purification therapy☆
