Totally intracorporeal robot-assisted urinary diversion for bladder cancer(part 2).Review and detailed characterization of the existing intracorporeal orthotopic ileal neobladder
Hugo Otaola-Ara , Kulthe Ramesh Seetharam Bhat ,Vipul R.Patel , Mario Covas Moshovas , Marelo Orvieto
a Department of Urology, Cl?′nica Alemana, Santiago, Chile
b School of Medicine, Cl?′nica Alemana-Universidad del Desarrollo, Santiago, Chile
c Department of Robotic Surgery, AdventHealth Global Robotics Institute, Celebration, FL, USA
Abstract Objective: To review the most used intracorporeal orthotopic ileal neobladder(ICONB) after radical cystectomy for bladder cancer and create a unified compendium of the different alternatives, including new consistent images.Methods: We performed a non-systematic review of the literature with the keywords“bladder cancer”, “urinary diversion”, “radical cystectomy”, and “neobladder”.Results: Forty studies were included in the analysis.The most frequent type of ICONB was the modified Studer “U” neobladder (70%) followed by the Hautmann “W” modified neobladder(7.5%), the “Y” neobladder (5%), and the Padua neobladder (5%).The operative time to perform a urinary diversion ranged from 124 to 553 min.The total estimated blood loss ranged from 200 to 900 mL.The rate of positive surgical margins ranged from 0%to 8.1%.Early minor and major complication rates ranged from 0% to 100% and from 0% to 33%, respectively.Late minor and major complication rates ranged from 0% to 70% and from 0% to 25%, respectively.Conclusion: The most frequent types of ICONB are Studer“U”neobladder,Hautmann“W”neobladder, “Y” neobladder, and the Padua neobladder.Randomized studies comparing the performance of the different types of ICONB, the performance in an intra or extracorporeal manner, or the performance of an ICONB versus ICIC are lacking in the literature.To this day, there are not sufficient quality data to determine the supremacy of one technique.This manuscript represents a compendium of the most used ICONB with detailed descriptions of the technical aspects, operative and perioperative outcomes, and new consistent images of each technique.
KEYWORDS Bladder cancer;Ileal orthotopic neobladder;Intracorporeal urinary diversion;Robot-assisted radical cystectomy;Surgical technique
1.Introduction
Bladder cancer is the 13th highest cause of cancer mortality worldwide (2.1%) and the 10th most commonly diagnosed malignancy (3%) [1].The gold-standard therapy for localized muscle-invasive bladder cancer (MIBC) [2] is to perform a radical cystectomy (RC) with pelvic lymph node dissection (PLND) and urinary diversion (UD).
Removal of the bladder is a life-changing event for most patients, who will have to endure lifelong use of a stoma or self-catheterization.Therefore,the possibility to replace the original bladder with a form of urinary diversion that allows the patient to void through the native urethra is remarkably appealing and has been investigated since the 1900s.Since the early times,various open-surgery lower urinary tract diversions attached to the native urethra have been developed;these were jointly named orthotopic neobladders(ONB).The Camey reservoir [3,4], the Hautmann “W” neobladder [5],the Studer pouch[6],the“T”pouch[7],stomach neobladder[8], cecal and ileocecal neobladders [9,10], and sigmoid reservoir[11]were amongst the most popular techniques.
With the advent of robotic surgery (DaVinci Surgical System?, Intuitive Surgical Inc., Sunnyvale, CA, USA),several groups embarked into performing robot-assisted radical cystectomy (RARC), whereas the ONB was usually completed extracorporeally.In 2003 Menon et al.[12] reported the first cases of RCs performed using the DaVinci robot.The first robot-assisted totally intracorporeal ileal ONB (ICONB) was performed by Beecken et al.[13] using a“W” configuration pouch following the principles of the Hautmann neobladder.Since then, the generation of new data has been slow, in line with the natural steep learning curve associated with these procedures.
The available literature reports that many totally intracorporeal reconstructive techniques have been attempted,without the supremacy of any being demonstrated.Besides,although each technique has its own illustrations, its complete understanding and graphic comparison sometimes are not simple, since each one has a different style.
Our objective was to review in two consecutive manuscripts the types of robot-assisted urinary diversions described in the literature in an attempt to create a unified compendium of the different alternatives, including detailed descriptions of the technical aspects, operative and perioperative outcomes (including complications and functional results),and to create new consistent images.In this second article, we focused on the most used robotassisted ICONB after RARC for MIBC.
2.Materials and methods
A non-systematic review of the publications in English or Spanish was performed using the PubMed electronic database.Search criteria included the keywords “bladder cancer”, “urinary diversion”, “radical cystectomy”, and“neobladder”.Additionally, a manual search of references in relevant published articles was performed.Only studies reporting robot-assisted ICONB techniques in human were included.
Similar to the methodology used in part one of our review, data were subdivided into baseline characteristics and operative and postoperative outcomes.Baseline characteristics included age and the proportion of males/females.Operative outcomes included operative time (OT),either total and/or for the intracorporeal urinary diversion(ICUD),estimated blood loss(EBL),and the rate of positive surgical margins(PSMs).Postoperative data included major and minor Clavien complications (early: <30 postoperative days;late:31-90 postoperative days),continence(daytime and nighttime) and potency rates.
Firstly, we started by describing the principles of each surgical technique.Then, we assessed baseline characteristics, operative and postoperative data of all types of ICONB.Lastly, we summarized the differences between intra and extracorporeal orthotopic neobladders, and between ICONB and intracorporeal ileal conduit (ICIC).
Data from all available studies were merged for combined analysis.Still,the results from large studies were also reported separately (greater than 30 patients).Data were summarized with ranges (minimum and maximum), and when sufficient information was available,weighted means of the percentages was calculated for qualitative variables(means were weighted according to the sample size of each study).
3.Results
We identified 40 studies in the literature that specifically addressed the use of totally ICONB in the treatment of bladder cancer.Among these,the most frequent type of UD described was the Studer “U” modified neobladder (twenty eight studies,70%)[14-43],followed by the Hautmann“W”modified neobladder (three studies, 7.5%) [13,44,45], the“Y" neobladder [46,47] and the Padua ICONB (two studies each, 5%) [48,49].The Camey reservoir [50], the pyramid pouch [51], the robot-assisted vescica ileale padovana (ra-VIP) [52], and the Florence robotic intracorporeal neobladder(FloRIN)urinary diversion[53]were reported in only one study each.In one study,the type of UD utilized was not specified [54].Outcomes were summarized in Table 1.
3.1.Surgical technique
In all described techniques, the patient is placed under general anesthesia in a low lithotomy position with a steep Trendelenburg tilt of 30.The arms are tucked to the side of the body for the RC and PLND.The patient is securely padded, strapped, and all exposed areas are wholly covered to prevent hypothermia.Pneumatic sequential calf compression devices are attached to the patient.

Table 1 Study characteristics, baseline, operative data, and complications after robot-assisted intracorporeal orthotopic neobladder.
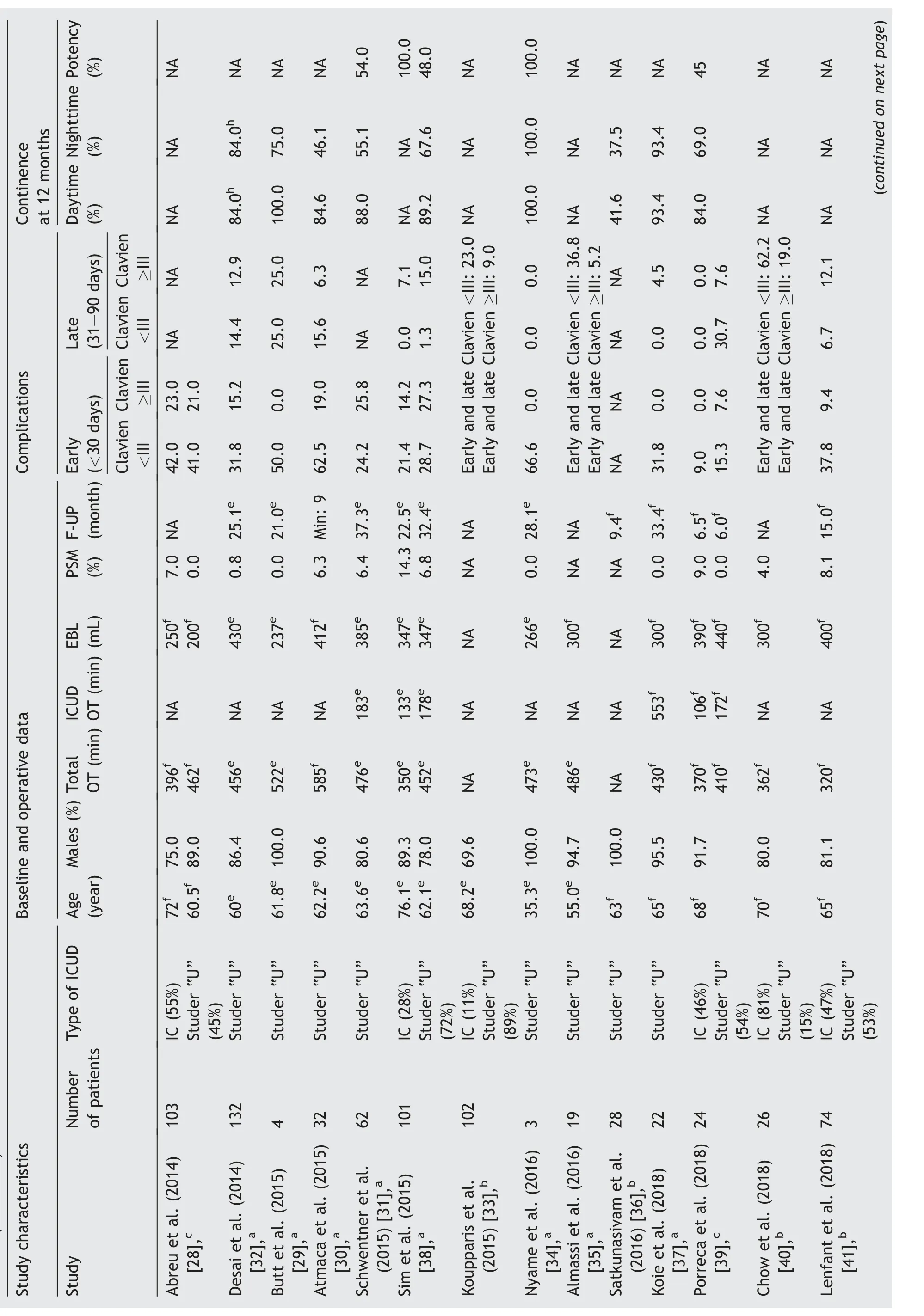
Table 1 (continued)
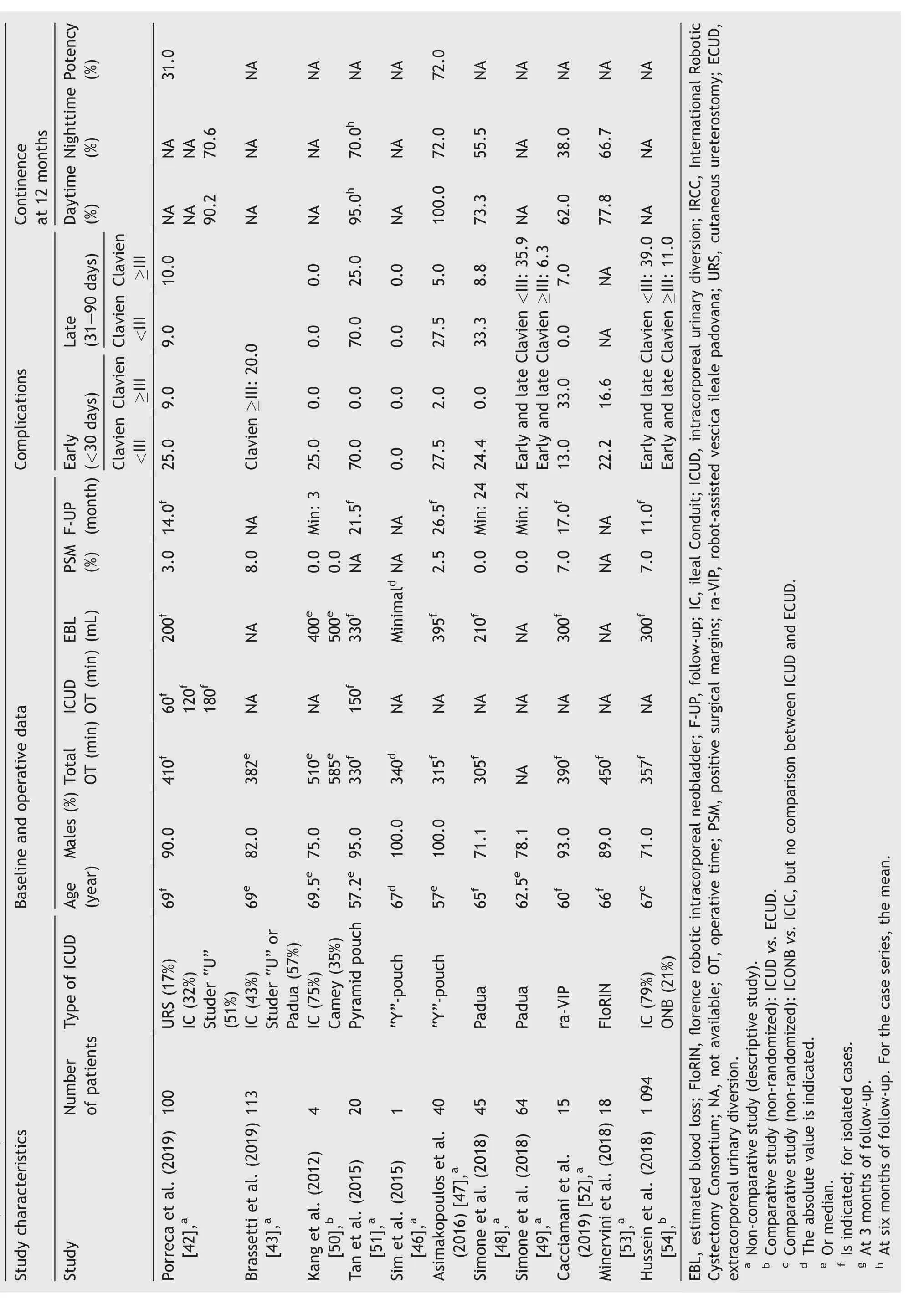
Table 1 (continued)
All techniques use a transperitoneal 6-port configuration, with the robot docked between the legs.After the extirpative part of the operation is completed,the robot is undocked briefly to reduce the Trendelenburg position to 10before performing the ICONB.This maneuver facilitates bringing the desired bowel segment down into the pelvis.
3.1.1.The robotic Hautmann “W” ICONB
The original,step-by-step description of this technique was published in 2003 [13] and updated in 2017 [45].After completing the RARC and the PLND,the ICUD is performed.
3.1.1.1.Port placement.A transperitoneal 6-port configuration is used: Four robotic ports (three 8-mm for the instruments, one 12-mm for the camera) and two assistant ports (one 5-mm and one 15-mm) (Fig.1A).The camera port is placed in the midline, 4-5 cm above the umbilicus.An additional 12-mm suprapubic port is placed to facilitate the restoration of the bowel continuity.
3.1.1.2.Identification and fixation of the bowel segment.A 45-50 cm ileal segment 15-20 cm proximal to the ileocecal valve is isolated and divided into a right and left limb (Fig.1B).Each limb has a descending and an ascending loop with a chimney.Six stay sutures are used to fix the W configuration.The first stay suture (Tag 1)should be at the most dependent part of the right limb.The second stay suture (Tag 2) is placed 12-15 cm proximal to the first and marks the upper end of the right chimney.The third stay suture (Tag 3) is placed 10-12 cm from Tag 1 on the ascending loop of the right half.These stay sutures are mirror-imaged(Tags 4-6)for the left limb.
3.1.1.3.Bowel detubularization and construction of the posterior plate.Excluding the 10-cm chimney, the bowel is detubularized by incising it along its antimesenteric border and folded so that the edges of the ascending and descending loop are sutured together(Fig.1C).The procedure is repeated on the left.Finally,the edges of the right and left limbs are oversewn to create the posterior plate; 3/0 barbed running sutures can be used.
3.1.1.4.Urethro-ileal anastomosis (UIA).A 2-arm Van Velthoven tension-free UIA is performed over a 22 Ch catheter after releasing the stay sutures (Tags 1-4) with 3/0 barbed sutures (Fig.1D).Then, the lower half of the anterior wall of the neobladder is sutured (Fig.1E).
3.1.1.5.Division of bowel continuity.The isolated bowel segment and its mesentery are then divided proximal to Tag 2 and distal to Tag 6 with a 45 or 60 mm Endo-GIA?stapler through the 15-mm assistant port.
3.1.1.6.Uretero-ileal anastomosis.Each ureter is spatulated and anastomosed end-to-side to a buttonhole incision on each chimney after the ureteral spatulation (4/0 polyglactin) (Fig.1F).The 8.5 Ch single-J ureteric stents are placed after the posterior aspect of the anastomosis is completed.A 3/0 chromic suture is used to secure each stent separately to the neobladder.
3.1.1.7.Closure of the anterior wall of the neobladder and restoration of the bowel continuity.The remaining anterior wall of the neobladder is closed in a T-shaped manner (Fig.1G).Finally, bowel continuity is restored.
3.1.2.The robotic Studer “U” ICONB
A robotic Studer “U” ICONB replicates the surgical principles of an open Studer ONB[55].There are two main Studer“U” ICONB variants that deserve further explanation.3.1.2.1.The Karolinska-modified Studer “U” ICONB.The characterization of the technique is based on described by Wiklund and Poulakis [18].
3.1.2.1.1.Port placement.A transperitoneal 6-port configuration is used: Four robotic ports (two 8-mm and one 15-mm for the instruments,and 12-mm for the camera)and two assistant ports (two 12-mm) (Fig.2A).The camera port is placed in the midline, 5 cm above the umbilicus.
A 15-mm port is placed in the third robotic arm just above and medial to the left anterior superior iliac spine.The assistant can disconnect the third robotic arm and use this port to insert the stapler.The assistant ports are placed on either side of the right robotic instrument port.
3.1.2.1.2.Identification of the bowel segment.In this technique, a 55-60 cm ileal segment, 20-25 cm proximal to the ileocecal valve, is used (Fig.2B).Both sites are marked with stay sutures of dyed 3/0 Vicryl.
3.1.2.1.3.Urethro-ileal anastomosis.A buttonhole incision is performed 15 cm proximal to the distal end of the chosen segment and as close as possible to the mesenteric border, avoiding injuring blood vessels.A twoarm tension-free UIA is completed over a 22 Ch catheter with 2/0 barbed sutures using a Van Velthoven technique.Alternatively, to help saturation of the bowel segment around the buttonhole incision can be opened before the UIA is performed.
3.1.2.1.4.Division and restoration of the bowel.The terminal ileum is divided 15-20 cm distal(0-cm point)and 40 cm proximal (55-cm point) to the UIA.A side ileo-ileal anastomosis is performed using an Endo-GIA? stapler through the left 15-mm port, and the bowel continuity is restored.
3.1.2.1.5.Bowel detubularization and construction of the posterior plate.The intestinal segment is detubularized along its antimesenteric border, except for the proximal 15 cm of the left ileal loop (chimney) (Fig.2C).To protect the UIA, detubularization around this area should be performed just off to the mesenteric border.The medial edges of the right and left ileal limbs are sutured together in a seromuscular fashion(avoiding suturing the mucosa)to create the posterior plate.The 3/0 barbed sutures can be used.
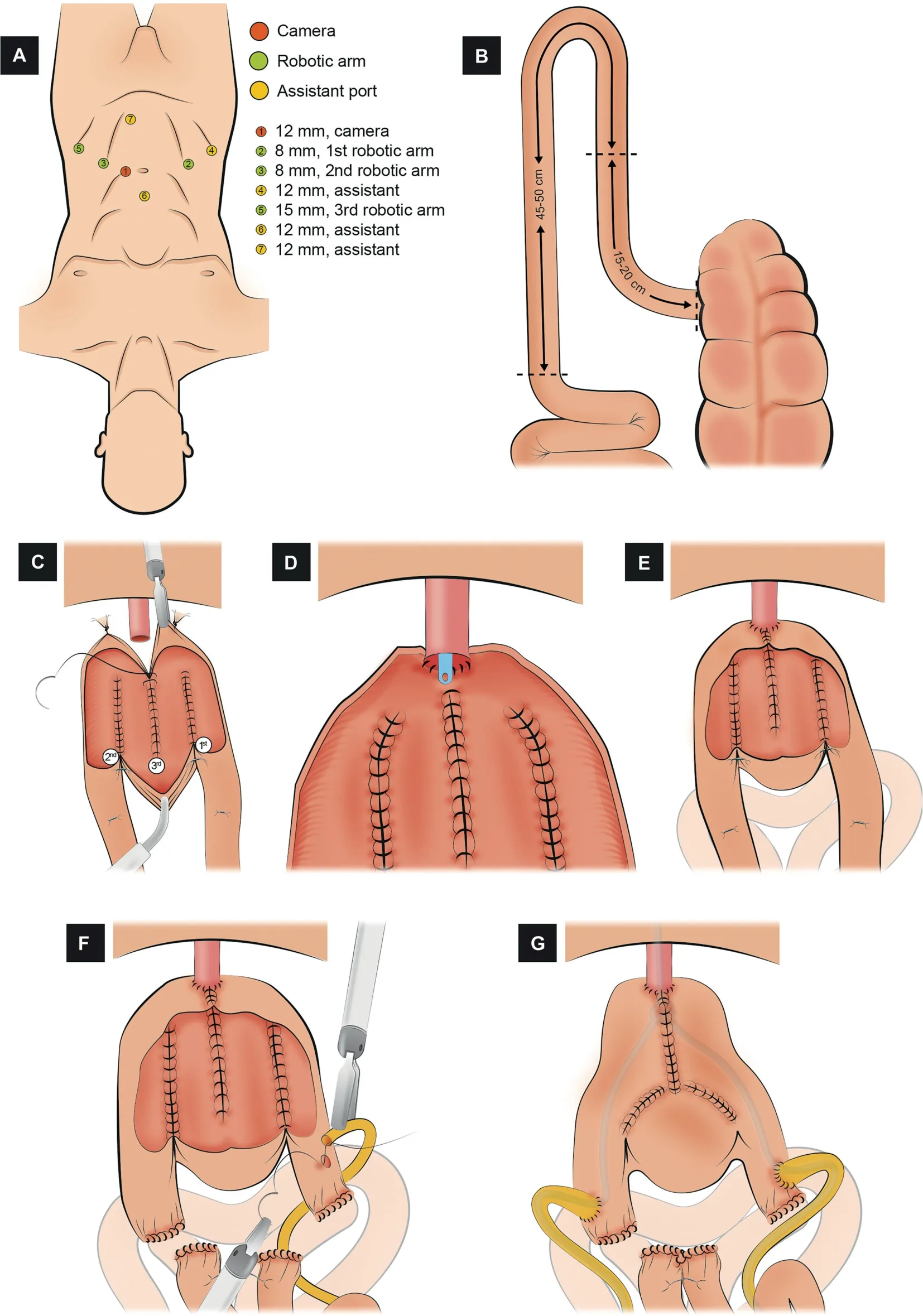
Figure 1 Schematic figure demonstrating the step-by-step creation of the robotic Hautmann“W” ICONB (Adapted from Hussein et al.[45]).(A) Port configuration.Four robotic and two assistant ports.An additional 12 mm suprapubic port is placed to ease restoration of bowel continuity.(B)Identification of the bowel segment.A segment of 45-50 cm of the ileum 15-20 cm proximal to the ileocecal valve is isolated and divided into a right and left limb.(C) Bowel detubularization and construction of the posterior plate.The bowel is detubularized by incising it along its anti-mesenteric border and folded so that the edges of the ascending and descending loop are sutured together.(D) Urethro-ileal anastomosis.Van Velthoven tension-free UIA is performed over a 22 Ch catheter.(E) Closure of the lower half of the anterior wall.Only the lower half of the anterior wall of the neobladder is sutured.(F) Uretero-ileal anastomosis.An end-to-side uretero-ileal anastomosis is made on each chimney after ureteral spatulation.(G) Closure of the anterior wall of the neobladder.The remaining anterior wall of the neobladder is closed in a T-shaped manner.
3.1.2.1.6.Closure of the anterior wall of the neobladder.The open edge of the right limb is folded anteriorly towards the left limb(Fig.2C),creating the spherical shape of the Studer neobladder.The lowermost part of the anterior wall of the reservoir is oversewn from the UIA upwards, while the uppermost 5-cm segment is kept open to facilitate passage of the ureteral stents.
3.1.2.1.7.Uretero-ileal anastomosis and closure of the anterior wall of the neobladder.A Wallace type anastomosis is preferred.Both ureters are spatulated and oversewn side-to-side in order to create the posterior wall plate(5/0 Biosyn suture).Simple-J ureteric stents are advanced using a Seldinger technique through two separate 4-mm incisions at the lower aspect of the abdominal wall.The Wallace anastomosis is completed, and the remaining part of the neobladder is then closed with a 3/0 barbed running suture.Both ureteral stents are secured separately with 4/0 chromic gut(Fig.2D and E).The window in the anterior wall of the neobladder is closed with a running 3/0 V-Loc?suture.
3.1.2.2.The University of Southern California (USC)-modified Studer “U” ICONB.The characterization of the technique is based on the study described by Chopra et al.[56].
3.1.2.2.1.Port placement.A transperitoneal 6-port configuration is also used, similar to the Karolinska technique [56], with the exception that the camera port is placed approximately 7 cm above the umbilicus.
3.1.2.2.2.Identification of the bowel segment.The authors start by identifying the most mobile and dependent loop of terminal ileum that reaches the urethra with the least tension.This site is marked as the point where the UIA will be performed(11-cm point)(Fig.3A).At this stage,five separate landmark points are marked as follows:
- 0-cm point:Located 11 cm distal to the UIA,towards the ileocecal valve; this is the distal end of the pouch.
- 22-cm point:Located 11 cm proximal to the UIA;this will be the future apex of the posterior plate (APP).
- 44-cm point: Located 22 cm proximal to the UIA; this is the proximal end of the pouch and beginning of the chimney (afferent limb).
- 60-cm point: Located 49 cm proximal to the UIA, the most proximal end of the chimney.
- 65-cm point: Located 5 cm proximal to the 60-cm point,this segment is discarded.
Each point on the pouch is marked with stay undyed sutures, whereas dyed sutures are used to mark the bowel segments that will later be anastomosed to restore the bowel continuity.
3.1.2.2.4.Bowel detubularization and creation of the posterior plate.A 24 Ch chest tube is used to cannulate the bowel segment to be detubularized and assure exact localization on its antimesenteric aspect.The bowel is first detubularized from the 44-cm to the 22-cm point, then from the 0-cm to the 22-cm point.The medial edges of the matching segments are oversewn from the APP to the chimney to create the posterior plate using 2/0 running barbed sutures(Fig.3B).The posterior plate is now rotated 90counterclockwise (Fig.3B and C).
3.1.2.2.5.Urethro-ileal anastomosis, cross folding of the pouch, and closure of the anterior wall.A “modified Rocco stitch” is placed between the 11-cm point and the distal cut edge of Denonvilliers’ fascia, posterior to the urethra(Fig.3D).A tension-free Van Velthoven type UIA is performed over a 24 Ch catheter with a 3/0 barbed suture with two needles,as previously described.The stay suture at the 22-cm point is used to cross-fold the pouch toward the 0-cm and 44-cm points.Running 2/0 barbed sutures are then used to close the anterior opening generated by cross folding of the pouch (Fig.3E).
3.1.2.2.6.Uretero-ileal anastomosis.An end-to-side uretero-ileal anastomosis (Bricker-type) is performed over the afferent limb after ureteral spatulation(4-0 polyglactin)(Fig.3F).The 6 Fr double-J ureteric stents are placed.
3.1.2.3.The robotic pyramid pouch ICONB.The characterization of the technique is based on described by Tan et al.[51].
3.1.2.3.1.Port placement.A transperitoneal 6-port configuration is used: Four robotic ports (two 8-mm and one 12-mm for the instruments, and one 12-mm for the camera), and two assistant ports (one 5-mm and one 12-mm) (Fig.4A).The camera port is placed 5 cm above the umbilicus.
3.1.2.3.2.Identification of the bowel segment.A 50-cm segment of the distal ileum, at least 15 cm from the ileocecal valve, is identified (Fig.4B).
3.1.2.3.3.Urethro-ileal anastomosis.A tension-free UIA is performed in the middle of the identified segment over a 16 Ch catheter with 3/0 barbed suture;the selected segment remains as two ileal limbs (Fig.4C).
3.1.2.3.4.Division and restoration of the bowel continuity.The isolated bowel segment and its mesentery are divided proximally and distally, with a 60-mm Endo-GIA?stapler inserted through the left 12-mm assistant port.The robotic third arm is temporarily removed while this maneuver is performed.A side-to-side stapled anastomosis using a 60-mm Endo-GIA?is accomplished in order to restore the bowel continuity.
One day the Prince went out hunting, and going in pursuit of a wild boar he soon lost the other huntsmen, and found himself quite alone in the middle of a dark wood
3.1.2.3.5.Bowel detubularization and construction of the posterior plate.The intestinal segment is detubularized along the antimesenteric border, except for the 2-cm uppermost portion of both limbs(Fig.4C).Detubularization near the UIA is carried out next to the mesenteric border.The medial edges of both ileal limbs are sutured together(from the outer (serosal) to the inner (luminal) aspect) to create the posterior plate (3/0 barbed suture).
3.1.2.3.6.Closure of the anterior wall of the neobladder.The first 10-cm of the anterior plate is sutured,starting from the UIA cephalad and folding the bowel in the sagittal plane (Fig.4D).Two lateral stay sutures of 2/0 polyglactin are placed midway along the remaining bowel segment on each anterior leaf.Then, the stay sutures are tensioned,and the folded segment is closed from lateral to medial in the coronal plane using a 3/0 running barbed suture(Fig.4E).The closure of each limb overlaps past the midline in order to reduce the chance of suture breakdown and leakage at this point.
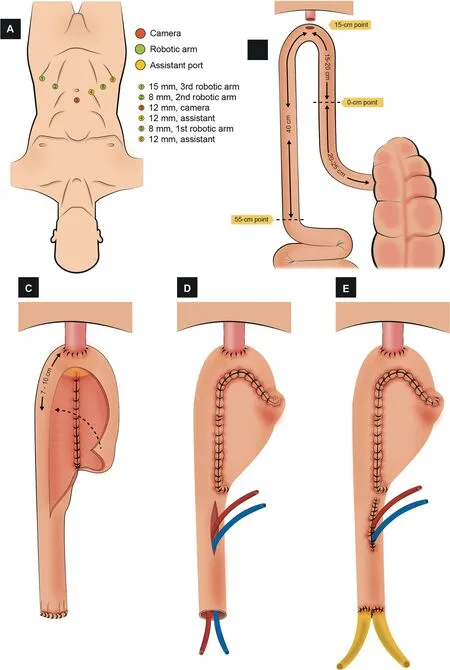
Figure 2 Schematic figure demonstrating the step-by-step creation of the robotic Karolinska-modified Studer “U” ICONB(Adapted from Wiklund and Poulakis [18]).(A) Port configuration.Four robotic and two assistant ports.(B) Identification of the bowel segment.A segment of 55-60 cm of the ileum 20-25 cm proximal to the ileocecal valve is isolated.(C)Construction of the posterior plate and closure of the anterior wall of the neobladder.The intestinal segment is detubularized.The medial edges of the right and left ileal limbs are sutured together to create the posterior plate.The open edge of the right limb is folded anteriorly towards the left limb.The cranial part of the right limb is folded and sutured to the left limb to create the anterior wall.The most proximal part of the anterior wall is kept open to facilitate the passage of ureteral stents.(D)Stent placement.Simple-J ureteric stents introduced through the window in the anterior wall.(E) Uretero-ileal anastomosis and closure of the anterior wall of the neobladder.Wallace anastomosis is made over the ureteric stents,and the window in the anterior wall of the neobladder is closed with a running barbed suture.
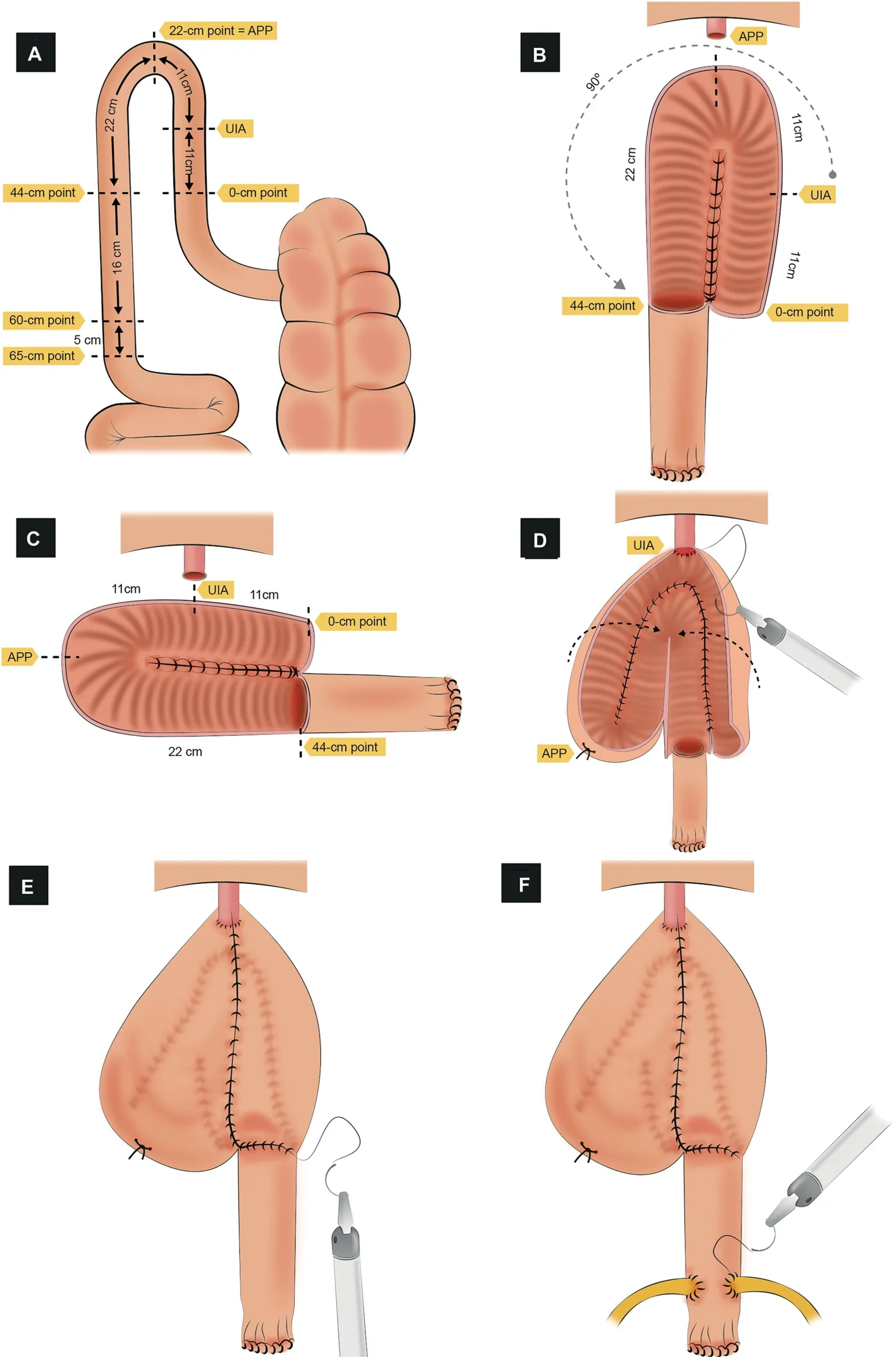
Figure 3 Schematic figure demonstrating the step-by-step creation of the robotic University of Southern California (USC)-modified Studer“U” ICONB(Adapted from Chopra et al.[56]).(A)Identification of the bowel segment.A segment of 65 cm of the ileum is isolated.The most mobile and dependent loop that reaches the urethra is marked as the UIA point.Posteriorly, five separate landmark points are marked.(B) Bowel detubularization, creation of the posterior plate, and rotation.The bowel detubularized(from the 44-cm to 0-cm point)and the medial edges of the matching segments are sutured to create the posterior plate.The posterior plate is rotated 90° counterclockwise.(C) Bowel segment layout after rotation.(D)Urethro-ileal anastomosis and cross folding of the pouch.An urethro-ileal anastomosis is made on the 11-cm point, and the pouch is cross folded to approximate the intestinal edges that will create the anterior wall.(E) Closure of the anterior wall.The cross folded intestinal edges are sutured together to create the anterior wall.(F) Uretero-ileal anastomosis.An end-to-side uretero-ileal anastomosis is performed.APP, apex of the posterior plate; UIA, urethro-ileal anastomosis.
3.1.2.3.7.Uretero-ileal anastomosis.Both ureters are adequately spatulated.An end-to-side uretero-ileal anastomosis (Bricker-type) is performed over the proximal end of both ileal limbs (4/0 polydioxanone suture) (Fig.4F).Single-J ureteral stents are advanced and externalized through an opening in the neobladder.An 18 Ch suprapubic catheter is inserted into the neobladder, and closure is completed.
3.1.2.4.The robotic Y-shaped ICONB.The characterization of the technique is based on the study described by the group of Dr.Gaston in Bordeaux [47].
Again, a transperitoneal 6-port configuration is used,similar to a robotic radical prostatectomy port configuration, but all trocars are shifted cranially by 2 cm.A 40-cm ileal segment is isolated 15-20 cm proximal to the ileocecal valve (Fig.5A).A tension-free UIA is performed in the middle of the identified segment over a 20 Ch catheter with 3/0 V-Loc?suture; the selected segment remains as two ileal limbs (Fig.5B).Then, the detubularization of the antimesenteric border of both limbs is carried out(Fig.5C).The posterior plate is closed with running barbed sutures(Fig.5D).The proximal part of the posterior plate is folded anteriorly, and two running sutures continue to close the anterior part bilaterally, creating a “heart shape” (Fig.5E and F).Ureters are spatulated and end-to-side anastomosed with no antireflux mechanism to the two side limbs(Fig.5G).Double-J 8 Ch ureteral stents are left in place.
3.1.2.5.The robotic Padua ICONB.The characterization of the technique is based on described by Simone et al.[48].
3.1.2.5.1.Port placement.A transperitoneal 6-port configuration is used: Four robotic (three 8-mm for the instruments and one 12-mm port for the camera), and two assistant ports (one AirSeal? and one 12-mm) (Fig.6A).An additional suprapubic miniport is placed to introduce double-J stents.
3.1.2.5.2.Identification of the bowel segment.A 42-cm segment of the most dependent portion of the ileum is isolated using two loads of a 60-mm Endo-GIA?stapler(Fig.6B).The neobladder is constructed according to the following measures:
- 8 cm for the right plate
- 10 cm for the neck configuration
- 8 cm for the left plate
- 16 cm folded in a ‘‘U’’ configuration to create an 8-cm dome
The optimal point to perform the UIA is approximately 13 cm proximal to the distal margin of the ileal segment.
3.1.2.5.3.Detubularization and configuration of the neobladder (Fig.6C-H).A 10-cm inverted U-shaped neobladder neck is created with a stay suture approximating the ileal segment at 8 cm and 18 cm from the distal margin of the ileum.The first 8-cm of distal ileum is now detubularized along the antimesenteric border.The neobladder neck is created with Endo-GIA?stapler from the two branches of the inverted U (5 cm+5 cm).The remaining 24 cm of the ileum is then detubularized, and the neobladder is then folded in order to create a triangular shape with 8 cm sides and the vertex at the inverted U-shaped neobladder neck.
3.1.2.5.4.Urethro-ileal anastomosis.A tension-free UIA is performed in the neobladder neck over a 22 Ch catheter with 2/0 Monocryl sutures (Fig.6I).
3.1.2.5.5.Uretero-ileal anastomosis and completion of the neobladder.The uretero-ileal anastomosis is performed using a modified split-nipple technique (Fig.6I).Both ureters are adequately spatulated,and anastomosis is performed using interrupted stitches of 4/0 Monocryl.The 6/7 Ch double-J stents are advanced through a prepubic miniport.The anterior wall of the neobladder is sutured using 2/0 V-Loc?running sutures.
3.2.Global results of robot-assisted ICONB
Seven studies out of forty made a non-randomized comparison of the performance of an ONB in an intracorporeal or extracorporeal fashion [14,33,36,40,41,50,54].Five out of forty studies compared the accomplishment of an ICONB or an ICIC[16-18,21,26,28,39].The remaining reports were only descriptive in nature.In one-third of the studies[14,20,22,33,40,41,43,50,54], intraoperative and postoperative outcomes were reported combined for patients who underwent an ileal conduit and patients who received an ONB, making it impossible to compare results between these techniques.
3.2.1.Baseline characteristics
In the included studies, the age of patients ranged from 55 to 79 years, with the exception of one study, which only included young patients (mean age: 35.3 years) [34].The percentage of males ranged from 70% to 100% (weighted mean 76.9%).A quarter of the studies included only males,and half of them included >90% proportion of males.
3.2.2.Operative outcomes
In the reviewed studies,total OT(skin to skin)ranged from 305 min to 720 min, whereas ICUD OT ranged from 124 min to 553 min.Half of the studies(49%)reported an OT longer than 7 h (420 min).Among the studies with larger cohorts(n>30), the maximum total and ICUD OT were shorter at 585 min and 184 min, respectively.Total EBL (skin to skin)ranged from 200 mL to 900 mL.The majority of studies(82%) reported an EBL lower than 500 mL.The rate of PSM ranged from 0% to 8.1% (weighted mean 4.8%), and was comparable between smaller and larger studies (weighted mean 5%).
3.2.3.Perioperative outcomes
3.2.3.1.Complications.Among the articles included in this review, twelve of them did not include complications data, did not classify the complications between early and late, or did not separate the information into minor(Clavien <III) and major (Clavien ≥III) complications.
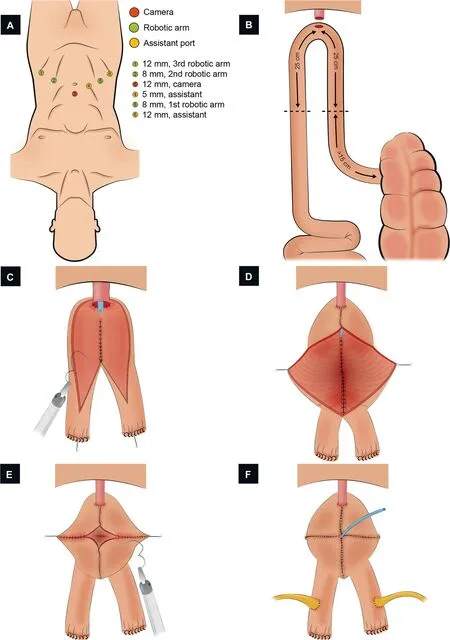
Figure 4 Schematic figure demonstrating the step-by-step creation of the robotic pyramid pouch ICONB(Adapted from Tan et al.[51]).(A) Port configuration.Four robotic and two assistant ports.(B) Identification of the bowel segment.A segment of 50 cm of the ileum >15 cm proximal to the ileocecal valve is isolated.(C)Urethral-ileal anastomosis,bowel detubularization,and formation of the posterior plate.After performing the urethral-ileal anastomosis,the bowel is detubularized(except for the 2 cm uppermost portion of both limbs), and the medial edges of both segments are sutured together.(D) Closure of the distal part of the anterior wall.The first 10 cm of the anterior plate is sutured, from distal to proximal.Two lateral stays are placed in the midway of the remaining bowel segment.(E) Neobladder construction.The closure of the folded bowel was made from lateral to medial in the coronal plane.(F) Uretero-ileal anastomosis.An end-to-side uretero-ileal anastomosis is performed over the proximal end of both ileal limbs.
Early minor and major complication rates ranged from 0%to 100% and from 0% to 33%, with weighted means of 29.4%and 17.7%,respectively.Late minor and major complication rates ranged from 0% to 70% and from 0% to 25%, with a weighted mean of 15.6%and 13.8%,respectively.The rate of complications achieved similar results in the larger studies,with weighted means of 28.2%and 18.8%for early minor and major complications,and 15.0%and 14.5%for late minor and major complications, respectively.The follow-up period ranged between 3.0 months and 37.3 months.
Collins et al.[27] in their report of 70 RARC with Studer“U” ICONB indicated that the most common early complications were urosepsis (8.6%), lymphocele (5.7%),abdominal abscess (4.3), uretero-ileal leakage (4.3%),paralytic ileus (2.9%), bleeding from major vessels (2.9%)and deep vein thrombosis/pulmonary embolism(2.8%).The predominant late complications were urinary tract infection (9.8%), obstructive uropathy (4.3%), uretero-ileal stricture (4.3%), and neobladder stones (2.8%).
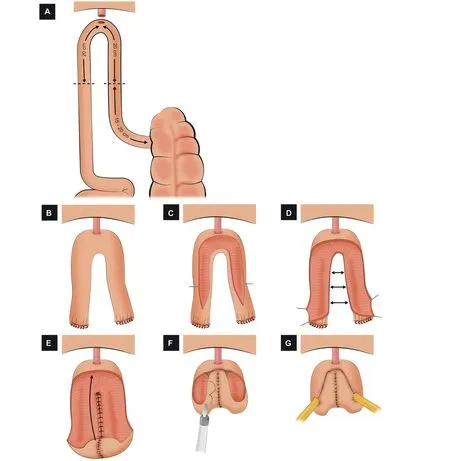
Figure 5 Schematic figure demonstrating the step-by-step creation of the robotic Y-shaped ICONB(Adapted from Asimakopoulos et al.[47]).(A)Identification of the bowel segment.A segment of 40 cm of the ileum 15-20 cm proximal to the ileocecal valve is isolated.(B) Urethro-ileal anastomosis.After performing the urethro-ileal anastomosis, the ileal segment remains as two ileal limbs.(C) Detubularization of the bowel.The two limbs are detubularized in the antimesenteric border.(D) Construction of the posterior plate.The medial edges of the detubularized limbs are sutured together to create the posterior plate.(E) Folding the pouch.The proximal part of the posterior plate is folded anteriorly.(F) Creation of the anterior wall.The anterior wall is closed with running sutures, creating a “heart shape”.(G) Uretero-ileal anastomosis.An end-to-side uretero-ileal anastomosis is performed over the proximal end of both ileal limbs.
3.2.3.2.Continence and potency.Only 22/40 and 10/40 articles included in this review reported data on continence and potency after the surgery, respectively.Daytime continence ranged from 41% to 100% (weighted mean 84.2%), whereas nighttime continence ranged from 0% to 100% (weighted mean 67.6%).Potency rates ranged from 25% to 100% (weighted mean 53.2%).Interestingly, the minimum ranges for all three parameters (daytime and nighttime continence and potency) increased significantly to 73%, 46%, and 31% when only large studies were considered.Maximum rates achieved similar results.
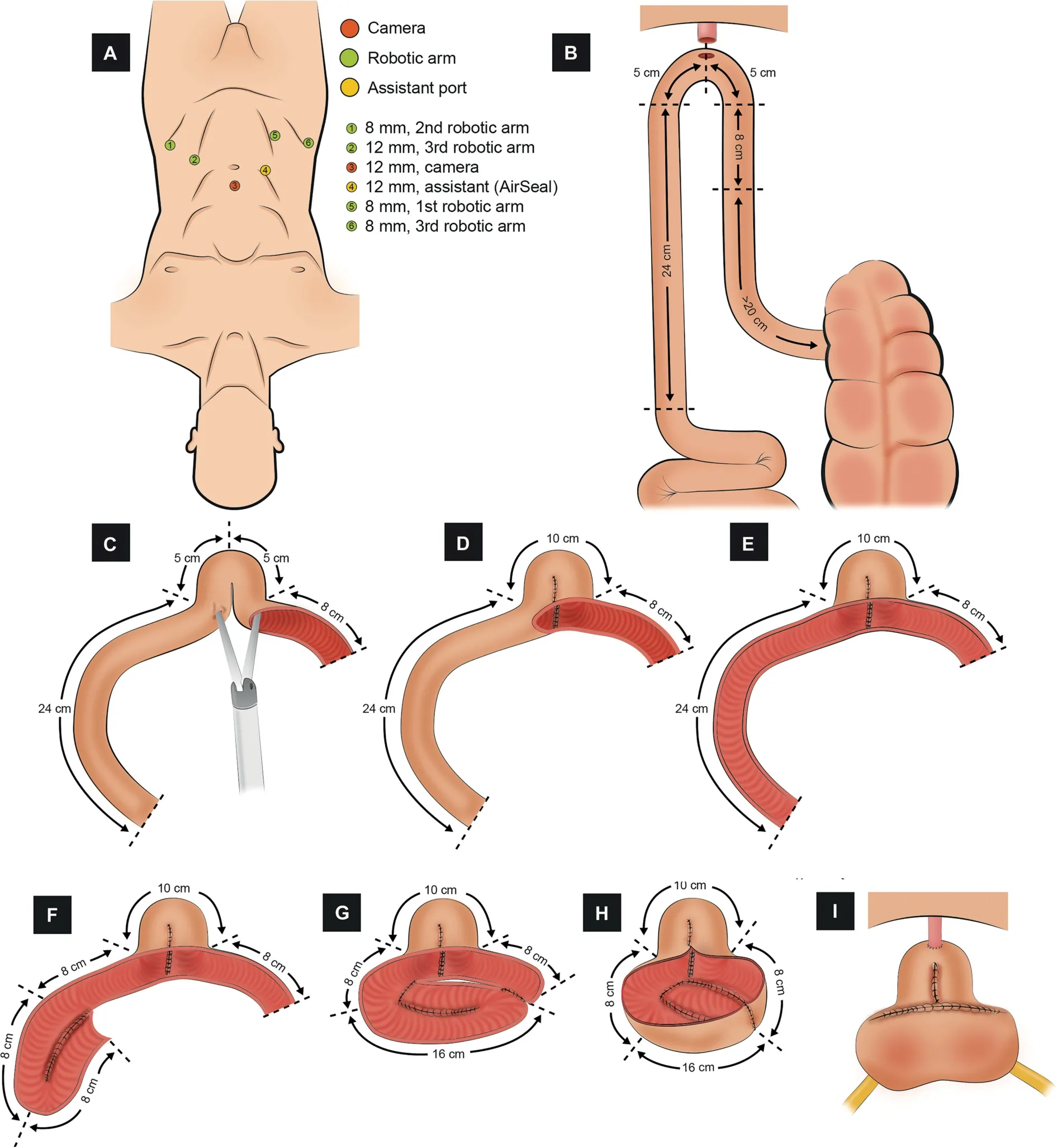
Figure 6 Schematic figure demonstrating the step-by-step creation of the robotic Padua ICONB (Adapted from Simone et al.[48]).(A) Port configuration.Four robotic and two assistant ports.(B) Identification of the bowel segment.A segment of 42 cm of the most dependent portion of the ileum.(C)Detubularization of the distal part of the ileum(8 cm).The most distal part(8 cm)of the small bowel is detubularized(8 cm),and an incision in the left horn is made to insert the stapler.(D)Creation of the neobladder neck.The neobladder neck is created with a stapler.(E)Detubularization of the proximal part of the ileum(24 cm).The remaining ileum (24 cm) is detubularized.(F) First fold.The first 8-cm ileum segment is folded and sutured to the second 8-cm segment.(G) Second fold.The next 8-cm ileum segment is folded over the previous to configure the posterior plate.(H) Completion of the posterior plate of the neobladder.The adjacent edges of the folded ileum are suture together.(I) Urethro-ileal anastomosis,uretero-ileal anastomosis, and completion of the neobladder.A tension-free urethro-ileal anastomosis is performed in the neobladder neck, an end-to-side uretero-ileal anastomosis is performed over the posterior wall, and the neobladder is finally completed with the closure of the anterior wall.
3.3.Intracorporeal versus extracorporeal ONB
This topic is widely discussed in part one (Table 2 in part one,which is in the same issue).As mention there,it stands out that none of the studies randomized the patients to intra or extracorporeal manner, and all the studies registered the data for both approaches together, so the conclusions must be drawn with caution.In this manuscript,we also include the study published by Satkunasivam et al.[36], which compares the urodynamic data of 28 males subjected to ICONB with a previously characterized cohort of 79 open ONB procedures.They concluded that ICONB had adequate urodynamic characteristics and comparable bladder cancer-specific health-related quality-of-life scores to open ONB.However, pad size and daytime wetness were worse for ICUD, which they hypothesized could be driven by significantly shorter follow-up on the ICUD group.
3.4.ICONB versus ICIC
To this day there are not sufficient quality data to determine the supremacy of either ICONB or ICIC.In this section,we summarize the results of those studies that compared the performance of ICONB and ICIC [16-18,21,26,28,39](Table 1).It is essential to highlight that none of these studies randomized the patients to the treatment group nor analyzed the IC and the ONB separately, so the conclusions must be drawn carefully.
Worth mentioning that in all studies that compared ICIC and ICONB, the ICONB was performed following the principles of Studer “U” modified neobladder.
Most of the studies showed younger patients in ICONB group (Jonsson et al.[16]: 73 vs.60 years, p<0.001; Desai et al.[26]: 75 vs.62 years, p=0.008; Abreu et al.[28]: 72 vs.60.5 years, p<0.001).Only one study [16] showed a higher proportion of males in ICONB (55% vs.91%,p=0.022), whereas the rest of the studies had a similar male rate in ICIC and ICONB.
Regarding operative data, two studies [28,39] found longer total OT in the performance of the ICONB versus ICIC(396 vs.462 min, p<0.001; 370 vs.410 min, p=0.002); the rest of the studies did not find a significant difference in total OT.OT referred exclusively to ICUD is rarely reported in the literature.Porreca et al.[39] found ICUD OT to be 66 min faster in ICIC (106 vs.172 min, p<0.001).Additionally, they demonstrated a progressive reduction over time in both groups:In the ICIC group it took 370 min for the case#1 and 280 min for the case#11;in the ICONB group,it took 440 min for the case #1 and 380 min for the case #13.The same author was the only one to find statistically significant differences in the EBL (390 mL vs.440 mL,p=0.047), while the rest did not.
Early and late minor and major complications were similar between ICIC and ICONB groups in all reviewed studies.The follow-up period ranged between 3 months and 32 months, and no statistically significant differences were found.
4.Discussion
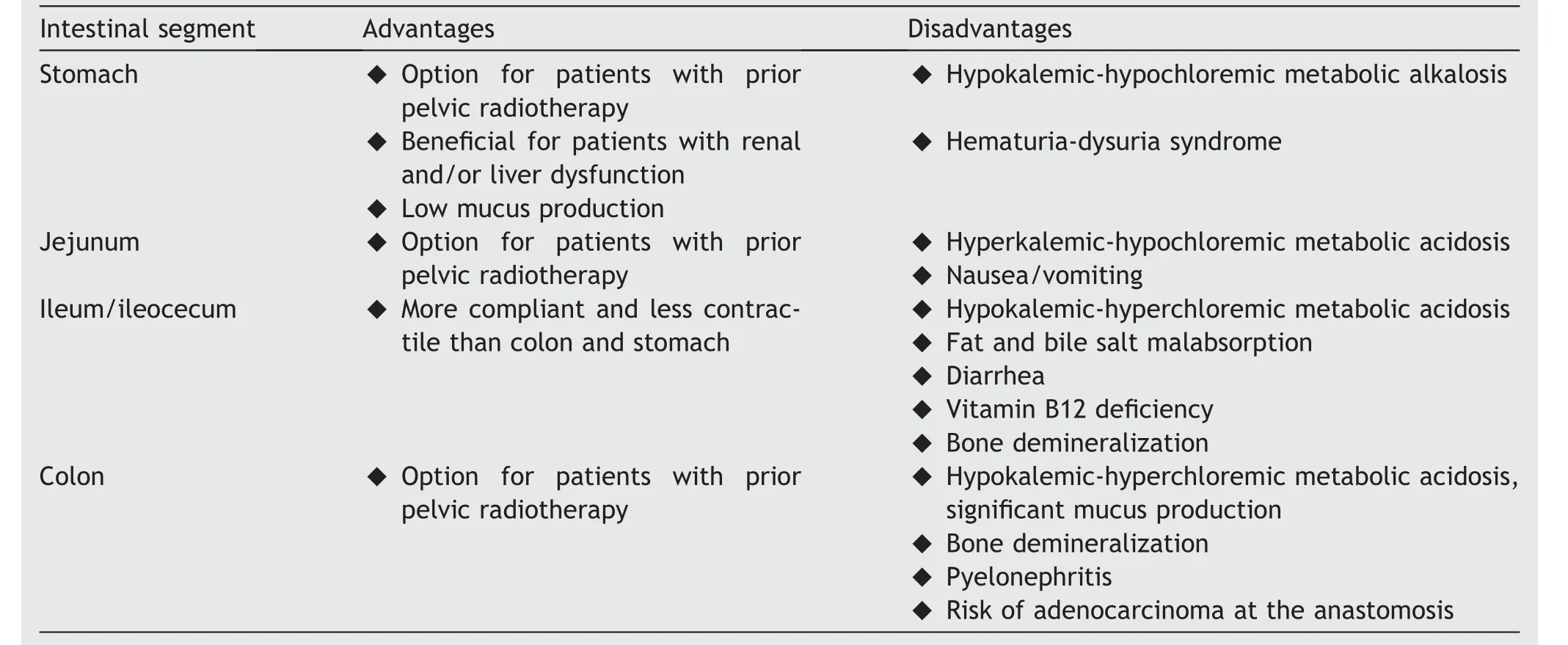
Table 2 Comparison of intestinal segments used for the construction of the neobladder (adapted from Tan et al.[60]).
RC with ONB offers preservation of body image along with a continent urinary system.Since the first RARC with ICONB described in 2003, different reports have confirmed the feasibility of performing a variety of ICUD types.In this review, we identified 40 studies that specifically addressed the use of totally ICONB in the treatment of bladder cancer.Among these, the most frequent type of ICONB was Studer“U” modified neobladder, Hautmann “W” modified neobladder, the “Y" neobladder, and the Padua neobladder.However, no randomized studies have been published comparing the performance of the different types of ICONB, the performance in an intra or extracorporeal manner, nor comparing the different types of ICONB with ICIC.At the time of this publication, there is an open clinical trial aiming to compare perioperative outcomes and complications after RARC with intra or extracorporeal UD in a prospective randomized fashion (https://clinicaltrials.gov/ct2/show/NCT02252393).However, since the study started in 2015, it has yet to recruit a single patient.The vast majority of series reported have been restricted to single-institution case series, with a limited sample size.In sum, high-quality evidence to draw meaningful conclusions is lacking.
We hypothesize that the lack of high-quality data could be driven by factors such as the relatively low incidence of MIBC, the technical difficulty associated with a robotic ICONB, and patients’ factors and tumor characteristics.In this regard, adequate knowledge of the technical aspects of these procedures is critical to master the techniques.This manuscript constitutes a compendium of the most used ICONB, with detailed descriptions of the technical aspects, operative and perioperative outcomes, and new consistent images.We hope that this work will facilitate having an in-depth knowledge of the ICONB techniques and the performance of randomized comparative studies to enable the establishment of the advantages and disadvantages of each intracorporeal approach accurately.
Regarding baseline characteristics,the patients included in our review are between their 6th and 8th decade of life.These data are consistent with the fact that although age alone is not an exclusion criterion to perform an ONB,being over 80 years is usually a contraindication[2].The majority of patients subjected to ICONB were males.This is consistent with the fact that historically the ICONB was restricted to males because it was believed that continence in females was dependent on an intact bladder neck.However,more recent anatomical studies have demonstrated that women can still remain continent despite preserving the urethra only [57,58], so performing an ICONB in females is not a limitation to achieve good continence.Follow-up is reported in only two-thirds(27/40)of the reviewed studies.Of these, one third (9/27) had a follow-up of less than 1 year.This could be particularly relevant for the analysis of medium/long term oncologic data.
Based on the literature, the ideal reservoir should have adequate capacity, low-pressure storage to avoid kidney damage and high compliance to help continence.Moreover,it should allow voluntary emptying at convenient intervals without residual urine and a low reabsorption capacity of hydrogen chloride.To obtain this, the reservoir should be spherical and made of small bowel [55,59].
The most popular intestinal segment used to create a continent reservoir has been the ileum, followed by ileocecum and colon.The stomach and jejunum have been used in the past, but their use has now been abandoned.Table 2 summarizes the advantages and disadvantages of the different intestinal segments.To perform an ileal neobladder,a sufficient length of the intestine must be first detubularized to prevent peristaltic contractions and to allow a large volume (500 mL) and low-pressure reservoir[60].The isolation of the correct and most mobile ileal segment is essential to ensure a tension-free, watertight,and well-vascularized UIA.Correct management of the ureters also includes handling the ureter gently, avoiding thermal damage of the mesoureter,and creating a tensionfree anastomosis.These are vital tips to prevent the development of an uretero-ileal anastomosis stricture.
Although the ideal bladder substitute is still to be developed, the ileal ONB most closely resembles the original bladder in both location and function.Voiding with this form of UD is accomplished by the use of concomitant abdominal straining (Valsalva maneuver) and relaxation of the pelvic floor musculature.
Various forms of upper urinary tract reflux protection have been described, including ileal intussusception, a simple isoperistaltic tunnel, direct submucosal or subserosal ureteral implantation, and tapered ileal prolongation implanted subserosally.However,due to the existence of very few reports in open surgery and none in robotic surgery,it is not clear whether it is necessary to perform an anti-reflux mechanism and, where appropriate, which of the methods described is the best.
5.Conclusion
Robot-assisted ICONB is doable, yet complex and challenging procedure.The most frequent types of robotassisted ICONB in the treatment of bladder cancer reported in the literature are Studer “U” modified neobladder, Hautmann “W” modified neobladder, the “Y"neobladder, and the Padua neobladder.Adequate knowledge of the technical aspects of these procedures is critical to master these techniques.
Randomized studies comparing outcomes from different types of ICONB, the performance in an intra or extracorporeal manner or the performance of an ICONB versus ICIC are lacking in the literature.The vast majority of studies reported have been restricted to single-institution case series,with limited sample sizes.To this day,there are not sufficient quality data to determine the supremacy of one technique.
The compendium of the most used ICONB created in this manuscript, with detailed descriptions of the technical aspects, operative and perioperative outcomes, and the new consistent images designed of each technique, will facilitate the knowledge of ICONB techniques.We encourage our peers to continue exploring them and to perform randomized comparative studies to enable the establishment of the advantages and disadvantages of each intracorporeal approach accurately.
Author contributions
Study concept and design: Hugo Otaola-Arca, Marcelo Orvieto.
Data acquisition: Hugo Otaola-Arca.
Data analysis: Hugo Otaola-Arca
Drafting of manuscript: Hugo Otaola-Arca.
Critical revision of the manuscript: Hugo Otaola-Arca,Kulthe Ramesh Seetharam Bhat, Marcio Covas Moschovas,Vipul R.Patel, Marcelo Orvieto.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors express their acknowledgments to Santiago Otaola because of his invaluable support.
 Asian Journal of Urology2021年1期
Asian Journal of Urology2021年1期
- Asian Journal of Urology的其它文章
- Single-port technique evolution and current practice in urologic procedures
- Robotic urologic surgery: Past, present and future
- Magnetic resonance imaging-guided prostate biopsy-A review of literature
- Totally intracorporeal robot-assisted urinary diversion for bladder cancer(Part 1).Review and detailed characterization of ileal conduit and modified Indiana pouch
- The robot-assisted ureteral reconstruction in adult: A narrative review on the surgical techniques and contemporary outcomes
- An unusual scrotal mass:Morphological clues
