Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients:a retrospective observational study
1 MiZ Company Limited, Kamakura, Japan
2 Clinic C4, Tokyo, Japan
3 Division of Transplantation Immunology, National Institute for Child Health and Development, Tokyo, Japan
4 Department of Advanced Technology for Transplantation, Osaka University Graduate School of Medicine, Osaka, Japan
5 Department of Renal Transplantation Center, Kansai Medical Hospital, Osaka, Japan
6 Faculty of Environment and Information Studies, Keio University, Fujisawa, Japan
Abstract Although intensity-modulated radiation therapy (IMRT) has been developed as an alternative to conventional radiotherapy, reducing bone marrow damage is limited.Thus, a novel technology is needed to further mitigate IMRT-induced bone marrow damage.Molecular hydrogen(H2) was recently reported as a preventive and therapeutic antioxidant that selectively scavenges hydroxyl radical (·OH) and peroxynitrite(ONOO–).This observational study aimed to examine whether H2 gas treatment improves IMRT-induced bone marrow damage in cancer patients.The study was performed at Clinic C4 in Tokyo, Japan between May 2015 and November 2016.During this period, all enrolled patients received IMRT once per day for 1 to 4 weeks.After each time of IMRT, the patients of control group (n = 7, 3 men and 4 women,age range:26–70 years) received mild hyperbaric oxygen therapy in health care chamber for 30 minutes, and the patients of H2 group (n =16, 8 men and 8 women, age range:35–82 years) received 5% H2 gas in health care chamber for 30 minutes once per day.Radiation-induced bone marrow damage was evaluated by hematological examination of peripheral blood obtained before and after IMRT, and the data were expressed by the ratio after to before treatment.The total number of radiation times and total exposure doses of radiation were similar between the control and H2 groups.IMRT with health care chamber therapy significantly reduced white blood cells and platelets, but not red blood cells, hemoglobin and hematocrit.In contrast, H2 gas treatment significantly alleviates the reducing effects of white blood cells and platelets (P = 0.0011 and P = 0.0275, respectively).Tumor responses to IMRT were similar between the two groups.The results obtained demonstrated that H2 gas inhalation therapy alleviated IMRT-induced bone marrow damage without compromising the anti-tumor effects of IMRT.The present study suggests that this novel approach of H2 gas inhalation therapy may be applicable to IMRT-induced bone marrow damage in cancer patients.The study protocol was approved by an Ethics Committee Review of Tokyo Clinic and Research Institute ICVS Incorporated (Tokyo, Japan) on February 1, 2019, and was registered in the University Hospital Medical Information Network (UMIN)Clinical Trials Registry (UMIN ID:UMIN000035864) on February 20, 2019.
Key words:bone marrow damage; cancer patient; hydrogen gas; IMRT; intensity-modulated radiation therapy; platelet; radiation-induced damage; retrospective observational study; white blood cell
INTRODUCTION
When the shape of a tumor is irregular and complex,conventional radiotherapy is unable to maintain a high tumor control rate and simultaneously attenuate the associated complications, because normal tissues and organs surrounding the tumor receive the same dose of irradiation.As an alternative to conventional radiotherapy, intensity-modulated radiation therapy (IMRT) has been developed to only irradiate tumors.1However, bone marrow damage still occurs in cancer patients with multiple tumor lesions and large irradiation volumes.Thus, novel technology is needed to mitigate further IMRTinduced bone marrow damage.To attenuate the symptoms associated with end-stage cancer, patients are also housed in a health care chamber (HCC, a mild hyperbaric oxygen chamber)2; however, satisfactory outcomes have not yet been achieved.
The direct effects of radiation are due to the changes it induces in bioactive macromolecules such as proteins and nucleic acids.Ionization, excitation, chemical bond rupture, and changes in molecular structures occur during this process, leading to abnormal functions and metabolic disorders.3Indirect effects also occur via the generation of free radicals by water radiolysis.Hydroxyl radicals (·OH),the strongest reactive oxidant species, are formed from this radiolysis and react with nucleic acids, lipids, and proteins.Approximately 65% of DNA damage is caused by the indirect effects of free radicals such as ·OH.4Therefore, selective and high concentrations of ·OH scavengers have potential as radioprotective agents.
Molecular hydrogen (H2) was recently identified by Ohsawa et al.5as a preventive and therapeutic antioxidant that selectively scavenges ·OH and peroxynitrite (ONOO–).However, in 2005, 2 years before Ohsawa’s study,5Yanagihara et al.6reported that the consumption of neutral H2-rich water produced by electrolysis may effectively reduce the oxidative stress induced by chemical oxidants in rats, which is pioneering research in H2medicine.Due to its small size and electrically neutral properties, H2easily reaches target organs.H2has also been proposed as a treatment for various oxidative stressrelated diseases.7-9Previous studies demonstrated that H2exerted radioprotective effects in various animal models10,11and improved the quality of life (QOL) of patients treated with radiotherapy for liver tumors.12However, there is currently no definitive therapy to attenuate radiation-induced bone marrow damage in cancer patients.Therefore, this retrospective observational study was designed to investigate whether H2gas treatment mitigates IMRT-induced bone marrow damage in cancer patients.
SUBJECTS AND METHODS
Subjects and designs
The study was a retrospective observational examination performed at Clinic C4 in Tokyo, Japan between May 2015 and November 2016.During this period, 26 patients with endstage cancer receiving two different treatments, IMRT with or without H2gas inhalation (the H2group and control group,respectively), were registered.Patients who did not receive all planned IMRT were excluded.One patient in the control group and two patients in the H2group did not receive all of the planned IMRT.Therefore, the data were collected from 23 patients, and the number of patients in the control group and H2group consisted of 7 and 16 patients, respectively.

Figure 1:Subject flow chart
The study protocol and materials were approved by an Ethics Committee Review of Tokyo Clinic and Research Institute ICVS Incorporated (Tokyo, Japan) on February 1, 2019 (Additional file 1), and all patients provided written informed consent (Additional file 2) prior to receiving therapy.All methods were performed in accordance with the relevant guidelines and regulations.The present study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID:UMIN000035864) on February 20, 2019.
Patients were subjected to 5 to 20 minutes of Tomo Therapy(HI-ART, Japan Accuray Inc., Tokyo, Japan) once per day for 1 to 4 weeks, except Saturday and Sunday.After each time of IMRT, the control group (n= 7, 3 men and 4 women) were housed in HCC (APF2, Air Press Co., Ltd., Tokyo, Japan) for 30 minutes under the environmental conditions of 1.35 atm and 27% O2.The H2group (n= 16, 8 men and 8 women) were also housed in HCC and received 5% H2gas inhalation for 30 minutes.H2gas was prepared by mixing H2gas and air using a 3.5% H2gas inhaler (MHG-2000, MiZ Co., Ltd., Kanagawa,Japan) and 6.5% H2gas inhaler (MHG-2000α, MiZ Co., Ltd.)connected in parallel.H2gas in both groups was produced by the electrolysis of water, and concentrations were controlled under the detonation limit of the mixture of H2gas and air.13Patients received mixed gas via a nose cannula at a flow rate of 4 L/minute in HCC.All patients in this study did not received chemotherapy or other standard therapies other than IMRT.
Evaluation of bone marrow damage
Bone marrow damage was evaluated by a hematological examination of peripheral blood.Blood samples drawn from the median cutaneous vein were obtained from all patients on the first day of IMRT (before the treatment) and after 1 to 4 weeks of IMRT (after the treatment).A blood hematology test for the counts of red blood cells (RBC), white blood cells(WBC), and platelets (PLT), the concentration of hemoglobin(HGB), and the hematocrit (HT) was conducted before and after the treatment using standard assays in a contract clinical examination laboratory, because these hematological markers can rapidly and easily assess the bone marrow damage in the daily work of hospital.
Evaluation of tumor responses
Patients underwent dynamic CT scans 1 month after completion of IMRT using iodine-based angiographic contrast agent,and then tumor response was checked at 2 to 3 months intervals.The tumor response was determined by the criteria established by Kwon et al.14using the dynamic CT scans.Described briefly, complete response was defined as the disappearance of any intratumoral arterial enhancement in all target lesions.Partial response was defined as at least a 30% decrease in the sum of the diameters of viable target lesions.Progressive disease was defined as at least a 20% increase in the sum of the diameters of viable target lesions or the appearance of a new lesion.Stable disease was defined as a tumor status that did not meet any of the above criteria.
Statistical analysis
Hematological data were expressed as ratios of after to before the treatment.The data including the number of radiation times, total exposure dose of radiation calculated by multiplying the tumor volume of an individual patient by the radiation(×103cm3·Gy), and hematological examinations were presented as means ± standard errors of the means (SEM) and analyzed statistically by an unpaired Student’st-test or the Mann-WhitneyUtest where appropriate using BellCurve for Excel (Version 3.0, Social Survey Research Information Co.,Ltd., Tokyo, Japan).P< 0.05 was considered to be significant.
RESULTS
Patient characteristics
Patient characteristics, including sex, age, tumor origin, stage of cancer, and number of metastases, are shown in Table 1 and Additional Table 1, and were not significantly different between the control and H2groups.

Table 1:Baseline data of patients from control and H2 groups
Radiation conditions
As shown in Figure 2A the number of radiation times for the control and H2groups was 10.7 ± 1.3 and 11.2 ± 1.1, respectively, which was not significantly different between the two groups (P= 0.8064).In addition, as shown in Figure 2B, the total exposure doses of radiation calculated by multiplying the tumor volume of an individual patient by the radiation were 446.0 ± 101.8 × 103cm3·Gy and 410.2 ± 124.4 × 103cm3·Gy,respectively, which were also not significantly different between the two groups (P= 0.8607).

Figure 2:Radiation conditions of patients in control and H2 groups
Tumor responses
Tumor responses to IMRT were similar between the two groups, and 4 of 7 (57%) patients in the control group and 7 of 16 (44%) patients in the H2group achieved a complete or partial response.In addition, 3 of 16 (19%) patients in the H2group achieved stable disease.These results indicated that tumor responses to IMRT were similar between the two groups,and that H2gas inhalation did not compromise the anti-tumor effects of IMRT.Moreover, the QOL, such as fatigue, sleep,and gastrointestinal symptoms, assessed based on the results of medical interviews was similar between the two groups,indicating that H2gas inhalation did not compromise the QOL of patients receiving IMRT.
Bone marrow damage
In the control and H2treatment groups, RBC ratios were 1.108 ± 0.124 and 0.985 ± 0.027, HGB ratios were 1.078 ±0.126 and 0.966 ± 0.029, and HT ratios were 1.073 ± 0.121 and 0.953 ± 0.027, respectively.These values were close to 1, and no significant differences were observed between the two groups (Figure 3), indicating that IMRT did not influence these hematological markers.In contrast, WBC ratios were 0.145 ± 0.041 and 0.612 ± 0.088 for the control and H2groups, respectively, and were significantly different (P=0.0011; Figure 4A).PLT ratios were 0.337 ± 0.106 and 0.752± 0.130 for control and H2groups, respectively, and were also significantly different (P= 0.0275; Figure 4B).These results indicated that IMRT selectively reduced hematological markers, such as WBC and PLT, in the control group, whereas the H2gas treatment protected against these reductions.
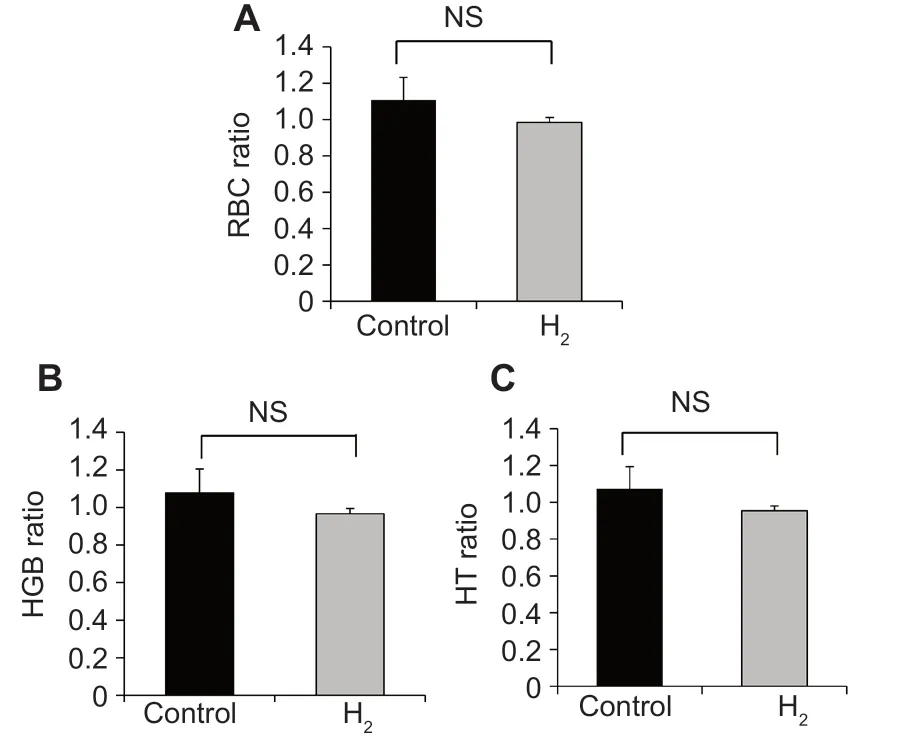
Figure 3:Effects of the hyperbaric hydrogen (H2) gas treatment on the red blood cell counts (RBC, A), hemoglobin levels (HGB, B), and the hematocrit(HT, C) of patients in control and H2 groups
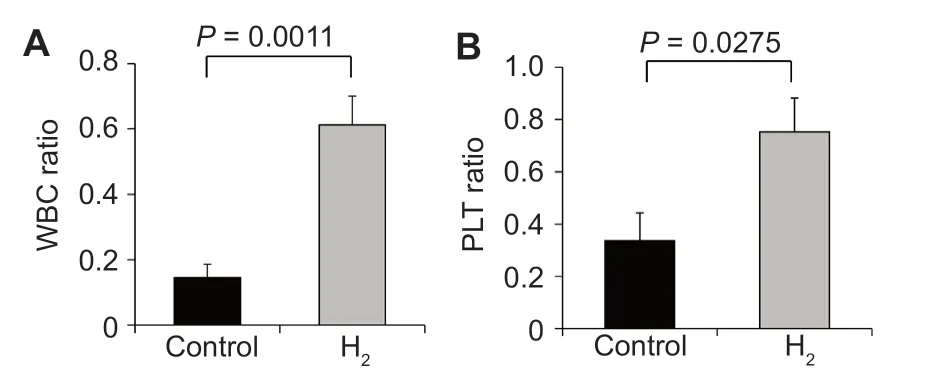
Figure 4:Effects of the hyperbaric hydrogen (H2) gas treatment on the white blood cell counts (WBC, A), and platelet counts (PLT, B) of patients in control and H2 groups
DISCUSSION
IMRT has been performed for cancer patients to alleviate the adverse effects associated with increased oxidative stress and inflammation1; however, bone marrow damage still occurs in patients with multiple tumor lesions and large irradiation volumes.Thus, the development of safe and more effective technology is needed to mitigate further IMRT-induced bone marrow damage.H2was recently reported as a preventive and therapeutic antioxidant that selectively scavenges ·OH.5The present study investigated whether H2, a selective ·OH scavenger, mitigates IMRT-induced bone marrow damage in end-stage cancer patients, because selective and high concentrations of ·OH scavengers have potential as radioprotective agents.The patients received 5% H2gas or air for 30 minutes after each time of IMRT.These H2concentration and inhalation time were enough to demonstrate the mitigation effects,in the extrapolation based on the area under the curve from the H2concentration of blood or tissue in rats treated with H2gas.15,16The results obtained demonstrated that the H2gas treatment alleviated IMRT-induced bone marrow damage without compromising the anti-tumor effects of IMRT.To the best of our knowledge, this is the first study to report the benefits of H2gas on IMRT in cancer patients.
Since the number of radiation times and total exposure doses of radiation need to be selected according to the size and number of tumors in individual patients, the number of radiation times performed in the present study ranged between a minimum of 5 (1 week) and maximum of 20 (4 weeks).Therefore,blood sampling to examine the adverse effects of radiation was performed from 1 to 4 weeks.However, the average number of radiation times and total exposure doses of radiation in the control and H2groups were equivalent, indicating that the design of the present study allowed for the protective effects of H2gas inhalation to be evaluated.
The present study demonstrated that the QOL, such as fatigue, depression, sleep, and gastrointestinal symptoms, was similar between the control and H2groups, and the inhalation of H2gas did not improve the QOL.However, since the main purpose of the present study was to mitigate radiation-induced damage, long-term inhalation may be needed to improve the QOL.Moreover, although the effects of H2gas inhalation were not examined in a non-radiated control group (patients not undergoing radiotherapy), based on the findings of an animal study showing that H2-rich saline did not affect hematological data in non-radiated control mice,11H2gas inhalation may not have an impact on hematological data in patients.
The radioprotective effects of H2have been reported in different systems, including a cell-free system and various organs.In the cell-free system, Chuai et al.10showed that the levels of ·OH produced by water radiolysis and the Fenton reaction were reduced by H2solution.Moreover, Yang et al.11demonstrated that a H2-rich medium pretreatment decreased·OH levels in AHH-1 cells, a human lymphocyte cell line.On the other hand, Yang et al.11also noted radiation-induced hematological changes in WBC and PLT, but not in RBC,HGB, or the mean corpuscular volume in mice subjected to total body radiation, and found that an intraperitoneal injection of H2-rich saline significantly attenuated the depletion of WBC and PLT.They also showed that H2reduced radiationinduced apoptosis in thymocytes and splenocytes in mice.These findings suggest that H2reduced radiation-induced·OH levels by directly affecting ·OH levels, and simultaneously reduced radiation-induced oxidative stress, apoptosis,and inflammation by indirect effects on ·OH.In the present study, the H2gas treatment in combination with HCC therapy did not suppress the anti-tumor effects of IMRT in cancer patients because the response rates between the two groups were similar.This result is supported by the findings of Kang et al.12who demonstrated that the consumption of H2-rich water reduced radiation-induced oxidative stress and the QOL in patients treated with radiotherapy for liver tumors without compromising anti-tumor effects.Therefore, the mechanisms underlying the radioprotective effects of H2gas may involve not only direct effects on ·OH, but also indirect effects on·OH via the activation of the host-mediated antioxidant and anti-inflammation systems.These mechanisms have been supported by many papers reporting the radioprotective effects of H2in animal experiments.10,11,17-20
In the present study, patients received the H2gas treatment after each time of IMRT.Previous studies reported the preventive (H2gas inhalation before radiation), but not therapeutic effects of this treatment.Furthermore, H2selectively scavenges·OH and ONOO–.5Since these reactive oxygen species are rapidly generated, however, many chemical agents generally exert stronger effects via prophylactic rather than therapeutic administration.Thus, the therapeutic administration protocol employed in the present study appears to accurately reflect the effects of H2gas inhalation because the mechanisms underlying the radioprotective effects of H2gas may involve not only direct, but also indirect effects on ·OH via the activation of host defense systems.
Hyperbaric oxygen therapy that involves housing patients for 1 hour in a chamber containing 100% O2at 2 atm (1 atm=101.325 kPa) has been performed, which is effective for patients with decompression, peripheral circulation failure,wound dysfunction, and radiation-induced damage.In contrast, therapy using HCC is based on the principle of hyperbaric oxygen therapy, and patients are housed in a chamber containing 1.35 atm and air.2In the present study, based on Henry’s law, patients in the H2group received 1.35-fold more H2and O2under 1.35 atm environmental conditions,21which is equivalent to patients receiving 6.8% H2gas inhalation therapy under normal pressure.In additional experiments, the radioprotective effects of 6.5% H2gas inhalation therapy at normal pressure (data not shown) were found to be similar,suggesting the importance of inhaling higher H2concentrations to attenuate bone marrow damage.Although H2gas concentrations for the detonation limit in a mixture of H2and air are less than 4%, we recently demonstrated that the detonation limit was less than 10% in our previous experiment22and a literature search.23Therefore, 6.5% H2gas therapy without HCC appears to be a clinically convenient, effective, and safe method for mitigating IMRT-induced bone marrow damage.
The development of safe and more effective radioprotective agents is very important in view of their potential application during radiotherapy for cancer patients.The radioprotective effects of many synthetic and natural agents have been investigated in the past 50 years.Nutraceuticals, including vitamin C, vitamin E succinate, α-lipoic acid, and N-acetyl cystine,in addition to hematopoietic growth factors and cytokines,such as stem cell factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and interleukin 3, have been reported to exert radioprotective effects in animal models.24-28Amifostine, known as Ethyol or WR2721, is the only clinically accepted radioprotective agent,but is not considered to be a viable option as a radioprotective agent because of its inherent dose-limiting toxicities.29-31Although several drugs are in different stages of evaluation,none possess all of the requisite qualities of an optimum radioprotective agent.Thus, there are no safe and effective non-toxic radioprotective agents available for human use.H2has been reported to exert radioprotective effects in various animal models.10,11Moreover, H2improves the QOL of patients treated with radiotherapy for liver tumors.12However, there is currently no definitive therapy to improve radiation-induced bone marrow damage in cancer patients.Therefore, the present study investigated whether H2gas mitigates IMRT-induced bone marrow damage in cancer patients, and the results obtained demonstrated that H2gas inhalation therapy with or without HCC alleviated IMRT-induced bone marrow damage without compromising the anti-tumor effects of IMRT.Clinical studies have demonstrated that H2has no adverse effects.7-9Therefore, these findings may provide the foundation for a clinically applicable, effective, and safe strategy for a H2gas to mitigate IMRT-induced bone marrow damage.
Bone marrow damage, such as reductions in WBC (leukopenia) and PLT (thrombocytopenia), frequently occurs during cancer radiotherapy, including IMRT, and this is a limiting factor for radiotherapy.32,33Since leukopenia and thrombocytopenia may cause infection and gastrointestinal bleeding,caution is required.If bone marrow damage may be attenuated by H2gas inhalation, it will lead to the prevention of infection and gastrointestinal bleeding.Therefore, H2gas inhalation may improve the prognosis of cancer patients.
In conclusion, the present study investigated whether H2gas inhalation mitigates IMRT-induced bone marrow damage in cancer patients.The results obtained demonstrated that H2gas inhalation therapy alleviated IMRT-induced bone marrow damage without compromising the anti-tumor effects of IMRT.However, this study had some limitations related to the number of patients, the retrospective observational analysis, and data collection from a single hospital.Although further largescale clinical studies involving many hospitals are required,the present study suggests that this novel approach of H2gas inhalation therapy may be applicable to IMRT-induced bone marrow damage in cancer patients.
Acknowledgements
The authors are grateful to Mr.Fumitake Satoh, Ms.Yoko Satoh,Mr.Bunpei Sato, and Dr.Yusuke Ichikawa (MiZ Co., Ltd.) for their excellent advice on the writing of this manuscript.
Author contributions
SH and YA designed the study and analyzed the data; SH wrote the main text, analyzed the data, and prepared figures; YT, YA, XKL, NI,and ST supported this study by collecting data and giving advice.All authors read and approved the final manuscript.
Conflicts of interest
The authors declare that they have no conflicts interests.
Financial support
None.
Institutional review board statement
The study was approved by an Ethics Committee Review of Tokyo Clinic and Research Institute ICVS Incorporated (Tokyo, Japan) on February 1, 2019, and was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN ID:UMIN000035864) on February 20, 2019.
Declaration of patient consent
The authors certify that they have obtained patients’ consent forms.In the form, patients have given their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials not be published and due efforts will be made to conceal their identity.
Reporting statement
The writing and editing of the study report were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement
The statistical methods of this study were conducted and reviewed by the biostatistician of Clinic C4, Tokyo, Japan.
Author statement
This paper has been posted as a preprint on Research Square with doi:https://doi.org/10.21203/rs.3.rs-16275/v1, which is available from:https://www.researchsquare.com/article/rs-16275/v1.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files
Additional Table 1:Patient characteristics of control and H2groups.
Additional file 1:Hospital ethics approval (Japanese).
Additional file 2:Informed consent form.
- Medical Gas Research的其它文章
- Ventilation with the noble gas argon in an in vivo model of idiopathic pulmonary arterial hypertension in rats
- Neuroprotective effect of helium after neonatal hypoxic ischemia:a narrative review
- Role of hydrogen in traumatic brain injury:a narrative review
- Efficacy of xenon anesthesia in preventing postoperative cognitive dysfunction after cardiac and major non-cardiac surgeries in elderly patients:a topical review
- Comparison of vital capacity rapid inhalation and tidal ventilation induction with sevoflurane in adults:a prospective cohort study
- Spontaneous breathing for managing analgesia during balanced anesthesia with remifentanil and desflurane:a prospective, single center randomized controlled trial

