An unusual scrotal mass:Morphological clues
Poojan Agarwal, Anila Sharma, Sunil Pasricha, Anurag Mehta
Department of Laboratory Services,Rajiv Gandhi Cancer Institute&Research Centre,New Delhi,India
A 21-year-old male presented with complaints of swelling and dragging sensation in the scrotum for 6 months.Local examination revealed a firm 10 cm×6 cm×4 cm lump with nonpalpable right testis.Overlying skin was free.Ultrasonography showed a mass lesion in the right testicular area with multiple heterogeneous foci and mild increased vascularity,however, without any cystic region and calcification.Right epididymis was also bulky and measured 1.6 cm×0.7 cm with heterogeneous texture.No significant inguinal lymphadenopathy was present.Features were suggestive of a neoplastic lesion.Patient’s serum lactate dehydrogenase(LDH), alpha feto-protein (AFP), beta-Human chorionic gonadotropin (β-HCG), complete blood count, liver and kidney function tests were within normal limits.A high inguinal orchidectomy was subsequently done.Gross examination revealed a firm oval mass with glistening external surface.Cut surface showed residual normal testis as a discrete nodule in center measuring 2.8 cm×2.5 cm×0.8 cm,encased by a large firm white lesion.Layers of tunica were embedded within the tumor.Microscopy revealed a pleomorphic tumor comprising of small undifferentiated cells having hyperchromatic nuclei and intermixed spindle cells.Many bizarre nuclei with evident multinucleation were also seen.Few cells with tadpole and strap cell like morphology were noted interspersed.A typical mitosis and foci of necrosis were present.Based on histomorphology, the differentials considered were rhabdomyosarcoma,undifferentiated sarcoma,sex cord stromal tumor and anaplastic seminoma.A panel of immunohistochemistry was put up to differentiate amongst these lesions(Table 1).On immunohistochemistry,the tumor cells expressed desmin,myogenin(diffuse)and myoD1(weak and focal)while they were negative for cytokeratin(CK),sallike protein 4(SALL4),placental alkaline phosphatase(PLAP),s-100, Steroidogenic factor-1 (SF-1) and inhibin (Fig.1 and Fig.2).A final diagnosis of embryonal rhabdomyosarcoma--Anaplastic variant was made (pT2N0 stage 1B).A combination chemotherapy was given with vincristine,actinomycin and cyclophosphamide (VAC) alternating with ifosfamide and etoposide (IE) regimen every three weekly.Radiation consisted of external beam therapy.The patient was doing well on 3 month follow-up.
The paratesticular region is a complex anatomical area which includes the contents of the spermatic cord, testicular tunics,epididymis and vestigial remnants of epithelial,mesothelial or mesenchymal origin.Tumors affecting this region may be clinically indistinguishable from testicular tumors, thus resulting in initial misdiagnosis.On rare occasions, tumors from distant sites may metastasize to the paratesticular region [1].These lesions typically present as a rapidly growing and painless intrascrotal mass, eliciting only anatomically associated symptoms.
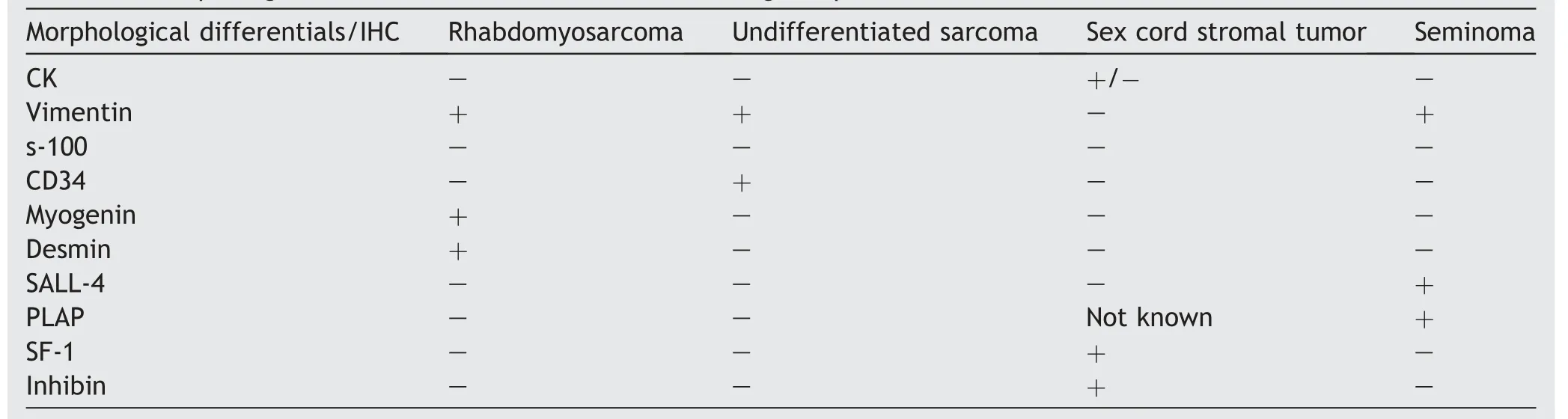
Table 1 Morphological differentials and their differentiating IHC profile.
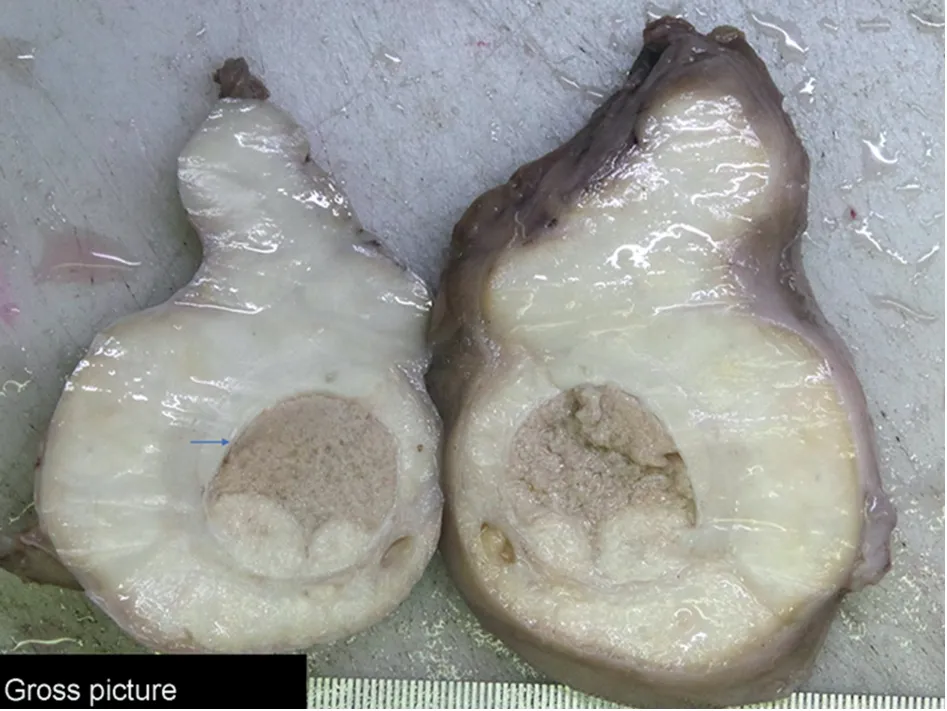
Figure 1 Gross picture of the testicular tumor.
Seventy percent of paratesticular tumors reported are benign and 30% are malignant.The common benign tumors reported in paratestis include lipomas, adenomatoid tumors and leiomyomas.Among the malignant tumors, the most common histological type reported is liposarcoma(46.4%), followed by leiomyosarcoma (LMS) (20%), malignant fibrous histiocytomas (MFH) (13.0%), and embryonal rhabdomyosarcoma (9.0%) [2].
Para testicular rhabdomyosarcoma (RMS)is a rare entity,accounting for 7% of all RMS reported and is uncommon in adults [3].Eighty percent of the lesions occur in individuals<21 years of age [4].Embryonal RMS (ERMS) is the most common subtype reported.ERMS further has botryoid and anaplastic variants.The anaplastic variant is a distinctive RMS subtype.The presence of markedly enlarged, atypical cells with hyperchromatic nuclei defines the anaplastic variant of rhabdomyosarcoma.Previously these tumors were stratified under alveolar or embryonal RMS,however,because of their poorer clinical outcome and lack of PAX-FOXO1 fusion gene,several authors now consider that these cases should be grouped separately as anaplastic RMS [5].Few studies have also found germline TP53 mutations in individuals diagnosed with anaplastic RMS at a young age.Early identification of germline mutationpavespathforsubsequentsurveillanceand early diagnosis of second malignancies[6].

Figure 2 Micro-photographs of the testicular tumor.(A)Tumor tissue abutting seminiferous tubules (100×; H&E); (B)Multinucleate tumor cells with abundant eosinophilic cytoplasm and peripherally pushed nuclei suggestive of rhabdoid differentiation(400×;H&E);(C)Scattered tadpole shaped cell seen in the center of field(400×;H&E);(D)Bizarre tumor cells with scattered mitotic figure (400×; H&E); (E) Immunohistochemical stain for desmin (400×); (F) Immunohistochemical stain for myogenin (400×).
We discuss a case of ERMS with anaplastic features as it is a rare entity and definitive management protocols are lacking.In the absence of protocols designed specifically for adult patients, it is imperative to follow pediatric therapeutic guidelines.Anaplastic RMS of the paratestis should be considered in differential diagnosis when dealing with scrotal masses of high grade histomorphology, for a better and aggressive patient management.
Author contributions
Study design: Poojan Agarwal, Anila Sharma.
Data acquisition: Anila Sharma, Anurag Mehta.
Data analysis: Anila Sharma, Sunil Pasricha, Poojan Agarwal.
Drafting of manuscript: Poojan Agarwal, Anila Sharma,Sunil Pasricha.
Critical revision of the manuscript: Poojan Agarwal.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2021年1期
Asian Journal of Urology2021年1期
- Asian Journal of Urology的其它文章
- Single-port technique evolution and current practice in urologic procedures
- Robotic urologic surgery: Past, present and future
- Magnetic resonance imaging-guided prostate biopsy-A review of literature
- Totally intracorporeal robot-assisted urinary diversion for bladder cancer(part 2).Review and detailed characterization of the existing intracorporeal orthotopic ileal neobladder
- Totally intracorporeal robot-assisted urinary diversion for bladder cancer(Part 1).Review and detailed characterization of ileal conduit and modified Indiana pouch
- The robot-assisted ureteral reconstruction in adult: A narrative review on the surgical techniques and contemporary outcomes
