Krukenberg tumor with concomitant ipsilateral hydronephrosis and spermatic cord metastasis in a man: A case report
Shu-Han Tsao, Cheng-Keng Chuang
Shu-Han Tsao, Cheng-Keng Chuang, Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Taoyuan City 333, Taiwan
Abstract BACKGROUND Tumors of the spermatic cord are rare, and approximately 25% are malignant neoplasms. Metastatic spermatic cord tumors are even rarer. Several studies have revealed that the most frequent primary tumors metastasizing to the spermatic cord and peritesticular tissues are neoplasms of the stomach and prostate.Furthermore, metastasis to the spermatic cord or epididymis may occur via retrograde lymphatic and hematic routes. We present the case of a man with gastric cancer that metastasized to the spermatic cord and epididymis, with concomitant ipsilateral hydronephrosis after surgical resection and chemotherapy for his primary tumor.CASE SUMMARY A 71-year-old man underwent total gastrectomy for pT4aN2 poorly differentiated gastric adenocarcinoma in December 2016. Two months after surgery, he received adjuvant chemotherapy with TS-1 from February 2017 to February 2018.Surveillance computed tomography (CT) was performed in June 2018, which did not reveal any sign of tumor recurrence. In November 2019, he presented with left lower quadrant abdominal pain and a palpable left inguinal-scrotal mass. CT revealed left mild hydronephrosis and a left scrotal mass measuring 4.0 cm × 1.7 cm. Tumor biomarkers, including alpha-fetoprotein (AFP), lactate dehydrogenase(LDH), beta-human chorionic gonadotropin (βHCG), carcinoembryonic antigen(CEA), and carbohydrate antigen 19-9 (CA19-9) were all normal. Renal and testicular echography showed left hydronephrosis and a left peritesticular soft tissue lesion with blood flow. Diagnostic ureteroscopy showed left lower ureter narrowing without an intraluminal lesion. A biopsy was obtained for the indurated spermatic cord and epididymis, which showed poorly differentiated adenocarcinoma. Immunohistochemical staining demonstrated that the tumor was diffusely and strongly positive for homeobox protein CDX2. The features were consistent with metastatic adenocarcinoma of a primary gastric tumor.CONCLUSION In patients with a history of primary cancer, an inguinal mass of unknown cause with accompanying ipsilateral hydronephrosis may be a sign of distant metastasis from a primary tumor, especially of gastrointestinal origin.
Key Words: Case report; Epididymis; Gastric cancer; Krukenberg tumor; Male; Spermatic cord
INTRODUCTION
Tumors of the spermatic cord are rare, and approximately 25% are malignant[1].Among the malignant neoplasms of the spermatic cord or epididymis, 8.1% are of metastatic origin, with the most frequent primary tumors originating in the stomach(42.8%) and the prostate (28.5%)[2]. Metastasis to the spermatic cord or epididymis can spread through the retrograde lymphatic and hematic routes. We present the case of a patient with gastric cancer that metastasized to the spermatic cord and epididymis,with concomitant ipsilateral hydronephrosis after surgical resection and chemotherapy for treatment of the primary tumor.
CASE PRESENTATION
Chief complaints
A 71-year-old man came to the hospital in November 2019 with a palpable left inguinoscrotal mass and symptoms of intermittent left quadrant abdominal pain for more than 6 mo.
History of present illness
The patient had received total gastrectomy and chemotherapy in December 2016 for pT4aN2 gastric cancer. The histopathology disclosed mixed poorly differentiated adenocarcinoma with signet ring cell components. Chemotherapy regimens against the primary gastric cancer was oral TS-1 20 mg (gimeracil + oteracil + tegafur) from February 2017 to June 2018.
History of past illness
The patient had been in good health.
Personal and family history
The patient denied any positive personal or family history.
Physical examination
The physical examination reveal ed a palpable, left inguinoscrotal mass with induration at the spermatic cord and epididymis. An abdominal examination showed no palpable mass nor tenderness.
Laboratory examinations
Tumor biomarkers were: AFP, 2.5 ng/mL; prostate-specific antigen, 1.11 ng/mL; LDH 265 U/L; βHCG < 1 mIU/mL; CEA 3.95 ng/mL; and CA19-9, 16.6 U/mL.
Imaging examinations
Renal and testicular echography showed left hydronephrosis and a left peritesticular soft tissue lesion with blood flow. CT revealed a left scrotal mass (Figure 1A) and left mild hydronephrosis (Figure 2A). Left retrograde pyelography revealed multiple stenosis and narrowing points causing left hydronephrosis and hydroureter (Figure 3).
Diagnostic ureteroscopy showed left lower ureter segmental narrowing without an intraluminal lesion. Scrotal exploration and biopsy of the indurated spermatic cord and epididymis disclosed metastatic poorly differentiated adenocarcinoma(Figure 1B). An immunohistochemical study showed nests of poorly differentiated adenocarcinoma diffusely and strongly positive for homeobox protein CDX2(Figure 4). The features of the tumor were consistent with metastatic adenocarcinoma of the primary gastric cancer.
Positron emission tomography with 2-deoxy-2-[fluorine-18]-fluoro-D-glucose integrated with computed tomography (18F-FDG PET/CT) from skull vertex to upper thighs was performed at 60 min after injection of18F-FDG. The examination revealed a gastric stump recurrence, positive celiac lymph nodes, and distant metastases to the spine, ribs, sternum, pelvic bones, bilateral scapulae, pelvic cavity, left spermatic cord,and epididymis (Figure 5).
FINAL DIAGNOSIS
The patient’s diagnosis was recurrent gastric cancer with multiple metastases from the gastric stump to the celiac lymph nodes, spine, ribs, sternum, pelvic bones, bilateral scapulae, pelvic cavity, left spermatic cord, and epididymis.
TREATMENT
Due to widespread metastatic cancer, the patient received palliative chemotherapy.The regimens were oral capecitabine 1000 mg twice a day and oxaliplatin 100 mg every two weeks from December 2019 to April 2020. After ten courses, this treatment was disrupted because the patient’s clinical condition deteriorated.
OUTCOME AND FOLLOW-UP
Follow-up abdominal CT in March 2020 showed stationary bone and spermatic cord metastasis. There were no enlarged lymph nodes in the neck, mediastinum, or abdomen, and the left hydronephrosis showed regression (Figure 2B). Unfortunately,the patient’s clinical condition deteriorated, and he became cachexic and passed away on June 2020.
DISCUSSION
Metastatic pathways of gastric cancer
Major routes of distant metastasis in gastric cancer are intraperitoneal, lymphatic, and direct invasion into neighboring organs[3]. The most common sites of metastasis are the liver (48% of patients), peritoneum (32%), lung (15%), and bone (12%). The histological type of gastric cancer affects metastasis. Compared with generic adenocarcinoma,signet ring adenocarcinoma more frequently metastasizes within the peritoneum, to the bone and ovaries, and less frequently, to the lungs and liver[4].
Krukenberg tumors
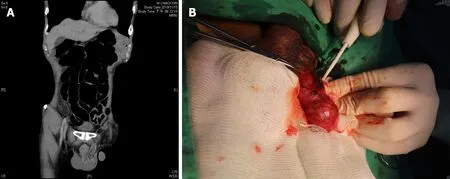
Figure 1 Tumor imaging and excision. A: Contrast-enhanced computed tomography of the abdomen reveals an irregular mass at the left spermatic cord extending to the epididymis; B: Intraoperative photograph of the indurated mass measuring 4.0 cm × 1.7 cm on the spermatic cord and epididymis, without invasion to the testis. A biopsy was obtained from the spot indicated by the pointer.
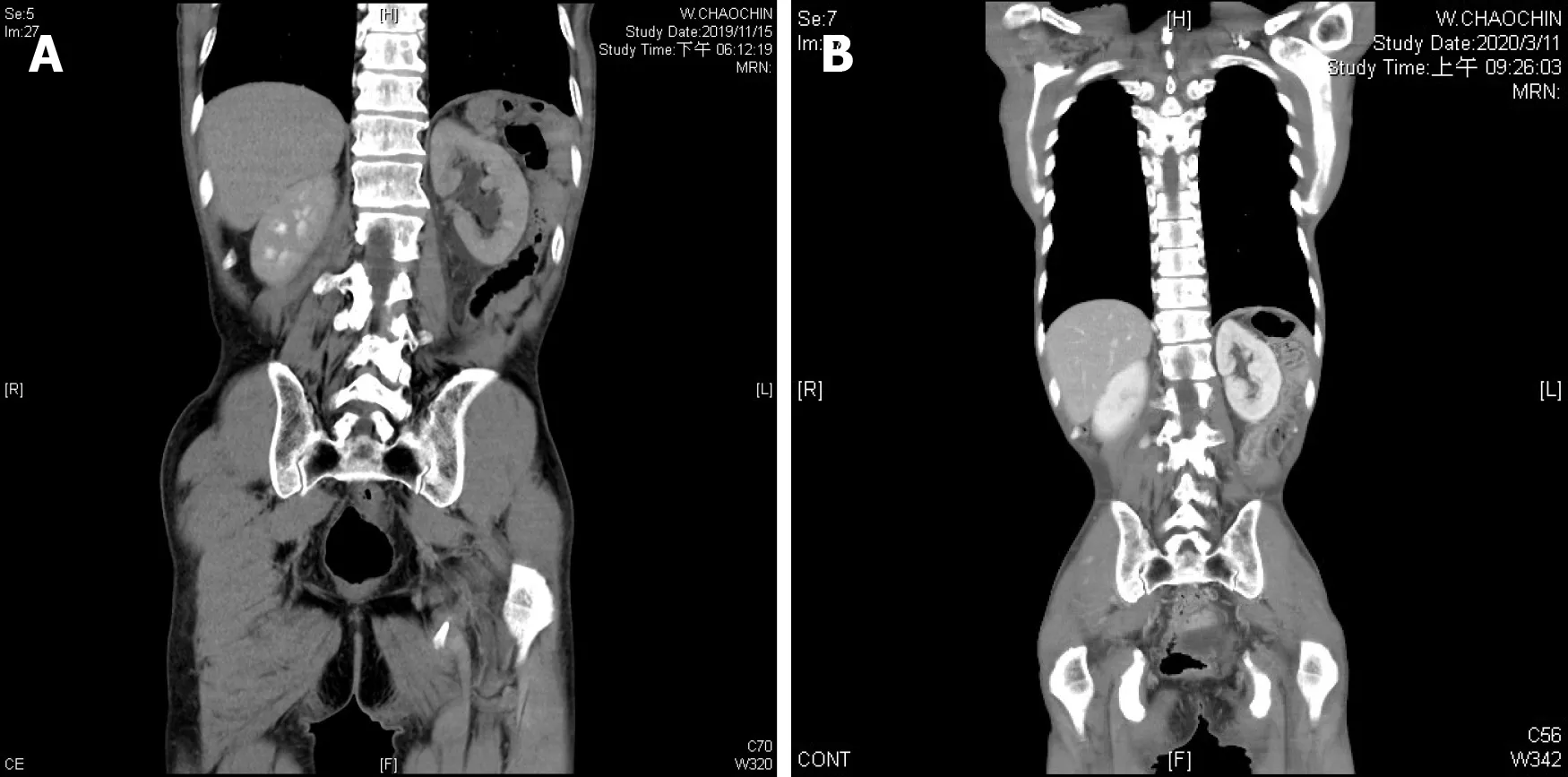
Figure 2 Ipsilateral hydronephrosis. A: Retrograde lymphatic metastasis-related left hydronephrosis in November 2019; B: Left hydronephrosis showed regression in March 2020, after systemic chemotherapy.
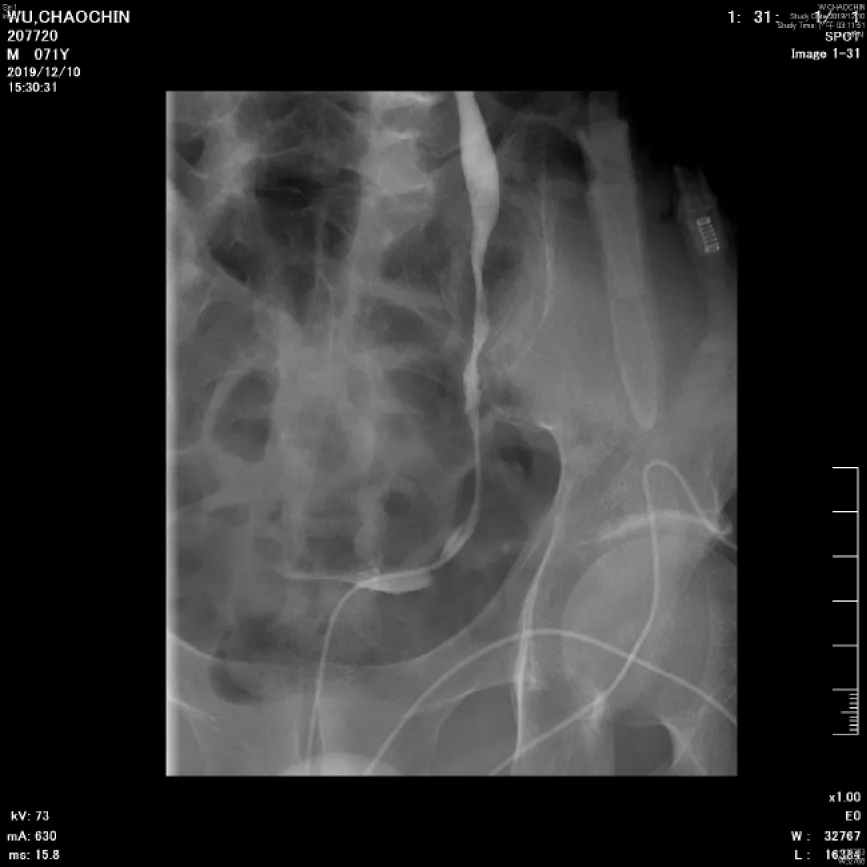
Figure 3 Left ureteral stenosis. Left retrograde pyelography showed multiple stenosis and narrowing points along middle to lower ureter, which led to left hydronephrosis and hydroureter.
Krukenberg tumors are secondary ovarian tumors defined as carcinomas that have a significant signet-ring cell component to the histopathology[5]. As previously mentioned, the major routes of distant metastasis in gastric cancer are intraperitoneal,lymphatic, and hematogenous. Lymphatic metastasis from the stomach to the ovaries predominates. The reason for this predominance could be the lymphatic vessel anatomy of urogenital lymph systems. The receptaculum chyli joins intestinal trunks,which connectviathe celiac lymph nodes to the gastric lymph nodes. The extensive tumor infiltration of the receptaculum chyli would have resulted in blockage of the thoracic duct, with subsequent retrograde flow. The retrograde lymphatic pathway enables the gastric cancer cells to metastasize easilyviathe receptaculum chyli to the urogenital lymph vessel trunks and the inguinal region[6,7].
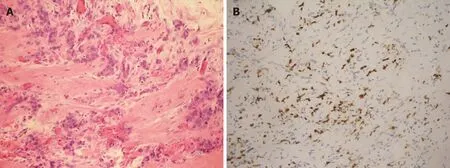
Figure 4 Histopathology. A: Hematoxylin and eosin–stained section showed fibrovascular tissue with infiltration of nests of poorly differentiated adenocarcinoma; B: The immunohistochemically stained section was diffusely and strongly positive for CDX2, which is a marker indicative of adenocarcinoma of intestinal origin.
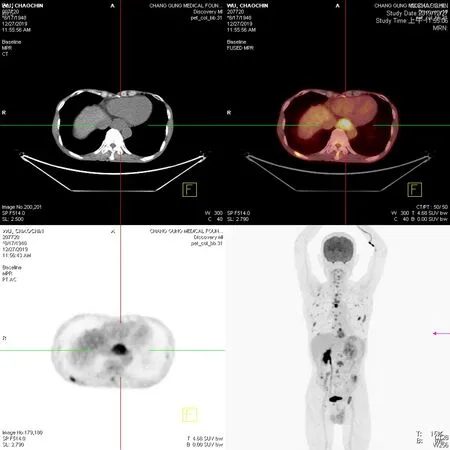
Figure 5 Tumor scan. Whole-body tumor scan from the skull vertex to upper thighs was performed at 60 min after injection of 18F-fluorodeoxyglucose. Purple arrow indicates gastric stump recurrence. Multiple faint nodules were observed in the pelvic cavity, suggesting peritoneal dissemination, as well as the cause of ureteral stenosis.
Krukenberg tumors in men
Metastatic dissemination of gastric signet-ring cell carcinoma to the ovaries is referred to as Krukenberg tumor. The counterpart in male organs derived from the sex cord is an exceedingly rare observation. Excluding lymphomas and leukemias, metastasis constitutes only 0.9% of all testicular tumors, and one of the possible dissemination pathways is retrograde lymphatic spread along the spermatic cord. In our patient, the histopathology disclosed signet-ring cell carcinoma from primary gastric cancer. The patient’s CT body scan revealed metastasis to the celiac lymph nodes, which supports the hypothesis of retrograde lymphatic metastasis. Based on the two points above, the clinical diagnosis of the patient is “male Krukenberg tumor” with distant metastasis to the ipsilateral spermatic cord and epididymis[8].
The prognosis of a metastatic tumor in the spermatic cord has been typically unfavorable. In a review of metastatic tumors in the spermatic cord, the 2-year survival rate after proven metastasis was 36% in a total of 16 patients with a median follow-up duration of 12 mo[9]. In our case, the patient passed away 8 mo after tumor recurrence. A review of the patient’s history showed that the inguinal mass was noted more than 6 mo earlier. We believe that the delay in diagnosis is the main reason for the patient’s inferior survival. The patient also had left hydronephrosis caused by external compression of the ureter. The ureteroscopic examination confirmed the segmental narrowing of the left lower ureter. Hydronephrosis secondary to compression by metastatic adenopathy in patients with Krukenberg tumor is previously reported[10]. Although it cannot be directly proven, we hypothesized that the cause of left hydronephrosis in our patient could have been retrograde lymphatic metastasis from his primary gastric cancerviaa pathway like that of metastasis of a Krukenberg tumor. Besides the preexisting inguinal mass, earlier detection of hydronephrosis could have contributed to a more favorable outcome of recurrent gastric cancer, especially in patients with normal tumor markers and hydronephrosis without notable urinary calculi. Although CT might not have detected all occult metastases in our patient, a whole-body tumor scan should be done to monitor tumor recurrence before symptoms such as a palpable mass or hydronephrosis caused by a metastatic tumor are found. To the best of our knowledge, this is the first to report of concomitant ipsilateral male genital distant metastasis with ipsilateral hydronephrosis from primary gastric cancer.
CONCLUSION
In patients with a history of primary cancer, an inguinal mass with an unknown cause of ipsilateral hydronephrosis may be a sign of distant metastasis from a primary tumor, especially one of gastrointestinal origin.
 World Journal of Clinical Cases2021年1期
World Journal of Clinical Cases2021年1期
- World Journal of Clinical Cases的其它文章
- Endoscopic salvage treatment of histoacryl after stent application on the anastomotic leak after gastrectomy: A case report
- Residual tumor and central lymph node metastasis after thermal ablation of papillary thyroid carcinoma: A case report and review of literature
- Necessary problems in re-emergence of COVID-19
- Simultaneous bilateral acromial base fractures after staged reverse total shoulder arthroplasty: A case report
- Intraparenchymal hemorrhage after surgical decompression of an epencephalon arachnoid cyst: A case report
- Immunosuppressant treatment for IgG4-related sclerosing cholangitis: A case report
