Capsaicin Regulates Mitochondrial Fission to Promote Melanoma Cell Apoptosis
Jing-Jing Ma,Yu-Qi Yang,Sen Guo,Hui-Na Wang,Xiu-Li Yi,Tao Zhao,Lin Liu,Qiao Yue,Yu Liu,Qiong Shi,Tian-Wen Gao,Wei-Nan Guo?,Chun-Ying Li?
Department of Dermatology,Xijing Hospital,The Fourth Military Medical University,Xi’an,Shaanxi710032,China.
Abstract
Keywords:capsaicin,melanoma,mitochondrial dysfunction,mitochondrial fission,TRPV channels
Introduction
Malignant melanoma is an aggressive form of skin cancer and accounts for approximately80%of skin cancer-related deaths.1Although both immunotherapy and therapy that targets mitogen-activated protein kinase inhibitors have improved the overall survival of patients with melanoma,the average survival rates remain poor because of disease recurrence and resistance to currently available therapies.2Therefore,identification of new therapeutic targets and agents for melanoma treatment is urgently needed.
Capsaicin(8-methyl-N-vanillyl-6-noneamide)(CPS),a major component of the red pepper,is a high-affinity agonist of the transient receptor potential vanilloid1(TRPV1)receptor,which belongs to the TRPV family.3TRPV6is a more recently identified receptor of CPS.4Once activated by CPS,these TRPV channels preferentially lead to augmented permeability of Ca2+flux,initiating intracellular Ca2+-dependent signaling cascades.5CPS is widely used in clinical practice for the treatment of pain and inflammation caused by various diseases.6Moreover,CPS can potently repress the growth of various cancers through the induction of apoptosis by TRPV-dependent or-independent pathways.7The tumor-suppressive role of CPS in melanoma has been proven both in vitro and in vivo.8–9Intriguingly,CPS does not have a prominent effect on the viability of normal cells,4,10making it an attractive agent for cancer therapy.However,the mechanism underlying its anti-tumor capacity in melanoma has not been fully clarified.
The mitochondrion is the crucial cytoplasmic organelle for maintaining homeostasis within eukaryotic cells;thus,targeting the mitochondria is a promising therapeutic strategy.Mitochondrial structure and function are dynamically regulated by two counteracting processes:fusion and fission.11Accumulated evidence has revealed that mitochondrial fission can be induced by various stresses and is tightly correlated with cell death via the regulation of mitochondrial function.12–13Although previous studies have demonstrated some mechanisms underlying the anticancer effect of CPS in cancers,whether mitochondrial fission is implicated in this effect remains unclear.
In the present study,we investigated the effects of CPS treatment on the vitality of melanoma cells,and its underline mechanism.Next,we investigated whether CPS treatment leads to mitochondrial dysfunction in melanoma cells.Finally,the implication of mitochondrial fission in the process of CPS-induced melanoma cell apoptosis was measured.
Material and methods
Cell culture and reagents
The human melanoma cell lines A2058and WM35were purchased from American Type Culture Collection(Manassas,VA,USA).Experiments were performed on cells passaged for no more than6months.The A2058cell line was cultured in DMEM/F12(Hyclone Laboratories/GE Healthcare,Chicago,IL,USA)supplemented with 10% fetal bovine serum(Gibco/Thermo Fisher Scientific,Waltham,MA,USA).The WM35cell line was cultured in RPMI-1640(Hyclone Laboratories/GE Healthcare)supplemented with10% fetal bovine serum at37°C in the presence of5% carbon dioxide.Both of these melanoma cell lines were authenticated by short tandem repeat fingerprinting by Beijing Microread Genetics Co.,Ltd.in 2016and tested for mycoplasma contamination.An immortalized normal human epidermal melanocyte cell line(PIG1)(a gift from Dr.Caroline Le Poole,Loyola University Chicago,Maywood,IL,USA)and human primary melanocytes(HPMs)isolated from human foreskin specimens obtained during surgical circumcision were cultured in Medium254(Cascade Biologics/Invitrogen,Portland,OR,USA)supplemented with human melanocyte growth supplement(Cascade Biologics/Invitrogen),5%fetal bovine serum(Invitrogen,San Diego,CA,USA),and a penicillin–streptomycin antibiotic mix at37°C in the presence of5% carbon dioxide.14
CPS and dimethyl sulfoxide were purchased from Sigma-Aldrich(St.Louis,MO,USA).Z-VAD-FMK(a pan-caspase inhibitor)and ruthenium red(RR)(a TRPV antagonist)were purchased from R&D Systems(Minneapolis,MN,USA).N-Acetyl-L-cysteine(NAC,an antioxidant)was purchased from Beyotime(Shanghai,China).
Cell vitality analysis
Cell vitality was monitored using a cell counting kit-8(CCK-8)assay according to the manufacturer’s protocol(Beyotime).In brief,melanoma cells were initially cultured at a density of5×103cells per well in96-well plates overnight.The cells were then incubated for indicated times with different treatments at37°C.Next,CCK-8 solution was added to each culture well and incubated for 20minutes at37°C.The absorbance at450nm was then measured with a microplate reader(Bio-Rad Laboratories,Hercules,CA,USA).All experiments were performed in triplicate,and the absorbance measured in treated cells was calculated as the percentage of the absorbance in untreated control cells.
Western blotting analysis
Cells that underwent the indicated treatment were washed once with ice-cold phosphate-buffered saline(PBS),and total proteins were extracted with RIPA Lysis Buffer containing the protease inhibitor phenylmethanesulfonyl fluoride(Beyotime).The homogenate was incubated on ice for10minutes and centrifuged at10,000g for15minutes to pellet large cellular debris.Protein concentrations were measured with a BCA Protein Assay Kit(Beyotime).Protein samples were denatured in5×sodium dodecyl sulfate(SDS)loading buffer by heating them at100°C for 5minutes before use.
For the western blotting analysis,30-μg protein samples were electrophoresed through SDS-polyacrylamide gels in Tris-glycine SDS running buffer.The separated proteins were electroblotted onto polyvinylidene difluoride membranes(Millipore,Billerica,MA,USA).After the membranes had been blocked in5% nonfat milk at room temperature for1hour,they were incubated with the following primary antibodies diluted at1:1000overnight at4°C:dynamin-related protein1(DRP1)(ab56788;Abcam Australia,Melbourne,Victoria,Australia),phospho-DRP1(pDRP1)(Ser616)(#3455;Cell Signaling Technology,Danvers,MA,USA),pDRP1(Ser637)(#4867;Cell Signaling Technology),mitofusin1(MFN1)(13798-1-AP;Proteintech Group,Rosemont,IL,USA),MFN2(12186-1-AP;Proteintech Group),fission 1(Fis1)(10956-1-AP;Proteintech Group),optic atrophy1(OPA1)(#67589;Cell Signaling Technology),and cleaved caspase-3(#9661;Cell Signaling Technology).As an internal standard between the samples,β-actin(CW0096 M;CWBio,Beijing,China)was used at a dilution of 1:5,000.The specific protein–antibody complex was detected using horseradish peroxidase-conjugated immunoglobulin G(Jackson ImmunoResearch Laboratories,West Grove,PA,USA)diluted at1:5,000for2hours at room temperature.Detection of the chemiluminescence reaction was carried out with an enhanced chemiluminescencekit(Pierce/ThermoFisherScientific).TheWesternblot data were quantified by analyzing the band intensity with Image Lab software,Version5.2.1(Bio-Rad Laboratories).
Annexin V-FITC/propidium iodide apoptosis assay
To quantify apoptotic and necrotic death,melanoma cells were plated in12-well plates at a density of1×104cells per well overnight,and the cells were then exposed to different treatments for indicated times.The cells were then collected and washed once with PBS.Apoptosis assay was conducted using an Annexin V-FITC Apoptosis Detection Kit(7Sea Biotech,Shanghai,China)according to the manufacturer’s instructions.The numbers of viable,apoptotic,and necrotic cells were quantified by flow cytometry(Beckman Coulter,Brea,CA,USA),and the analysis was performed with Expo32software(Beckman Coulter).Each treatment was repeated in triplicate.
Measurement of intracellular reactive oxygen species(ROS)
The production of ROS was monitored by flow cytometry using CM-H2DCFDA as the label.After undergoing the indicated treatments,the cells were loaded with CMH2DCFDA(Life Technologies,Carlsbad,CA,USA)at 37°C for30minutes and then incubated with culture medium for another5minutes.The intracellular ROS level was analyzed by flow cytometry(Beckman Coulter)at an excitation wavelength of488nm and emission wavelength of530nm.
Measurement of mitochondrial membrane potential(MMP)
The MMP(Δψm)was evaluated by a JC-1staining assay.Briefly,cells that had undergone the indicated treatments were collected and resuspended in0.5mL of fresh culture medium containing10μmol/L JC-1(Life Technologies)at 37°C for30minutes followed by analysis with flow cytometry(Beckman Coulter).The MMP was quantified by the ratio of red to green fluorescence staining signals.
Measurement of intracellular adenosine triphosphate(ATP)level
The intracellular ATP level was measured using an ATP Assay Kit(Beyotime)according to the manufacturer’s instructions.In brief,lysed cells were added to ATP detection buffer,and the ATP level was measured with a luminometer(Bio-Rad Laboratories).The measurement was performed three times for each sample.The ATP concentration was calculated from the luminescence value according to the standard curve.
Mitochondrial network imaging by confocal microscopy
The fluorescent dye MitoTracker Green FM(Invitrogen)was used to monitor mitochondrial morphology in living cells according to the manufacturer’s instructions.In brief,the cells were loaded with MitoTracker Green FM at37°C in serum-free media for30minutes,the media was exchanged for fresh media,and the cells were viewed with an Olympus FV1000/ES confocal microscope(Olympus Corporation,Tokyo,Japan).
Mitochondrial morphology can also be observed by immunofluorescence staining of TOM20.15Cells were fixed in4% paraformaldehyde for10minutes,washed twice with PBS,permeated with0.1%Triton X-100,and blocked with goat serum for1hour.The cells were then incubated with TOM20antibody(1:200;Proteintech)overnight at4°C.After being washed with PBS,the cells were incubated with secondary antibodies(FITC-tagged goat anti-rabbit,1:400)for1hour.The cells were then washed with PBS and further incubated with DAPI(1:1,000;Dako,Glostrup,Denmark)for15minutes.Fluorescent images were obtained with an FV1000/ES confocal microscope(Olympus).
Mitochondrial fission is indicated by a dramatic increase in mitochondria fragmentation.Therefore,the length of mitochondria,including fragmented,intermediate,and elongated mitochondria,was measured using ImageJ software(National Institutes of Health,Bethesda,MD,USA)to analyze the extent of mitochondrial fission or fusion.16
Statistical analyses
One-way analysis of variance and the Bonferroni method were used to detect differences among multiple groups.The unpaired two-tailed Student t test was used for comparisons between two groups.These analyses were performed with GraphPad Prism software,Version7.0(GraphPad Software,San Diego,CA,USA).Differences with P<0.05,P<0.01,or P<0.001were considered statistically significant.Data are presented as mean±standard deviation for at least three independent experiments.
Results
CPS induces apoptosis of melanoma cells through TRPV channels and caspase cascade
We first examined the effect of CPS on melanoma cells and elucidated the involvement of TRPV channels.We treated melanoma cells(A2058and WM35cell lines)and melanocytes(HPMs and PIG1cell line)with different concentrations of CPS for16hours.The results showed that the cell vitality of the two melanoma cell lines was significantly reduced when the concentration of CPS was≥120μmol/L(For A2058cells:0vs.120μmol/L:[100%±0%]vs.[51.02%±6.40%],P<0.05;For WM35cells:0vs.120μmol/L:[100%±0%]vs.[51.80%±3.45%],P<0.05),while CPS showed less impact on HPMs and the PIG1cell line(Supplementary Fig.1,http://links.lww.com/JD9/A2).These findings suggest that melanoma cells are more sensitive to CPS than are melanocytes.Additionally,the vitality of all of these cells decreased as the concentration of CPS increased(Supplementary Fig.2,http://links.lww.com/JP9/A12),indicating a dose-dependent effect of CPS on the vitality of melanoma cells and melanocytes.
Pretreatment with RR,a widely used TRPV channel antagonist,reversed the inhibitory effect of CPS on cell viability(For A2058cells:CPS vs.CPS+RR:[41.61%±2.11%]vs.[65.22%±3.78%],P<0.01;For WM35cells:CPS vs.CPS+RR:[26.95%±3.98%]vs.[43.28%±4.00%],P<0.05)(Fig.1A).Consistent with this,the flow cytometry analysis revealed that while CPS prominently promoted cell apoptosis in the two melanoma cell lines(For A2058cells:Control vs.CPS:[1.53%±0.49%]vs.[9.7%±0.46%],P<0.05;For WM35cells:Control vs.CPS:[5.33%±0.94%]vs.[20.13%±0.88%],P<0.01),pretreatment with RR protected the melanoma cells against CPS-induced cell apoptosis(For A2058cells:CPS vs.CPS+RR:[9.70%±0.46%]vs.[5.40%±0.50%],P<0.05;For WM35cells:CPS vs.CPS+RR:[20.13%±0.88%]vs.[7.03%±1.13%],P<0.01)(Fig.1B and1C).RR treatment alone had no effects on cell vitality or apoptosis(data not shown).Western blotting analysis showed that CPS promoted the expression of cleaved caspase3,whereas pretreatment with RR reduced its expression in a dose-dependent manner(Fig.1D).Notably,we used Z-VAD-FMK,a pan-caspase inhibitor and specific cell apoptosis inhibitor,to ensure that the CPSinduced cell death was mostly due to cell apoptosis and caspase signal-dependent(CPS vs.CPS+Z-VAD-FMK:[16.85%±0.55%]vs.[4.95%±0.65%],P<0.01)(Fig.1E and1F).Collectively,these results demonstrate that CPS treatment promotes apoptosis of melanoma cells through TRPV channels and the caspase cascade.
CPS-triggered ROS generation is mediated by TRPV channels and contributes to cell apoptosis
A previous study showed that CPS-induced tumor cell apoptosis is associated with the generation of ROS.17Therefore,we analyzed the production of intracellular ROS in melanoma cells after CPS treatment.We found that the level of ROS significantly increased as the concentration of CPS gradually increased in both cell lines(For A2058cells:0vs.140μmol/L:[1.00±0]vs.[1.70±0.08],P<0.05;For WM35cells:0vs.140μmol/L:[1.00±0]vs.[1.60±0.28],P<0.05)(Fig.2A and2B).
To explore whether CPS-triggered ROS generation is mediated by TRPV channels,we pretreated A2058and WM35cells with RR followed by CPS treatment and found that the generation of intracellular ROS was prominently diminished(For A2058cells:CPS vs.CPS+RR:[2.34±0.30]vs.[1.34±0.12],P<0.05;For WM35 cells:CPS vs.CPS+RR:[2.25±0.25]vs.[1.65±0.13],P<0.05)(Fig.2C and2D).This finding indicates that TRPV channels mediated CPS-triggered intracellular ROS production.RR treatment alone had no effect on ROS generation(data not shown).
We further investigated the role of ROS in CPS-induced apoptosis of melanoma cells.The cells were pretreated with different concentrations of the ROS scavenger NAC followed by CPS treatment.The results showed that the viability of melanoma cells was significantly suppressed with CPS treatment alone while it gradually recovered in the presence of NAC(For A2058cells:CPS vs.CPS+4 mmol/L:[46.44%±5.81%]vs.[84.75%±10.90%],P<0.05;For WM35cells:CPS vs.CPS+4mol/L:[40.77%±7.11%]vs.[95.34%±16.95%],P<0.01)(Fig.3A and3B).Similarly,while CPS treatment alone obviously induced cell apoptosis of A2058and WM35 cells,NAC pretreatment prominently abrogated this effect(For A2058cells:CPS vs.CPS+NAC:[22.65%±2.05%]vs.[8.70%±2.20%],P<0.05;For WM35cells:CPS vs.CPS+NAC:[16.05%±3.32%]vs.[7.78%±0.50%],P<0.05)(Fig.3C and3D).NAC treatment alone had no effect on cell vitality or apoptosis(data not shown).Therefore,the generation of intracellular ROS contributed to CPS-induced apoptosis of melanoma cells.
CPS treatment leads to TRPV channel-dependent MMP dissipation and ATP reduction in melanoma cells
Because mitochondrion is the main source of intracellular ROS,18we were interested in determining whether CPS treatment influences mitochondrial function and thereby regulates cell apoptosis.Through the flow cytometry assay,we found that CPS treatment prominently induced dissipation of the MMP(Control vs.CPS:[1.00±0]vs.[0.61±0.08],P<0.05)(Fig.4A and4B),which has been regarded as a characteristic of mitochondrial dysfunction and is highly correlated with the generation of ROS and induction of apoptosis.19Moreover,pretreatment with RR abolished the CPS-induced dissipation of the MMP(CPS vs.CPS+RR:[0.61±0.08]vs.[1.10±0.11],P<0.05)(Fig.4A and4B).RR treatment alone had no effects on MMP of melanoma cells(data not shown).

Figure1.RR,a TRPV antagonist,blocks CPS-induced apoptosis and caspase activation.A2058and WM35cells were pretreated with RR(20μmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.(A)The cell viability was determined by a CCK-8assay.(B and C)Cell apoptosis was analyzed with flow cytometry.(D)The protein level of cleaved caspase3in A2058cells was measured after the indicated treatment.β-Actin was detected as a loading control.(E and F)A2058cells were pretreated with Z-VAD-FMK(20μmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.Cell apoptosis was analyzed by flow cytometry.The data are presented as mean±standard deviation of three independent experiments.?P<0.05,??P<0.01.CCK-8:cell counting kit-8;CPS:capsaicin;RR:ruthenium red;TRPV:transient receptor potential vanilloid1.

Figure2.CPS increases intracellular ROS generation through TRPV channels.(A and B)A2058and WM35cells were treated with various concentrations of CPS for16hours,and the cellular ROS levels were detected.(C and D)A2058and WM35cells were pretreated with RR(20μ mol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.The intracellular ROS levels were analyzed by flow cytometry.The data are presented as mean±standard deviation of three independent experiments.?P<0.05,??P<0.01.CPS:capsaicin;RR:ruthenium red;TRPV:transient receptor potential vanilloid1.
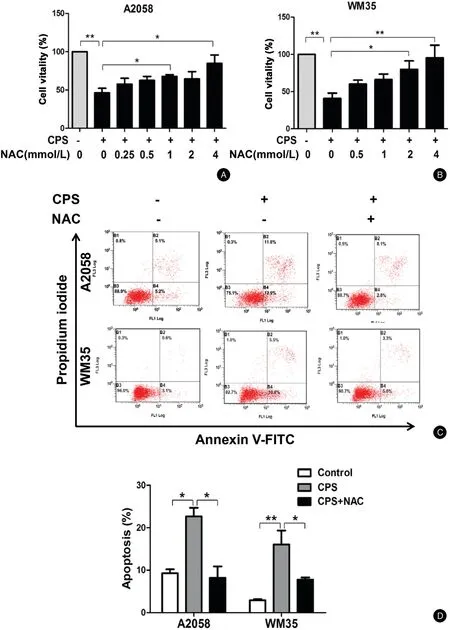
Figure3.ROS contributes to CPS-induced apoptosis of melanoma cells.(A and B)A2058and WM35cells were pretreated with various concentrations of NAC for1hour and then incubated with or without CPS(120μmol/L)for16hours.The cell viability was determined by CCK-8 assay.(C and D)A2058and WM35cells were pretreated with NAC(4mmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.Cell apoptosis was analyzed by flow cytometry.The data are presented as mean±standard deviation of three independent experiments.?P<0.05,??P<0.01.CCK-8:cell counting Kit-8;CPS:capsaicin;NAC:N-acetyl-L-cysteine;ROS:reactive oxygen species.

Figure4.CPS triggers MMP dissipation and ATP reduction through TRPV channels.(A and B)A2058cells were pretreated with RR(20μmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.The MMP level was examined by JC-1staining.(A)The scatter plot of the flow cytometry analysis shows the distribution of JC-1aggregates(red)and the JC-1monomer(green)cell population.(B)The histogram calculated the relative ratio of red to green fluorescence.(C)A2058and WM35cells were treated with various concentrations of CPS for16 hours,and the ATP levels were detected.(D)A2058and WM35cells were pretreated with RR(20μmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours;the ATP levels were then detected.The data are presented as mean±standard deviation of three independent experiments.?P<0.05,??P<0.01.ATP:adenosine triphosphate;CPS:capsaicin;MMP:mitochondrial membrane potential;RR:ruthenium red;TRPV:transient receptor potential vanilloid1.

Figure5.CPS promotes mitochondrial fission in a TRPV channel-dependent manner.(A–D)A2058and WM35cells were pretreated with RR(20μmol/L)for1hour and then incubated with or without CPS(120μmol/L)for16hours.The mitochondrial network was displayed with MitoTracker Green FM staining.(A and C)Representative confocal microscopic images of the mitochondrial network are shown.(B and D)The proportion of cells(n=100cells for each sample)with fragmented,intermediate,and elongated mitochondria was quantified.(E and F)A2058 and WM35cells were treated with various concentrations of CPS for16hours,and mitochondrial dynamics-related protein levels were examined by western blotting assay.The data are presented as mean±standard deviation of three independent experiments.?P<0.05,??P<0.01,???P<0.001.CPS:capsaicin;RR:ruthenium red;TRPV:transient receptor potential vanilloid1;DRP:dynamin-related protein1;p-DRP:phosphorylated-DRP;Fis1:fission1;MFN1:mitofusin1;OPA1:optic atrophy1.
Further,we examined the alteration of intracellular ATP to reveal the functional status of the mitochondria.Consistent with the trend of the MMP,CPS treatment prominently suppressed the intracellular ATP level,and this suppression was reversed by RR treatment(For A2058 cells:Control vs.CPS:[1.00±0.00]vs.[0.57±0.01],P<0.05;CPS vs.CPS+RR:[0.57±0.01]vs.[1.12±0.12],P<0.05;For WM35cells:Control vs.CPS:[1.00±0.00]vs.[0.05±0.02],P<0.01;CPS vs.CPS+RR:[0.05±0.02]vs.[0.35%±0.06%],P<0.05)(Fig.4C and4D).RR treatment alone had no effect on the ATP level(data not shown).Collectively,these results demonstrate that CPS treatment results in mitochondrial dysfunction characterized by the generation of ROS,dissipation of the MMP,and down-regulation of intracellular ATP in a TRPVdependent manner.
Mitochondrial fission connects CPS treatment to mitochondrial dysfunction
During the process of apoptosis,mitochondrial fission is an initial step that occurs before MMP dissipation and caspase activation.20Therefore,we speculated that CPS treatment may induce the alteration of the mitochondrial dynamics and thereby promote cell apoptosis.To this end,we employed immunofluorescence staining using the mitochondrial dye MitoTracker Green or TOM20to reveal the alterations of the mitochondrial dynamics.We found that CPS treatment led to dramatic mitochondrial fission in both the A2058and WM35melanoma cell lines as indicated by a dramatic increase in mitochondrial fragmentation(For A2058cells:Control vs.CPS:[6.35%±3.83%]vs.[72.10%±5.05%],P<0.001;For WM35 cells:Control vs.CPS:[2.5%±2.5%]vs.[77.21%±6.20%],P<0.001)(Fig.5A–D,Supplementary Fig.2,http://links.lww.com/JP9/A12).However,we did not observe significant mitochondrial fission in HPMs or the PIG1cell line treated with CPS(Supplementary Fig.3,http://links.lww.com/JD9/A14).We then found that pretreatment with RR obviously suppressed CPS-induced mitochondrial fission(For A2058cells:CPS vs.CPS+RR:[72.10%±5.05%]vs.[15.63%±5.98%],P<0.01;For WM35cells:CPS vs.CPS+RR:[77.21%±6.20%]vs.[12.57%±5.61%],P<0.01)(Fig.5A–D),illustrating the involvement of the TRPV channels.
Mitochondrial dynamics is governed by a series of regulators,including DRP1,Fis1,MFN1,MFN2,and OPA1.Among them,Fis1and DRP1facilitate mitochondrial fission while OPA1,MFN1,and MFN2promote mitochondrial fusion.11Notably,phosphorylation of DRP1at S616can promote its activity to facilitate mitochondrial fission,while phosphorylation of DRP1at S637suppresses its activity to inhibit mitochondrial fission.21Our western blotting analysis revealed that while the expressions of DRP1,Fis1,OPA1,MFN1,and MFN2were not significantly altered,the phosphorylation of DRP1at S616was prominently increased(For A2058 cells:0vs.100vs.120μmol/L:[1.00±0]vs.[2.50±0.13]vs.[3.78±0.22],P<0.01;For WM35cells:0vs.100vs.120μM:[1.00±0]vs.[1.86±0.15]vs.[1.95±0.15],P<0.05)(Fig.5E and5F).However,we did not observe an alteration of the phosphorylation of DRP1at S637(Fig.5E and5F).This result indicates that increased phosphorylation of DRP1at S616may be responsible for CPS-induced mitochondrial fission.
Discussion
In the present study,we found that CPS treatment significantly inhibited the vitality of melanoma cells whereas it exerted less impact on normal melanocytes,indicating that melanoma cells are more sensitive to CPS than are normal melanocytes.This makes CPS an attractive agent for melanoma therapy.We also proved that CPS induced apoptosis of melanoma cells through TRPV channels and the caspase cascade.Moreover,we proved that intracellular ROS generation contributed to CPS-induced melanoma cell apoptosis.We also showed that mitochondrial dysfunction,including dissipation of the MMP and downregulation of intracellular ATP,occurred after CPS treatment.Our data further revealed that mitochondrial fission connected CPS treatment to mitochondrial dysfunction in melanoma cells.Collectively,our findings demonstrate the role of mitochondrial fission and its related mitochondrial dysfunction in mediating the therapeutic effect of CPS on melanoma(Fig.6).
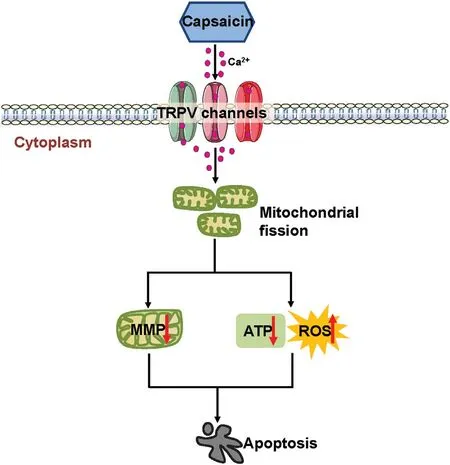
Figure6.Proposed model of the mechanism underlying the antitumor effect of CPS in melanoma.Once activated by CPS,TRPV channels preferentially lead to prominent Ca2+influx into melanoma cells,which triggers extensive mitochondrial fission,mitochondrial dysfunction including MMP dissipation,ATP reduction,and ROS generation.All of these effects finally result in melanoma cell apoptosis.ATP:adenosine triphosphate;MMP:mitochondrial membrane potential;ROS:reactive oxygen species;TRPV:transient receptor potential vanilloid1.
The suppressive role of CPS on the growth of melanoma has been proven,8-9but the underlying mechanisms are not well characterized.CPS reportedly inhibits the growth of melanoma via inhibition of NADH oxidase activity in the plasma membrane.22Our recent study showed that CPS treatment inhibited melanoma growth by activating p53 and inducing cell apoptosis.8The present study emphasized the involvement of the alteration of mitochondrial dynamics in CPS-induced apoptosis of melanoma cells.While mitochondrial fission may play an important oncogenic role during tumorigenesis and progression of melanomas,23-24excessive mitochondrial fragmentation leads to defective cellular metabolism,oxidative stress,mitochondrial dysfunction,and even cell death.21Importantly,we proved for the first time that mitochondrial fission connected CPS treatment to cell apoptosis in cancer cells.Consistent with a previous study demonstrating that CPS enhanced Ca2+-dependent mitochondrial fission through TRPV1in dorsal root ganglia,25our results showed that CPS-induced mitochondrial fission in melanoma is also TRPV channel-dependent,suggesting that calcium signaling plays a role in regulating the mitochondrial dynamics of melanoma cells.The two melanoma cell lines A2058and WM35used in the present study are metastatic and primary melanoma cell lines,respectively.We compared the effects of CPS on the cell vitality,cell apoptosis,mitochondrial fission,and intracellular ROS and ATP levels of these two melanoma cell lines.The results showed that the effects of CPS on these parameters were comparable between the two melanoma cell lines with the exception of the ATP level,which decreased more significantly in the WM35than A2058cell line(Supplementary Fig.4,http://links.lww.com/JD9/A15).
Mitochondrial fission is highly correlated with cell death via the regulation of mitochondrial function.13Our results showed that impaired mitochondrial function,characterized by dissipation of the MMP and reduction of ATP,occurred after CPS treatment through TRPV channels.This is consistent with some previous studies showing that CPS could induce MMP dissipation in urothelial cancer cells26and glioma cells.27Following MMP dissipation,cytochrome c is released from the mitochondria to the cytoplasm and activates caspase cascade-dependent apoptosis.19Therefore,dissipation of the MMP is correlated with CPS-induced activation of the caspase cascade in melanoma.
Massive mitochondrial fragmentation increases ROS production.18As expected,we observed increased intracellular ROS generation after CPS treatment.This increase contributed to CPS-induced apoptosis of melanoma cells and was dependent upon TRPV channels,indicating that intracellular Ca2+influx may mediate ROS generation.Indeed,TRPV1activation and sustained increase of intracellular Ca2+facilitate the production of ROS and trigger cell apoptosis.10Notably,research has shown that nitric oxide can also mediate CPS-induced apoptosis in A375melanoma cells,28suggesting the pivotal role of different types of ROS in the inhibitory effect of CPS on melanoma growth.
The bioavailability of CPS has been studied in rats,nude mice,and humans.29In humans,5g of capsaicinoids(primarily comprising CPS,dihydrocapsaicin,nordihydrocapsaicin,homohydrocapsaicin,homodihydrocapsaicin,and nonivamide)was administered orally.The halflife of CPS in the blood was found to be about25minutes,and the peak plasma concentration of CPS was2.5ng/mL(approximately8.2nmol/L)at45minutes.30Although the concentration of CPS used in the present study was high,CPS can be applied locally via direct injection into a tumor as described in our previous study,in which CPS was intratumorally administered and significantly inhibited melanoma growth in vivo.8
In conclusion,our data highlight the importance of mitochondrial fission and resultant mitochondrial dysfunction,which are induced through TRPV channels,in mediating CPS-induced apoptosis of melanoma cells.Our findings reveal novel mechanisms for understanding the anti-tumor activity of CPS,which may be a clinically useful agent for cancer therapy.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China(Nos.81625020,81402736,and 81902791)and the Key Research and Development Program of Shaanxi Province(No.2019sf-079).
- 國(guó)際皮膚性病學(xué)雜志的其它文章
- Mask on Followed by Gloves on:Do We Have a Choice?
- Mobilization of Melanocytes During NB-UVB Treatment of Vitiligo
- Consensus on the Diagnosis and Treatment of Melasma in China(2021Version)#
- Retiform Hemangioendothelioma
- Malignant Syphilis as an Initial Presentation of HIV Infection:A Case Report
- Treatment of Nevoid Basal Cell Carcinoma Syndrome by Surgery Combined With ALA-PDT:A Case Report

