Role of hydrogen in traumatic brain injury:a narrative review
1 Department of Neurosurgery & Brain and Nerve Research Laboratory, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
2 Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
Abstract Traumatic brain injury (TBI) is a serious global public health problem.Survivors of TBI often suffer from long-term disability, which puts a heavy burden on society and families.Unfortunately, up to now, there is no efficacious treatment for TBI patients in clinical practice.As a reducing gas, hydrogen has been shown to be neuroprotective in multiple cerebral disease models; however, its efficacy in TBI remains controversial.In this review, we will focus on the results of hydrogen in experimental TBI, elaborate the potential mechanisms, and put forward for future researches based on our current understanding and views.
Key words:anti-autophagy; anti-inflammation; anti-oxidation; experimental research; hydrogen; neuroprotection; therapeutic applications;traumatic brain injury; underlying mechanism
INTRODUCTION
Traumatic brain injury (TBI) is defined as an alteration in brain function, or other evidence of brain pathology caused by an external force.1Around the world, more than 50 million people suffer from TBI every year, and it is predicted that almost half of the world’s population will experience TBI once or more in their lifetime.2TBI is one of the leading causes of morbidity,disability and mortality of all age groups in all countries, which has increased burden on families and society and becomes a global public health and medical problem.2,3
The clinical symptoms of TBI vary in severity, mainly depending on the extent of brain damage.Survivors of TBI often suffer from long-term physical, cognitive and psychological dysfunction.4According to the pathophysiological mechanism of brain injury, TBI can be divided into two categories:primary and secondary brain injury.5Primary injury is directly caused by mechanical forces which occurs at the exact moment of insult and results in the disruption of the integrity of brain cells.6Then a series of events happened.It includes the release of excitatory amino acids and the opening of Ca2+channel, the generation of free radicals and lipid peroxidation, the release of inflammatory cell mediators, apoptosis and so on.7These mechanisms not only have simultaneous effects, but also constitute a chain reaction.Altogether, these events lead to brain edema, ischemia, cytotoxic cell swelling and intracranial pressure rise, and finally lead to secondary injury after TBI,which is considered to be the main cause of death after brain injury.8To sum up, for the primary brain injury, all we can do is to prevent and reduce the injury.However, investigations on the secondary injury after TBI are of great significance,which are helpful for us to determine the therapeutic target of TBI and to improve the prognosis.
Hydrogen is a kind of colorless, tasteless and reductive small molecular gas.It was thought that hydrogen was a physiologically inert gas in mammalian cells until 1975,when it was first reported in Science that hydrogen plays a therapeutic role in mouse skin cancer model by scavenging hydroxyl radicals.9In 2007, it was reported that hydrogen could reduce the cerebral ischemia-reperfusion injury by selectively reducing cytotoxic reactive oxygen species.10Since then,hydrogen has opened a new chapter in the field of medical research and application.And a large number of studies have shown that hydrogen plays a therapeutic role through the mechanism of antioxidant stress in a variety of diseases,such as central nervous system diseases,11respiratory system diseases,12cardiovascular system diseases,13digestive system diseases,14urinary system diseases15and other diseases.16Central nervous system diseases are the focus of hydrogen medicine research.Compared with other organs of the body,brain tissue is more vulnerable to oxidative stress because of its high oxygen consumption, low antioxidant enzymes and high content of unsaturated fatty acids.17Unfortunately, there is no ideal antioxidant for the treatment of nervous system diseases.However, hydrogen, as a reducing gas, has the advantages of easy access, convenient administration (such as inhalation and intraperitoneal injection of hydrogen, oral administration of hydrogen-rich water, and injection of hydrogen-rich sodium chloride solution), easy diffusion, quick onset and no obvious toxicity, which will provide a new idea for the prevention and treatment of nervous system diseases.18From January 2007 to May 2020, a search was performed at Web of Science database.In this review, we will discuss the role of hydrogen in TBI,elaborate the potential mechanisms and put forward our current understanding and views for future researches.
APPLICATION OF HYDROGEN ON CENTRAL NERVOUS SYSTEM DISEASES
Since 2007, due to the moderate reduction activity of hydrogen that can quickly diffuse through the blood-brain barrier,10more and more researches have focused on the application of hydrogen in central nervous system diseases.
Stroke is a severe acute cerebrovascular disease, which is caused by occlusion or rupture of cerebral blood vessels, including ischemic stroke and hemorrhagic stroke.19Oxidative stress,20inflammatory response,21,22mitochondrial damage23and apoptosis24,25are key elements of stroke pathophysiology.In 2007, Ohsawa and colleagues10first reported that inhalation of hydrogen markedly suppressed brain injury in a focal cerebral ischemia/reperfusion injury rat model.The potential mechanism might be associated with that hydrogen could selectively reduce the hydroxyl radical, which was the most cytotoxic of reactive oxygen species.10Since then, a large number of researches have reported that hydrogen plays a role of protection in stroke.In a common carotid artery occlusion rat model, inhalation of hydrogen alleviated cognitive impairment by decreasing the levels of oxidative stress products malondialdehyde (MDA) and 8-iso-prostaglandin-2α, and increasing the activities of anti-oxidative enzymes superoxidase dismutase and catalase.26In another study, after cerebral ischemia/reperfusion injury (middle cerebral artery occlusion rat model), the levels of pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukine-6 and interleukine-1β,were reduced by hydrogen treatment.However, transforming growth factor-1β (a kind of anti-inflammatory cytokine)increased.It indicated that hydrogen could ameliorate cerebral ischemia/reperfusion injury via anti-inflammation.27Lately,it was reported that hydrogen exerted neuroprotective effects on oxygen-glucose deprivation/re-oxygenation damaged neurons in rat hippocampal.And the underlying mechanism was protecting mitochondrial function via regulating mitophagy mediated by phosphatase and tensin homolog-induced kinase 1/Parkin signaling pathway.28Besides application on ischemic stroke, hydrogen can also applicate on hemorrhagic stroke.It is well known that hemorrhagic stroke including subarachnoid hemorrhage and intracerebral hemorrhage.After hemorrhage,microglia and inflammatory cells are activated and free radicals are produced.29,30Then a series of pathophysiological changes,such as hematoma formation, hemoglobin decomposition,can deteriorate oxidative stress.In 2019, it was reported that inhalation of 66% hydrogen increased 72-hour survival rate and improved 24-hour neurological deficits after subarachnoid hemorrhage in rats31; however, it had no detrimental effects on subarachnoid hemorrhage grade 24 hours after subarachnoid hemorrhage compared to air group.In a rat model of intracerebral hemorrhage, inhalation of 1.3% hydrogen for 1 hour significantly reduced the levels of MDA, tumor necrosis factor-α, interleukine-1β and Caspase-3 protein and increased brain-derived neurotrophic factor expression, suggesting that hydrogen exerted a neuroprotective effect against brain injury after intracerebral hemorrhage through antioxidative activity,anti-inflammatory, anti-apoptotic, and neuroprotective.32
The neurodegenerative disease is characterized as the loss of brain and spinal cord cells, which are usually not renewable.With the passage of time, neurological function deteriorated,and eventually irreversible neurological dysfunction appeared.Alzheimer’s and Parkinson’s diseases are two of the most common neurodegenerative diseases.And inflammation and oxidative stress are recognized as the main causes of Alzheimer’s and Parkinson’s diseases.33In a dementia mice model(transgenic mice (DAL101) lacking aldehyde dehydrogenase 2), drinking hydrogen-water could reduce oxidative stress, suppress the decline in learning and memory impairment, decrease neurodegeneration and extend the mean of lifespan of mice.34Recently, Zhang et al.35developed a small-sized palladium hydride nanoparticle that could release hydrogen sustainably in Alzheimer’s disease brain, and they demonstrated that it could overcome the cognitive impairment in Alzheimer’s disease mice via recovering mitochondrial dysfunction, inhibiting β-amyloid generation and aggregation, reducing oxidative stress and activating the anti-oxidative pathway.Additionally,drinking hydrogen water could alleviate cognitive impairment and inhibit hippocampal neurodegeneration in a mouse model of Parkinson’s disease.36In a 6-hydroxydopamine-induced Parkinson’s disease rat model, it has demonstrated that hydrogen could prevent both development and progression of the nigrostriatal degeneration.37
THERAPEUTIC EFFECTS OF HYDROGEN ON TRAUMATIC BRAIN INJURY
The application of hydrogen in the treatment of TBI began in 2010.The first study reported that hydrogen could exert neuroprotection in a rat model of controlled cortical impact(CCI) brain injury.It demonstrated that inhalation of 2%hydrogen from 5 minutes to 5 hours significantly attenuated blood-brain barrier damage, brain edema and lesion volume and improved neurological outcome after TBI.38In the following years, many studies on the therapeutic effect of hydrogen on TBI have been reported (Table 1).One study examined the role of hydrogen in brain injury induced by surgery.The results showed that inhalation of 2.9% hydrogen could also reduce brain edema and improve neurological function.However, through myeloperoxidase and lipid peroxidation assay,it was found that hydrogen failed to reduce oxidative stress and inflammation.Unfortunately, the authors did not give the possible reasons.39Recently, another study investigated the effects of different concentrations of hydrogen inhalation on the neurological function of a lateral fluid percussion injury model of diabetic rat after TBI.It found that inhalation of 42%hydrogen was shown to alleviate nerve damage and improve neurological function after TBI in diabetic rats; however,inhalation of 3% hydrogen did not.The potential mechanism might include reducing oxidative stress and neuron apoptosis.40In addition, it reported that 2% of hydrogen treatment could also improve the neurological outcome after TBI induced by CCI via increasing the expression of miR-21.41
In recent years, there were many kinds of researches on therole of other forms of hydrogen on TBI, such as hydrogenrich saline, hydrogen-rich water and hydrogen in drinking water.Hou et al.42reported that after mild TBI induced by fluid percussion injury, treatment with hydrogen-rich saline attenuated oxidative stress injury and cognitive impairment.In the same year, another study revealed that hydrogen-rich saline intraperitoneally injection also exerted the similar neuroprotective effects after TBI induced by CCI, and these protective effects were dose-dependent.43In 2014, Dohi and colleagues44found that the molecular hydrogen in drinking water relieved brain edema, blood-brain barrier disruption,and neuroinflammation after CCI induced TBI model of mice.Then, Tian et al.45studied the role of hydrogen-rich water in a CCI induced TBI rat model.They found that intraperitoneal injection of hydrogen-rich water could reduce the mortality rate, attenuate blood-brain barrier disruption and brain edema and improve the cognitive function of CCI-induced TBI rats.In a Feeny weight-drop model of the rat after TBI, compared with TBI group, intraperitoneal injection of hydrogen-rich water significantly increased the 7-day survival rate, ameliorated neurological severity score (modified neurological severity score) and lowered intracellular oxidative stress level.
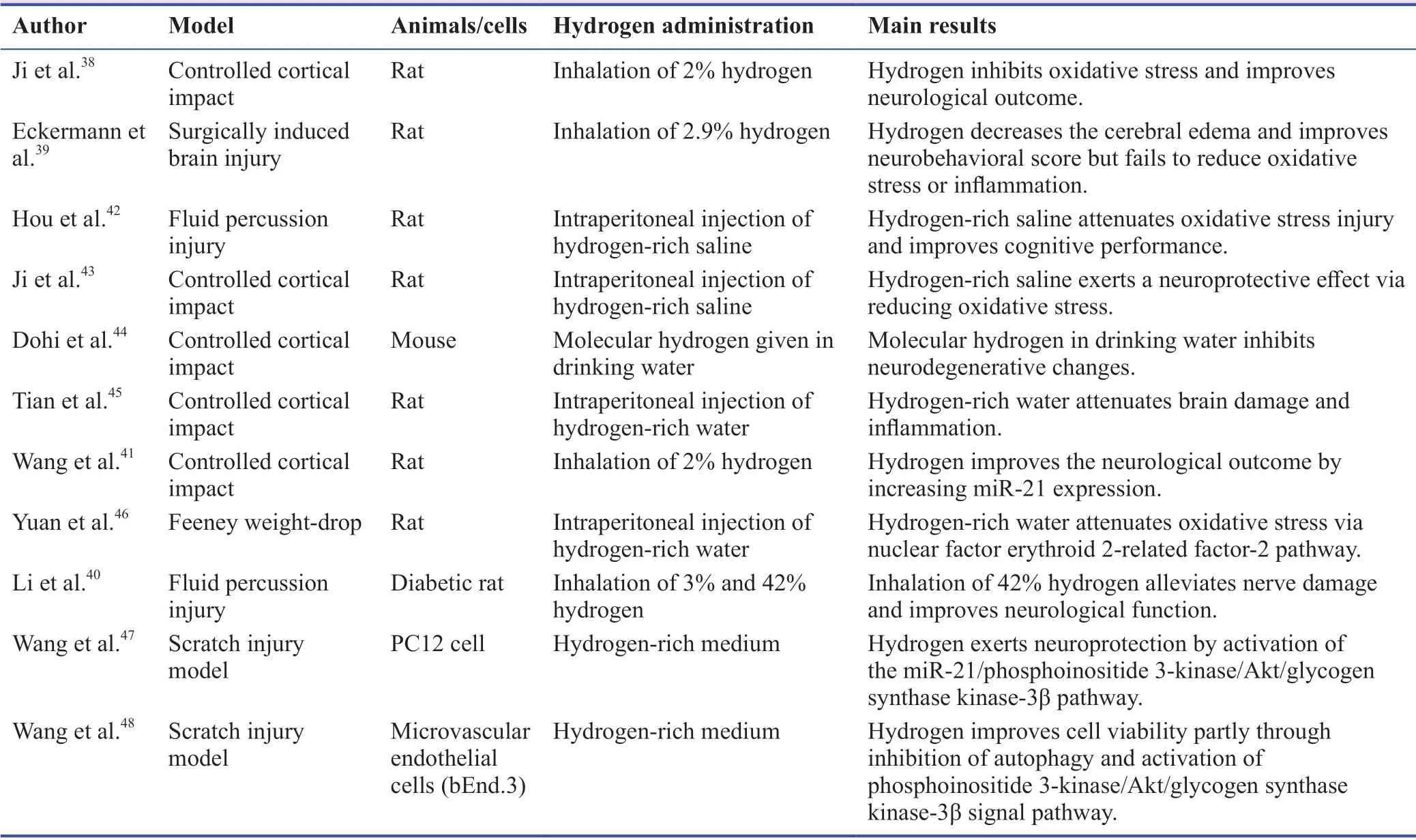
Table 1:Experimental studies regarding hydrogen in traumatic brain injury
Currently, the application of hydrogen-rich medium in anin vitromodel of TBI has been investigated.In 2020, using anin vitromodel of TBI (PC12 cells), a report indicated that the hydrogen-rich medium played a neuroprotective role against neuronal apoptosis and impaired nerve regeneration.47Another similar study revealed that hydrogen-rich medium improved the cell viability in a microvascular endothelial cell model of TBI (bEnd.3, an immortalized mouse brain endothelial cell line) though inhibition of autophagy.48
MECHANISMS OF THE HYDROGEN THERAPY IN TRAUMATIC BRAIN INJURY
It is well known that TBI is a major cause of death and disability among young people globally.49And the survivors after TBI often have neurological deficits, seizures, behavioral changes, cognitive impairment and so on, which seriously affect the quality of life and bring heavy burden to both family and society.50So far, to all organs in the body, TBI is one of the most complex diseases.Because of the high heterogeneity of brain injury and neuroendocrine dysfunction, the underlying pathophysiological mechanism occurring in TBI is more complex.51At present, it is believed that the brain tissue will have violent collision, acceleration, deceleration, or rotation movement when the head is subjected to external force.Then,the mechanical destruction of the brain tissue causes axon shearing and blood vessels tearing, which can lead to contusion and hemorrhage.All these events result in a secondary cascade of molecular and biochemical changes in minutes of the initial impact, which is called secondary brain injury.52The secondary injury mechanisms mainly include mitochondrial dysfunction, excitotoxicity, calcium overload, neuroinflammatory response and oxidative stress.These induce membrane cellular and vascular system destruction, which will lead to blood-brain barrier damage, cerebral blood flow alteration,ischemia and hypoxia, energy deficit, and eventually bring about apoptosis or necrosis.53Some experiments have shown that TBI rats can have brain edema, neurological dysfunction and prolonged remodeling time.Oxidative stress, neuroinflammation and apoptosis are important factors leading to these pathological changes.44,45,54
At present, many studies have shown that hydrogen plays neuroprotective roles in TBI through a variety of mechanisms.The potential mechanisms include anti-oxidation, anti-inflammation, inhibition of apoptosis and so on (Figure 1).55
Anti-oxidation
Increasing evidences have shown that oxidative stress is considered to be the key factor for secondary injury in the pathophysiology of TBI.Further studies found that excessive production of reactive oxygen species and reduction of antioxidant defense systems play an important role in the pathogenesis of TBI.Also, oxidative stress is involved in the development of cerebral edema, inflammation, and secondary neuronal damage.56-58No matter hydrogen, hydrogenrich water, hydrogen-rich saline or hydrogen in drinking water, they have been reported to have antioxidant stress effect in many studies.32,59,60Hydrogen plays a similar role in TBI model.MDA is the end product of lipid peroxidation induced by free radicals and its level is related to the degree of damage induced by free radicals.61Additionally, 8-isoprostaglandin-2α is a specific product after lipid peroxidation of arachidonic acid on free radical catalyzed biofilm, which can sensitively reflect the level of oxidative stressin vivoand is related to the severity of disease.62The determination of MDA and 8-iso-prostaglandin-2α is considered to be an ideal index to evaluate oxidative stress.61,63Meanwhile, the harmful effects of free oxygen radicals can be alleviated by the action of antioxidant enzymes superoxidase dismutase and catalase.64 Related reports showed that inhalation of hydrogen or intraperitoneal injection of hydrogen-rich saline after TBI exerted anti-oxidative stress effects by decreasing the level of MDA and 8-iso-prostaglandin-2α and increasing the activities of superoxidase dismutase and catalase.38,43Silent information regulator 2 (Sir2) is a nicotinamide adenine dinucleotide+-dependent deacetylase, which is involved in the regulation of cell cycle, energy metabolism, fatty acid oxidation and other processes.65And Sir2 may play an antioxidant stress role by promoting the antioxidant system.66Besides,Sir2 is involved in the regulation of oxidative stress after TBI.67In 2012, Hou et al.42confirmed that hydrogen-rich saline could play an anti-oxidative stress role by promoting Sir2 expression after TBI.Nuclear factor erythroid 2-related factor-2 is an important regulator factor of cellular antioxidants, which plays an important role in protecting neurons from oxidative stress after TBI.68A study demonstrated that intraperitoneal injection of hydrogen-rich water could attenuate oxidative stress in rats with TBI induced by Feeney weight-drop method via nuclear factor erythroid 2-related factor-2 pathway.46
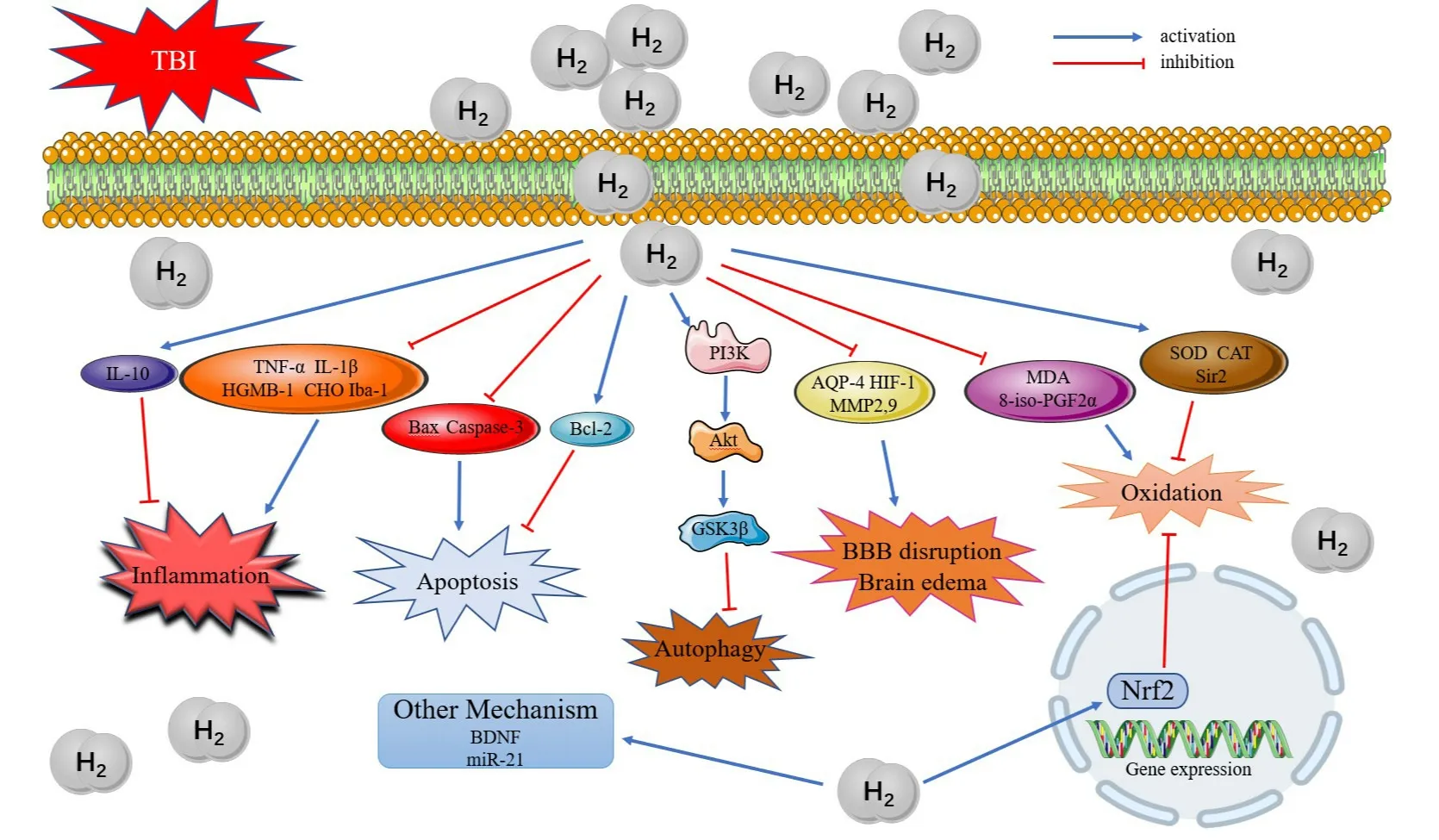
Figure 1:The potential mechanism of hydrogen against TBI.
Anti-inflammation
Inflammation is a common pathological process in most diseases.The activation of immune cells and the release of inflammatory cytokines are related to inflammation.Neuroinflammation is an immune response activated by microglia and astrocytes and it usually occurs under the stimulation of trauma, infection, toxin or the action of autoimmunity.Although transient neuroinflammatory signal transduction plays a protective role during development and tissue repair after injury, chronic neuroinflammation is related to the progression of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and multiple sclerosis.8Using hydrogen-rich water as the hydrogen source, a study found a significant difference between TBI and hydrogen-rich water-treated TBI rats in measures of inflammatory cytokines levels and inflammatory metabolites.45Intraperitoneal injection of hydrogen-rich water significantly attenuated the levels of pro-inflammatory cytokines (tumor necrosis factor-α, interleukine-1β and high mobility group box-1), inflammatory cells (ionized calcium-binding adapter molecule-1) and inflammatory metabolites (choline), while observably increased the level of anti-inflammatory cytokine (interleukine-10).45It suggested that hydrogen could play a neuroprotective role through antiinflammatory response after TBI.45
Anti-apoptosis
According current studies, neuronal apoptosis is the main pathological change type of secondary brain injury after TBI and plays an important role in delayed neuron loss after acute and chronic central nervous system injury.It has been reported that there are two pathways of neuronal apoptosis,one is caspase-dependent apoptosis by activating caspase family, the other is independent caspase pathway initiated by apoptosis-inducing factors in mitochondria.Both pathways are regulated by the Bcl-2 family of proteins.It is generally believed that Bcl-2 plays an anti-apoptotic role, while Bax and caspase-3 play a part in pro-apoptotic, which are commonly used to detect apoptosis markers.69,70Recently, a study showed that inhalation of 42% hydrogen could significantly decrease the expression of Bax and caspase-3 and increase the expression of Bcl-2 after TBI in diabetic rats.Besides, neurological function scores were also improved after hydrogen treatment.Therefore, it revealed that hydrogen could inhibit apoptosis exerting neuroprotective effects.40Similarly, the anti-apoptotic effect of hydrogen was also verifiedin vitro.In anin vitromodel of TBI (PC12 cells), after hydrogen-rich medium treatment, the researchers measured the expression of Bax and Bcl-2 proteins and detected the degree of apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling staining.The results showed that compared with TBI group, hydrogen intervention significantly decreased the expression of Bax and increased the level of Bcl-2, and reduced apoptosis.47
Anti-autophagy
Autophagy is a process in which eukaryotic cells mediate the degradation of damaged organelles, protein aggregates and invading pathogens through lysosome dependent pathways.71Some studies have shown that autophagy is involved in oxidative stress,72inflammatory response,73apoptosis74and other pathophysiological processes.Furthermore, autophagy pathway is also involved in brain injury after TBI.75Anin vitroexperiment found that hydrogen treatment would not change the autophagy level of normal microvascular endothelial cell(bEnd.3).While further study confirmed that the autophagy level in bEnd.3 cells was activated after TBI, which can be inhibited by hydrogen treatment through the up-regulation of phosphoinositide 3-kinase/Akt/glycogen synthase kinase-3β signal pathway.48
Other potential mechanisms
In addition to the above mechanisms, hydrogen also has been reported to play a role in TBI through the following potential mechanisms.The levels of aquaporin-4, hypoxia-inducible factor-1α, matrix metalloproteinase-2 & -9 were altered by molecular hydrogen in drinking water treatment after TBI in a CCI model, which could affect brain edema, bloodbrain barrier disruption and alterations in brain interstitial fluid circulation.44And the pathological phosphorylated tau changes induced by CCI were blocked by molecular hydrogen in drinking water intervention.Moreover, molecular hydrogen in drinking water increased adenosine triphosphate and nucleotide-binding after TBI and altered pathological gene expressions that regulate oxidation, carbohydrate metabolism and suppressed cytokine activation.44In another study, researchers demonstrated that hydrogen-rich saline treatment improved rat cognitive performance after mild TBI via increasing the expression of molecules associated with brain-derived neurotrophic factor-mediated synaptic plasticity.42MicroRNA is a kind of non-coding single-stranded RNA molecules with a length of about 22 nucleotides, which involved in the regulation of post-transcriptional gene expression and related to the pathophysiology of many diseases,including TBI.76More and more researches on micro-RNA in TBI have been carried out.Recently, relevant researches showed that miR-21 expression increased after TBI, and it could improve neurological outcome.77,78Further studies confirmed that hydrogen could play a neuroprotective role in TBIin vivoorin vitromodel via increasing the expression of miR-21.41,47
CONCLUSION AND PROSPECTS
To sum up, the neuroprotective effect of hydrogen after TBI has been confirmed by many experiments.Its potential mechanism may be related to anti-oxidation, anti-inflammatory,anti-apoptosis, anti-autophagy and regulation of cell signaling pathway.Because hydrogen is non-toxic and easy to diffuse and reductive, it is a promising gas in the treatment of TBI.At present, the researches on the role of hydrogen in TBI are mainly focused on antioxidant stress.However, the pathophysiological mechanism of TBI is complex.Whether hydrogen can play a protective role in TBI secondary brain injury through other mechanisms remains to be studied further, which provides a theoretical basis for the application of hydrogen in TBI.Moreover, the relationship between the neuroprotective mechanisms of hydrogen is also not clear.In addition, it is still lack of large-scale researches on the way of hydrogen administration, the optimal concentration, safety and clinical application.All of these are potential research directions in the future.
Acknowledgements
The authors would like to express our sincere thanks to all those who have lent our hands in the course of my writing this paper.We thank Professor Yu-Lun Huang (The First Affiliated Hospital of Soochow University) for the modification of the manuscript’s figure.We also acknowledge Qing-Zhi E (Soochow University) for the grammar review and editing.
Author contributions
Manuscript writing:HWH; manuscript revision:ZGC; manuscript drafting:JGL and GC.All authors read and approved the final version of the manuscript for publication.
Conflicts of interest
The authors declare that they have no competing interests.
Financial support
The work was supported by the Scientific Research of Jiangsu Commission of Health of China (No.H2018065) and Scientific Research of Guizhou Commission of Health of China (No.gzwjkj2019-1-150).
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- Medical Gas Research的其它文章
- Ventilation with the noble gas argon in an in vivo model of idiopathic pulmonary arterial hypertension in rats
- Neuroprotective effect of helium after neonatal hypoxic ischemia:a narrative review
- Efficacy of xenon anesthesia in preventing postoperative cognitive dysfunction after cardiac and major non-cardiac surgeries in elderly patients:a topical review
- Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients:a retrospective observational study
- Comparison of vital capacity rapid inhalation and tidal ventilation induction with sevoflurane in adults:a prospective cohort study
- Spontaneous breathing for managing analgesia during balanced anesthesia with remifentanil and desflurane:a prospective, single center randomized controlled trial

