Mechanisms implicated in the contralateral effect in the central nervous system after unilateral injury: focus on the visual system
Fernando Lucas-Ruiz, Caridad Galindo-Romero, Virginia Albaladejo-García,Manuel Vidal-Sanz, Marta Agudo-Barriuso
Abstract The retina, as part of the central nervous system is an ideal model to study the response of neurons to injury and disease and to test new treatments. During the last decade is becoming clear that unilateral lesions in bilateral areas of the central nervous system trigger an inflammatory response in the contralateral uninjured site. This effect has been better studied in the visual system where, as a rule, one retina is used as experimental and the other as control. Contralateral retinas in unilateral models of retinal injury show neuronal degeneration and glial activation. The mechanisms by which this adverse response in the central nervous system occurs are discussed in this review, focusing primarily on the visual system.
Key Words: bilateral effect; brain; glaucoma; inflammation; mirror effect; optic nerve axotomy; optic nerve crush; retina; spinal cord
Introduction
Neurodegenerative disorders are a growing problem in our society because mammalian neurons have a limited capacity of regeneration and they are not able to divide and proliferate.To date, there are no effective treatments for these diseases,and so extensive research is being carried out on preclinical animal models, mostly rodents.
The visual system, and more specifically the retina, is widely used to study neurodegeneration and neuroprotection. The retina has several advantages over other regions of the central nervous system (CNS) such as: (1) a well-known anatomy(Salinas-Navarro et al., 2009a, b; Galindo-Romero et al., 2011,2013a; Nadal-Nicolás et al., 2014, 2015a, 2018; Ortín-Martínez et al., 2014; Valiente-Soriano et al., 2014), (2) it can be treated intravitreally, systemically or topically (Lindqvist et al., 2004;Parrilla-Reverter et al., 2009; Rovere et al., 2015; Deng et al.,2019; Lucas-Ruiz et al., 2019a), (3) it is easily accessible, and(4) it allows to performin vivoanatomical, functional and behavioural analyses (Kasi et al., 2019; Gallego-Ortega et al.,2020; Sims et al., 2020).
There are several models of retinal degeneration that are either hereditary (photoreceptor degeneration or ocular hypertension) or induced (axonal trauma, excitotoxicity,ischemia or ocular hypertension). These models are not only used to decipher how CNS neurons respond to a given insult or trauma, but also to understand the progression of human diseases such as glaucoma. Glaucoma is one of the main causes of blindness in the world, affecting more than 80 million people (Tham et al., 2014). This disease produces the progressive degeneration of retinal ganglion cells (RGC)and their axons (Vidal-Sanz et al., 2001, 2007, 2015, 2017;WoldeMussie et al., 2001; Galindo-Romero et al., 2013b;Nadal-Nicolás et al., 2015a; Valiente-Soriano et al., 2015;Calkins et al., 2018; Zhang et al., 2019). Because RGC axons are the only afference of the retina and they project to several areas of the brain, those retinorecipient regions also respond to RGC death due to the retrograde degeneration of their axons (Dekeyster et al., 2015; Smith et al., 2018).
Oftentimes, the contralateral uninjured retinas as well as the contralateral parts of other bilateral regions of the CNS are used as controls in experiments involving unilateral lesions.However, it is becoming increasingly clear that their use may lead to error, since molecular changes, glial activation and significant neuronal death have been observed in contralateral retinas and other CNS areas (Bodeutsch et al.,1999; L?nngren et al., 2006; Chan et al., 2010, 2014; Kerr et al., 2012; Niesman et al., 2014; Sánchez-Migallón, 2016, 2018;Ulbrich et al., 2016; Brown and Martinez, 2018; de Hoz et al.,2018; Lucas-Ruiz et al., 2019b; Pischiutta et al., 2019). The response of the uninjured contralateral tissue to a lesion on the fellow one has been named mirror or contralateral effect/response and in it the glial cells play an essential role. The contralateral effect has also been described for lesions that do not affect primarily the nervous system. In this situation, the coordination between the two sides of the body is believed to be orchestrated by the spinal cord (Shenker et al., 2003).
Glial cells, astrocytes, microglial cells and, in the retina Müller cells, play a homeostatic role in healthy subjects. Upon neuronal degeneration, glial cells activate, in an effort to reduce damage and further control neuronal death, triggering an inflammatory environment that resolves when the tissue heals (van der Merwe et al., 2019; Guttenplanet al., 2020).However, if the homeostasis does not resume, inflammation becomes chronic. This uncontrolled inflammatory state is known as neuroinflammation and it is damaging to neurons,causing their progressive degeneration. Glial over-activation is thus counterproductive to cell viability as it causes an imbalanced pro-inflammatory environment that impairs neuronal survival (Colonna and Butovsky, 2017; Voet et al.,2019). Neuroinflammation is a hallmark of glaucoma (Williams et al., 2017) and other neurodegenerative diseases such as Alzheimer’s or Parkinson’s (Williams et al., 2017; Illes, 2020;Iovino et al., 2020; Kim et al., 2020) and it is thought to be the primary mechanism of the contralateral effect.
This review is focussed on the contralateral response in the CNS and we will discuss the possible mechanisms by which it occurs. We have performed a PubMed literature search of articles published from 1990 to 2020 on: contralateral response OR contralateral effect OR mirror effect OR mirror response AND retina OR visual system OR brain OR spinal cord OR hippocampus OR central nervous system.
Contralateral Effect: Biological Meaning and Properties
Shenker et al. (2003) proposed that the biological significance of the contralateral response may be tissue protection and damage limitation. Hence, the body prepares the unharmed region to better fight the damage that has already occurred on the mirror site. This is a precise mechanism and thus,they theorise, it is economical because it prevents a systemic response saving energy and avoiding the (possible) deleterious effect of an excessive inflammatory reaction.
Shenker et al. (2003) also speculated on the properties of the contralateral response. Firstly, the damage must be of a minimum magnitude before triggering a reaction in the contralateral region. Secondly, the contralateral effect must be topographically precise. Thirdly, the contralateral response is stimuli-specific, i.e. if the injury elicits an inflammatory response in the damaged area, then the contralateral response is also inflammatory. Fourthly, the contralateral response is a shadow of the original injury, both in terms oftime and magnitude. As we discuss below, all the contralateral effects reported in the CNS comply with these properties.
Retina
Although the mechanisms involved in the contralateral effect are being investigated using animal models, the contralateral response has also been observed in humans. In fact,sympathetic ophtalmia is a clinical manifestation observed in patients that had suffered a traumatic damage or the enucleation of one eye that proceeds with an inflammatory response in the undamaged eye (Cunningham et al., 2017).
It has been also reported that in healthy subjects the experimental reduction of perfusion in one eye produced a bilateral decrease of retinal function (Lovasik et al., 2005).The loss of functionality in the contralateral eye was lower than in the experimental one, and the functional recovery took longer. In patients with bilateral diabetic macular edema,an unilateral injection of anti-vascular endothelial growth factor had a positive outcome in both eyes (Hanhart et al.,2014). Kyncl et al. (2019) published a case report of a patient who suffered a traumatic optic neuropathy in the right optic nerve. The patient had a decreased function in the left retina and a considerable decrease in visual cortex activity after stimulation of the left eye. The authors proposed that this bilateral response was due to glial activation along the visual tract upon RGC death in the affected retina.
In rats and mice a contralateral response has been shown following optic nerve crush (ONC) (Macharadze et al., 2009;Lehmann et al., 2010; Huang et al., 2018; Sánchez-Migallón,2018; Lucas-Ruiz et al., 2019b; Mesentier-Louro et al., 2019),optic nerve transection (ONT) (Sharma et al., 2012; Choe et al., 2014; Sánchez-Migallón et al., 2018), ocular hypertension(OHT) (Wang et al., 2000; Ramírez et al., 2010; 2020a, b;Gallego et al., 2012; De Hoz et al., 2013; Frankfort et al., 2013;Chen et al., 2015; Sapienza et al., 2016; De Hoz et al., 2018;Tribble et al., 2019), ischemia/reperfusión (Kerr et al., 2012;Ulbrich et al., 2016), intravitreal injection (Lam et al, 1996;Di Pierdomenico et al., 2016) and ocular trauma (Lam et al.,1996; Bricker-Anthony and Rex, 2015).
The best studied models are OHT, ONC and ONT. In the injured retina these insults cause the massive death of RGCs and glial activation. Conversely, in the uninjured retina astrocytes and Müller cells hypertrophy, microglial cells activate, phagocytic microglia appears, and the major histocompatibility complex II (MHC-II) is over-expressed, a glial response similar to that observed in the injured retina but with less intensity(Bodeutsch et al., 1999; Gallego et al., 2012; De Hoz et al.,2013, 2018; Galindo-Romero et al., 2013b; Rojas et al., 2014;Cen et al., 2015; Sapienza et al., 2016; Lucas-Ruiz et al., 2019b;Tribble et al., 2019; Ramírez, et al., 2020a, b). Interestingly,after ONT both microglial activation (Cen et al., 2015) and the appearance of phagocytic microglia in the uninjured retina is an early response that is constant and independent of the rate of RGC death in the uninjured retina (Galindo-Romero et al.,2013b).
Due to the magnitude of RGC loss in the injured retina, it is thus not surprising that the contralateral retina shows signs of RGC degeneration. Huang et al. (2018) observed that after ONC in rats the contralateral retinas showed a thinning of the nerve fibre layer and a decrease in its reflectance, a sign of RGC degeneration. Sanchez-Migallón et al. (2018) showed that some RGCs in the contralateral retina expressed the highly phosphorylated axonal neurofilament subunit H in their somas, a hallmark of RGC degeneration and death (Parrilla-Reverter et al., 2009; Rovere et al., 2015). Furthermore, as occurs in the injured retinas, following ONC or ONT there is a significant increase of active-caspase 3 in the ganglion cell layer of the uninjured retina (Sánchez-Migallón et al., 2016;Lucas-Ruiz et al., 2019b). Again, all these degenerative signs were significant compared to control retinas, but smaller than those reported in injured retinas.
Regarding actual RGC loss in the uninjured retina, some authors have described that in mice there is a decrease in the number of these neurons after OHT or optic nerve damage(Sawada and Neufeld, 1999; Ahmed et al., 2001; Macharadze et al., 2009; Frankfort et al., 2013; Chen et al., 2015;Moisseiev et al., 2016; Sapienza et al., 2016). All these authors concluded that the loss of RGCs is not as pronounced as in the injured retina and that it may become significant in the long term. Our Laboratory first described in mice the course of RGC death in uninjured retinas following unilateral ONC(Lucas-Ruiz et al., 2019b). We performed ONC in left optic nerve close to the optic nerve head (0.5 mm), or the furthest away that the optic nerve can be accessed intraorbitally in mice (2 mm). In this experimental setting, the 0.5 mm lesion was further away from the contralateral retina than the 2 mm one. In the injured retinas, we observed that RGC death was not modulated by the lesion site, and in both lesions was first significant at 3 days. In the contralateral eyes there was small and consistent (although non-significant) decrease of RGCs at 3 and 5 days. RGC death became significant when reaching 15% of the original population which was 9 days for the 2 mm ONC and 45 days for the 0.5 mm ONC. This loss did not progress further, at least up to 90 days. Thus, RGC loss in the contralateral retina is delayed with respect to the injured retina, it is not progressive and it is modulated by the physical distance since the closer the injury is performed to the uninjured eye, the earlier the degeneration appears. In addition to RGC loss, we also observed in the contralateral retinas a transient decrease in the expression of RGC markers. Then, the expression of these markers returned to their physiological state or they were overexpressed, may be in an attempt to overcome the injury. Furthermore, we reported that the loss of contralateral RGCs was prevented by treatment with minocycline (inhibitor of microglial cells) or with a non-steroidal anti-inflammatory drug, suggesting that the neuroinflammatory environment produced by activated microglial cells may play a role in the loss of these neurons in the contralateral retinas.
Functional studies are scarcer. Bilateral electroretinographic changes have been observed following unilateral ocular hypertension and it is hypothesised that this is due to a modification in central visual centres (Tang et al., 2016). It has also been reported that the photopic responses in the opposite eye decrease after unilateral manipulation of one eye, either by laser or even only by scleral depression (Francis et al., 2013).
Mechanisms Involved in the Contralateral Response
The mechanisms by which the contralateral response causes the degeneration of RGCs are not completely understood.Nadal-Nicolás et al. (2015b) and Ramírez et al. (2015)proposed some theories that alone or in combination could explain this effect: (1) the direct death of retino-retinal projecting RGCs (ret-ret RGCs) (Figure 1); (2) stress signals liberated by the damaged ret-ret RGCs that cause a neurotoxic environment in the contralateral retina (Figure 1); (3) the propagation of a glial reaction through the optic chiasm(Figure 2); (4) a retrograde degeneration spreading from the deafferentated retinorecipient areas in the brain (Figure 2);and/or (5) a systemic inflammatory reaction (Figure 3).
Death of the retino-retinal projecting RGCs
RGCs project their axons to different nuclei of the brain. A minute proportion of RGCs project as well to the contralateral retina, the so-called ret-ret RGCs (Bunt and Lund, 1981; Müller and Holl?nder, 1988; Avellaneda-Chevrier et al., 2015; Nadal-Nicolás et al., 2015b; Tang et al., 2016; Murcia-Belmonte et al., 2019). Because ret-ret RGCs project from one retina to the other, they are directly injured in the unilateral lesion and thus they die as a consequence of the axotomy (Figure 1). However, their death does not account for the 15% of the RGC loss observed in the contralateral retinas, because in adult rodents the total number of ret-ret RGCs is almost negligible. Albino rats have 1 to 4, and pigmented mice from 3 to 12, which represents between 0.006% and 0.03% of the RGC population, respectively (Nadal-Nicolás et al., 2015b).However, we cannot exclude the possibility that death of the ret-ret RGCs is the first trigger of the contralateral response,as we discuss below.
Stress signals released by the injured ret-ret RGCs
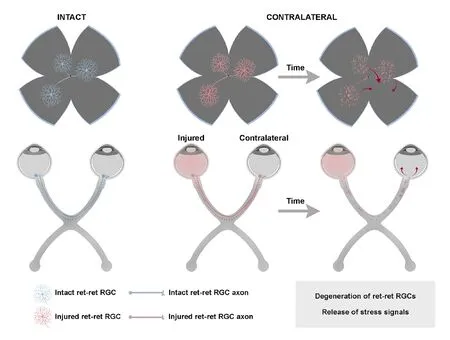
Figure 1 |Mechanisms involved in the contralateral response: the retino-retinal projection.
Another mechanism to explain the contralateral effect is that the degeneration of ret-ret RGCs causes the release of neurotoxic stress signals that in turn result in the death of a greater number of RGCs (Figure 1). Several authors have shown that in contralateral retinas there is overexpression of certain markers of neuronal death, as well as proinflammatory cytokines that are released to the extracellular space favouring inflammation (Bodeutsch et al., 1999;Macharadze et al., 2009; Kerr et al., 2012; Mesentier-Louro et al., 2017, 2019; Lucas-Ruiz et al., 2019b). Lam et al. (1996)found that there was a significant increase in free radicals in both retinas following a unilateral puncture. Later, it was discovered a bilateral increase in c-Jun, a pro-inflammatory mediator (Bodeutsch et al., 1999). After ischemia/reperfusion,it has been shown that there is a transient increase in Cx43, a gap junction protein that is overexpressed after CNS damage and allows the passage of apoptotic and necrotic signals to adjacent cells (Cronin et al., 2008; Kerr et al., 2012; Cooper et al., 2020). Extracellular ATP is a sign of tissue stress caused by tissue injury and inflammation (Wilhelm et al.,2010; Barberá-Cremades et al., 2012). Nadal-Nicolás et al.(2016) demonstrated, using mice lacking the P2X7 receptor(extracellular ATP receptor) that the deficit of this protein delays the death of contralateral RGCs. They suggest that the release of ATP by the ret-ret RGCs may cause an inflammatory environment leading to microglia activation and astrocyte hypertrophy. Therefore, even though the ret-ret projection is very small it possibly is part of the contralateral effect puzzle. A further pillar to support the involvement of retret RGCs is that in albino rats which have fewer ret-ret RGCs than pigmented mice, no significant RGC death nor microglial activation has been observed in the contralateral retinas even long term after axotomy (Nadal-Nicolás et al., 2015a).However, after OHT in albino rats, the contralateral retinas show signs of glial activation, suggesting that the inflammatory response is higher after OHT than ONC, reaching the minimum necessary to trigger a contralateral response (Ramírez et al.,2010).
Propagation of the glial reaction from the injured optic nerve along the visual pathway
It has been postulated that the signal for inflammation travels from the damaged eye to the contralateral one, “recruiting”the glia on the way through the optic chiasm (Figure 2). This theory is supported by some works showing a delay in the response of the contralateral retina with respect the same effect in the injured one. In an OHT model in mice some signs of morphological activation of microglial cells, such as loss of processes and over-expression of Iba1, appear earlier in the injured than in the contralateral retina (Ramírez, et al.,2020a) in agreement with the course of RGC death (see above and Lucas-Ruiz et al., 2019b) and with the hypertrophy of astrocytes observed after ONC (Mesentier-Louro et al., 2019).The delayed response of the contralateral retina may be dependent on the physical distance, in accord with the finding that RGC death in the contralateral retina is observed earlier when the axotomy is performed closer to the contralateral eye (Lucas-Ruiz et al., 2019b). Thus, astrocytes and microglial cells will be activated in a chain reaction by the inflammation that would start in the injured nerve and that would travel through the chiasm to the contralateral optic nerve, reaching the retina. This is further supported by a recent work by Cooper et al. (2020) showing that astrocytes play a crucial role in bilateral maintenance upon neurodegeneration. They elegantly demonstrated that there is a redistribution of metabolites from the healthy eye to the damaged eye through the astrocyte Cx43 paired gap junctions. Furthermore, it is understood that this is a bilateral mechanism that travels through the astrocytes from one eye to the other. This chain reaction may also spread to the retinorecipient areas(Dekeyster et al., 2015; Smith et al., 2018) and have an additive effect, as we discuss in the next point.
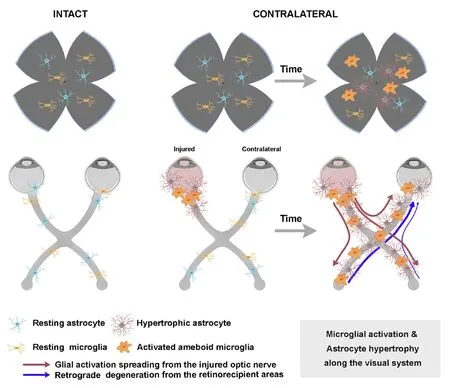
Figure 2|Mechanisms involved in the contralateral response: propagation of the glial reaction from the injured optic nerve along the visual pathway.
Retrograde degeneration from the deafferentated retinorecipient areas in the brain to the retinas
Both in humans and animal models, inflammatory events induced by glaucoma do not only occur in the retina and the optic nerve, but rather they spread along the visual pathway.In the human brain of glaucoma patients, inflammation has been observed in retinorecipient regions such as dorsolateral geniculate nucleus (Gupta et al., 2006, 2007) and in rodent models of glaucoma induces as well the loss of synapses in the superior colliculi (SC) and visual cortex (Dekeyster et al., 2015).In rodents, after unilateral optic nerve damage both SCs show an increase in microglial and macroglial cells which also show signs of activation (Zhang et al., 2009; Sapienza et al., 2016;Li et al., 2018; Tribble et al., 2019). After deafferentation,there is as well expression of cell death markers in the SC,such as c-fos (proto-oncogene expressed in dying neurons)(Herrera and Robertson, 1996) and p38 (active apoptosis pathway) (Kikuchi et al., 2000; Sapienza et al., 2016). These same authors showed that glial activation occurred in both retinas after injection of tumor necrosis factor α into the right SC, demonstrating the bilateral spreading of the unilateral inflammatory insult from the brain to the retinas. Thus,another possible mechanism to explain the contralateral effect would be that the inflammation and degeneration occurring in the deafferented retino-recipient areas would travel retrogradely to both retinas causing RGC death and glial activation (Figure 2).
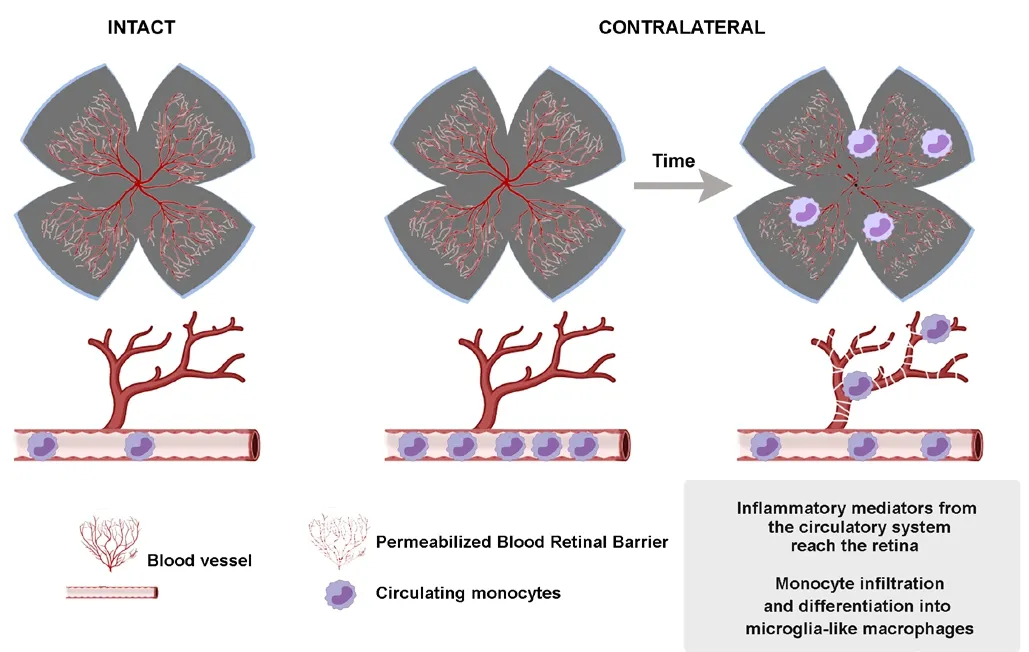
Figure 3 | Mechanisms involved in the contralateral response: systemic effect.
In rodents, the vast majority of RGCs (~95%) project to the contralateral SC (Salinas-Navarro et al., 2009a, b; Chang et al.,2011) and the rest project to the ipsilateral one. Therefore,RGC death in the left retina would massively deafferent the right SC where the inflammatory response would affect the ipsilateral projection of the right retina. Conversely, the death of the ipsilateral RGC axons from the left retina would alter the left SC where the contralateral axons of the right retina project. Thus, SC deafferentation could cause a retrograde degeneration of the surviving RGC axons, and eventually the death of their parent neurons. In albino animals the ipsilateral projection is significantly smaller than in pigmented ones(Lund, 1964; Dr?ger and Olsen, 1980; Balkema and Dr?ger,1990; Salinas-Navarro et al., 2009a, b; Nadal-Nicolás et al.,2012). This difference may explain as well why in albino animals, the loss of RGCs in the contralateral retina is not significant (Nadal-Nicolás et al., 2015a; Sánchez-Migallón,2018).
Systemic effect
Finally, the possibility of a systemic effect involving proinflammatory mediators and infiltration of monocytes into the uninjured retina is considered (Figure 3).
In adult untouched rats, unilateral topical instillation of saline to one eye resulted in an increased expression of fibroblast growth factor 2 mRNA levels in the contralateral eye (L?ngren et al., 2006). This response was thought to be an unspecific stress-response attributed to the handling of awake animals during topical administration.
Monocyte infiltration has been observed in glaucoma patients and animal models (Howell et al., 2012; Williams et al.,2017, 2019; Margeta et al., 2019). In rodents, 24 hours after inducing OHT, a large number of highly rounded Iba1+MHCII+cells that could be infiltrated monocytes were observed (De Hoz et al., 2018; Ramírez et al., 2020a, b). A more recent work supports these results (Tribble et al., 2019). These authors demonstrate that there is infiltration of monocytes in the first stages of the lesion in the undamaged retina. Later they observed an increase in microglia cells, sustaining the hypothesis that monocytes infiltrate the retina and become derived microglia-like macrophages during glaucoma.
The systemic effect may have a stronger impact in old animals or patients because the blood-retinal barrier is seriously affected by age (Chan-Ling et al., 2007), thus easing the entry of monocytes and inflammatory mediators from the systemic circulation. In this regard, Ramírez et al. (2020b) have shown that after OHT older animals show an increase in the number of Iba1+cells (resident microglia and microglia-like macrophages) in both retinas, compared to young individuals.
Contralateral Effect in Other CNS Areas: Spinal Cord and Brain
As above mentioned, most studies on the contralateral response have been carried out in the visual system. However,several authors have observed that there is a contralateral response to unilateral damage in specific areas of the CNS.After a lateral hemi-section of the spinal cord, motor function was hindered bilaterally (Nishimura and Isa, 2012; Brown and Martinez, 2018). Motor function recovered gradually because the ipsilateral motor cortex (contralateral to the damaged cortex) reorganized to support recovery of motor function, even though this area is not innervated by the damaged spinal cord axons. After hemi-section of the spinal cord the serotonergic fibres responsible for locomotion drop to 1% compared with the total number in intact animals. In the contralateral zone, the effect is similar but with a less pronounced effect, as the number of fibres drops to 50%(Leszczynska et al., 2015). Li et al. (2017) found an anatomical mechanism in which some axons of the spinal cord connect the damaged area with its contralateral, and hypothesize that it is a form of communication where, both the damage signal and regeneration and/or protection signals, are sent from the injured region to its uninjured contralateral.Several studies have shown that there is also a contralateral response after traumatic brain injury (Niesman et al., 2014;Taib et al., 2017; Pischiutta et al., 2018; Zhao et al., 2019).In this model a substantial morphological deterioration has been observed in the cortex, corpus callosum, thalamus and hippocampus in the damaged hemisphere (Zhao et al., 2019).The degeneration of the contralateral corpus callosum reflects the damaged hemisphere in both time and intensity (Taib et al., 2017) where an activation of astrocytes and phagocytic microglia has been observed (Pischiutta et al., 2018; Zhao et al., 2019). Furthermore, there here is a significant increase in pro-inflammatory cytokines in the undamaged hemisphere (Niesman et al., 2014; Taib et al., 2017) caused by the activation of glia since in mice deficient in caveolin(a suppresor of glial activity), there is a greater release of cytokines (Niesman et al., 2014).
Another interesting effect has to do with the plasticity of high visual order centres in the brain that compensate and optimize the visual function in response to RGC degeneration.Using functional tests it has been shown that in patients with monocular glaucomatous disease and a deficit in visual evoked potential latency, there is a latency bias between paired eyes. Both low-contrast and high-contrast responses in the visual evoked potential are shown to be complementary and demonstrate a minimization of the pathological condition through the CNS (Sponsel et al., 2017). Moreover, as a consequence of this natural compensatory process, visual field patterns are also modulated, showing a maximization of the binocular visual field in patients with bilateral glaucomatous damage (Sponsel et al., 2014; Reilly et al., 2015) which is a clear evidence of the implication of CNS compensating the glaucomatous degeneration.
Conclusion
Why the contralateral response is so specific and precise still remains unknown. However, it is clear that contralateral retinas (or hemispheres) should not be used as controls in neurodegenerative models. All the mechanisms described above may be involved in the pro-inflammatory and glial reaction in the uninjured retina and be part of the eventual death of RGCs. In conclusion, extreme care must be taken when using contralateral retinas and, rather than using them as controls, they could be considered experimental and test on them the neuroprotective therapies that are being only tested on the injured ones.
Author contributions:Drafting the article: FLR, MAB; concept and design:FLR, CGR and MAB; acquisition of data: FLR, CGR, VAG; revising the article critically for important intellectual content: MVS; funding acquisition: MAB and MVS. All authors revised the article critically for important intellectual content and approved the final version of the paper.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional “Una manera de hacer Europa” (PI19/00071 [to MAB], PID2019-106498GB-I00 [to MVS], RD16/0008/0026 [to MVS] and RD16/0008/0016 [to MVS]) and by the Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia (19881/GERM/15) (to MVS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Liang Li, Stanford University, United States.
Additional file:Open peer review report 1.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Toward three-dimensional in vitro models to study neurovascular unit functions in health and disease
- Apolipoprotein A1, the neglected relative of Apolipoprotein E and its potential role in Alzheimer’s disease
- Oral frailty and neurodegeneration in Alzheimer’s disease
- Non-coding RNAs and other determinants of neuroinflammation and endothelial dysfunction:regulation of gene expression in the acute phase of ischemic stroke and possible therapeutic applications
- Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies
- Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review

