Reporting quality of systematic review protocols of interventions for knee osteoarthritis: a systematic review protocol
Jie-Yun Li, Li-Yao Tang, Hao Tang, Tao He, Qi Su,Yao-Hui Su, Cun-Cun Lu
Reporting quality of systematic review protocols of interventions for knee osteoarthritis: a systematic review protocol
Jie-Yun Li1 #, Li-Yao Tang2 #, Hao Tang2 #, Tao He3, Qi Su2,Yao-Hui Su2, Cun-Cun Lu1, 4 *
1School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, China.2Clinical College of Chinese Medicine, Gansu University of Chinese Medicine, Lanzhou 730000, China.3Gansu Provincial Rehabilitation Central Hospital, Lanzhou 730000, China.4Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China.
Knee osteoarthritis is a chronic degenerative disease responsible for a substantial disease burden worldwide. Multiple protocols of systematic reviews of interventions for knee osteoarthritis have been published, but their reporting quality remains unknown, with potential influencing factors failing to be explored. Therefore, the present systematic review was designed to fill those gaps in our knowledge.The protocols of systematic reviews of interventions for knee osteoarthritis will be searched systematically through the PubMed and Embase online databases from inception to May 2021. Two reviewers will independently screen the literature and abstract data, and cross-check the results. The reporting quality of protocols included in the review will be evaluated using the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols checklist, and potential influencing factors (e.g., methodologist involvement and number of authors) for reporting quality will be explored using univariable and multivariable linear regression methods. The general information of the protocols included in the review will be reported qualitatively. Excel 2019 and Stata 13.0 software will be used to manage and analyze the data.-values < 0.05 will be considered statistically significant.The results of this methodological systematic review will be submitted to a peer-reviewed journal for publication.The systematic review will provide evidence of the reporting quality of protocols of systematic reviews of interventions for knee osteoarthritis. The evidence from the present review will be available to inform the reporting of future protocols.
Knee osteoarthritis, Systematic reviews, Reporting quality, PRISMA-P, Protocols
Background
Knee osteoarthritis is a chronic progressive condition characterized by pain in the knee joint, stiffness, and functional disability [1, 2]. Long-term consequences of the disease are an inevitable reduction in physical activity, sleeping disorders, depression, and fatigue, etc. [1]. Evidence reported in the published literature suggests that 263 million individuals around the globe suffer from knee osteoarthritis, which represents a considerable disease burden [3]. In particular, a recent meta-analysis reported that the prevalence was 16.0% (95% confidence interval: 14.3%–17.8%) with an incidence of 203 per 10,000 person-years (95% confidence interval: 106–331) worldwide [2]. At present, both pharmacologic and nonpharmacologic treatments are available for knee osteoarthritis [1], the former including interventions such as nonsteroidal anti-inflammatory drugs [1, 4] and Chinese medicines [5], the latter represented by exercise [1], physiotherapy (e.g., acupuncture) [1, 6], and surgery [1, 7].
Using the concept of evidence-based medicine, clinical decision-making should be conducted using the best evidence currently available [8]. Systematic reviews incorporate both qualitative and (or) quantitative research and synthesize all available studies that have focused on a specific research problem [9]. High-quality systematic reviews and meta-analyses are considered the highest level of evidence and the basis to inform clinical judgments of clinicians [10].
The protocol for a systematic review is a detailed report, before formal implementation, that contains the key methodological considerations regarding a systematic review [11, 12]. It can guide the implementation and reporting of systematic reviews and meta-analyses. Over recent years, researchers have accepted the importance of preregistration protocols, which improve the quality of systematic reviews generally, reducing research waste caused by duplication and selective reporting [11, 12]. However, several studies have shown that preregistration of the protocols of systematic reviews and meta-analyses is not ideal [13, 14]. In addition to registering protocols on relevant websites, publishing protocols in peer-reviewed academic journals (e.g.,,,) has become quite common over recent years, but few studies have investigated the reporting quality of these published protocols.
At present, multiple protocols for systematic reviews of interventions for knee osteoarthritis have been published [15–19]. However, to our knowledge, no study has so far evaluated the reporting quality of these protocols. The Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) is a reporting checklist developed and issued by a group of international experts [20]. They designed it to allow authors to report their protocols of systematic reviews by reference to the tool [20], aiming to promote the reporting quality of protocols for systematic reviews and meta-analyses. Other researchers can also use it to evaluate the reporting quality of published protocols [21].
The present review will utilize the PRISMA-P checklist to systematically evaluate the reporting quality of published protocols of systematic reviews of interventions for knee osteoarthritis. We will use regression analyses to explore the association between the reporting quality of the protocols and pre-specified study characteristics which may influence the reporting quality [22]. The research results can be used to inform the development and reporting of protocols of systematic reviews of interventions for knee osteoarthritis in the future, and improve the quality of the full-publications of corresponding systematic reviews. Ultimately, we hope that patients with knee osteoarthritis will benefit clinically from high-quality systematic reviews and meta-analyses. The present report outlines a protocol for this systematic review. All deviations from this report and the reasons will be reported in the following full publication.
Methods
Study registration
We designed the present study in line with Preferred Reporting Items for Systematic Reviews and Meta-analyses Guidelines [23], and prepared this report according tothe PRISMA-P 2015 reporting checklist [20]. In addition, this systematic review has been registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols (registration number: INPLASY202110111). This systematic review will not include any animals, patients, or members of the general public. The present systematic review will only include data extracted from the literature published in peer-reviewed journals, so ethical review approval is not required.
Information sources
The protocols of systematic reviews and meta-analyses of interventions for knee osteoarthritis will be systematically searched via the PubMed and Embase databases in May 2021. The comprehensive search strategy was developed through Boolean logic using Medical Subject Headings and keywords, including “osteoarthritis, knee”, “knee osteoarthritis”, “knee osteoarthritides”, “knee osteoarthrosis”, “osteoarthritis of knee”, “knee osteoarthroses”, “knee arthritis”, “degenerative knee”, “review*”, “meta*”, “protocol*”, and “plan*”. The detailed strategy for searching the PubMed database is presented in Table 1. In addition, thereferencesofprotocolsincludedinthereviewwill be checked for additional relevant articles.
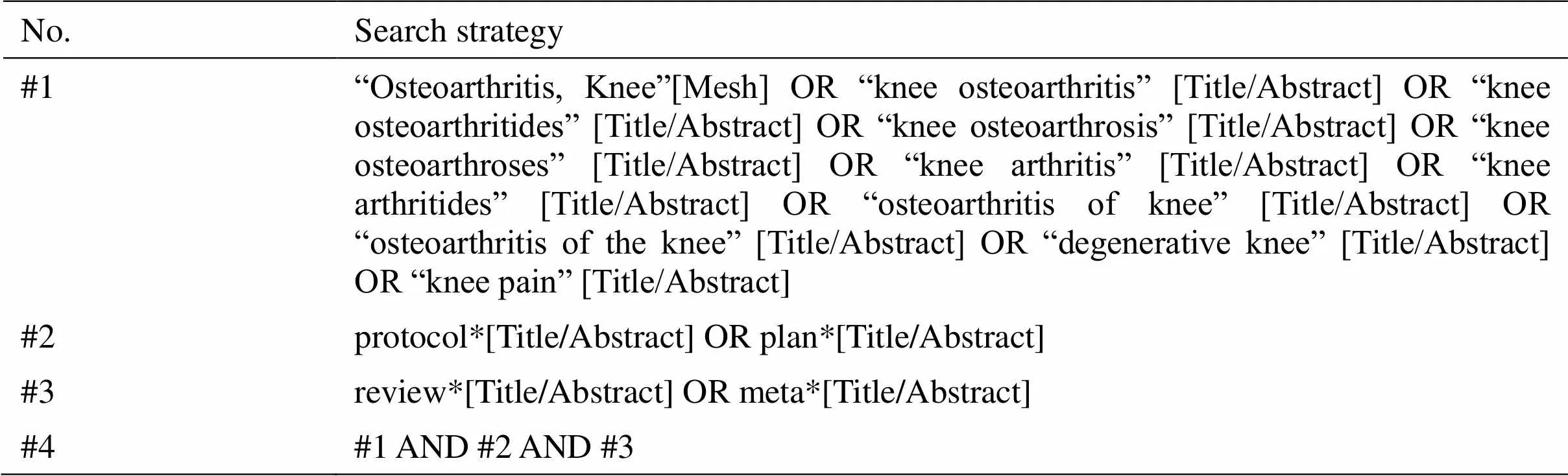
Table 1 PubMed search strategy.
Eligibility criteria
Study design.We will include the protocolsof systematic reviews of interventions for knee osteoarthritis published in English in this planned review. For this study, we define a systematic review as: “a review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyze data from the studies included in the review [24].” A meta-analysis is defined as: “the statistical combination of at least two independent studies to produce a single estimate of the effect of the healthcare intervention under consideration [24].” Furthermore, a meta-analysis can be part of a systematic review, but not always [9].
Participants. We will select studies in which participants have osteoarthritis of the knee confirmed by clinical judgment. Selection criteria will be based on clinical manifestations and imaging examinations. We will impose no restrictions, such as age, gender, race, ethnicity, or nationality.
Interventions. All available treatments described in the protocols of systematic reviews will be considered for inclusion in the present review. These treatments include but are not limited to, traditional therapy (e.g., Chinese medicines, exercise, massage, and acupuncture), drugs (e.g., nonsteroidal anti-inflammatory drugs and chondroitin sulfate), surgery, and other interventions, e.g., stem cell therapy.
Outcomes. We will consider all available outcomes mentioned in the included protocols. These include efficacy (e.g., pain reduction or improvement in quality of life), safety (i.e., unexpected complications), and economic data (e.g., hospital costs).
Study selection
The study selection process will consist of four stages in this systematic review. Firstly, all protocols identified from online databases will be imported into Endnote (Version X9, Clarivate Analytics) to remove duplicates. Secondly, two independent reviewers will select protocols based on titles and abstracts using the eligibility criteria specified above. Thirdly, they will read the full-text of protocols from the second stage to further assess eligibility. Finally, we will check the references of eligible articles to identify papers not found by the online search (Figure 1). Any discrepancies will be resolved through discussion between the reviewers.
Data extraction
Two reviewers will independently extract key information from the included protocols and enter into an Excel spreadsheet.The reviewers will resolve any disagreements through discussion. The extracted information will include: title, first author, publication date, country of corresponding author, number of authors, affiliation of authors (to judge whether a statistician or an epidemiologist as a methodologist was involved a protocol), country of affiliation, registration information (i.e., website and registration number), journal of publication and impact factor (IF2019), study objectives, databases searched, whether PRISMA-P was mentioned, types of knee osteoarthritis, types of intervention, types of outcome, funding information, and other information relevant to reporting quality assessment.
Reporting quality assessment
The PRISMA-P checklist, with 17 items (and 26 sub-items) [20], will be used by two independent reviewers to assess the reporting quality of the protocols of systematic reviews of interventions for knee osteoarthritis. Any disagreements will be resolved through discussion between reviewers. Each item will be potentially answered as “Yes”, “Partial Yes”, “No”, or “Not applicable”, which will be scored “1”, “0.5”, or “0” for the three former responses (“Not applicable” will be scored a “1” for avoiding punishment) [25], respectively. Our primary outcome will be the proportion of the aforementioned responses according to each item of the PRISMA-P checklist.
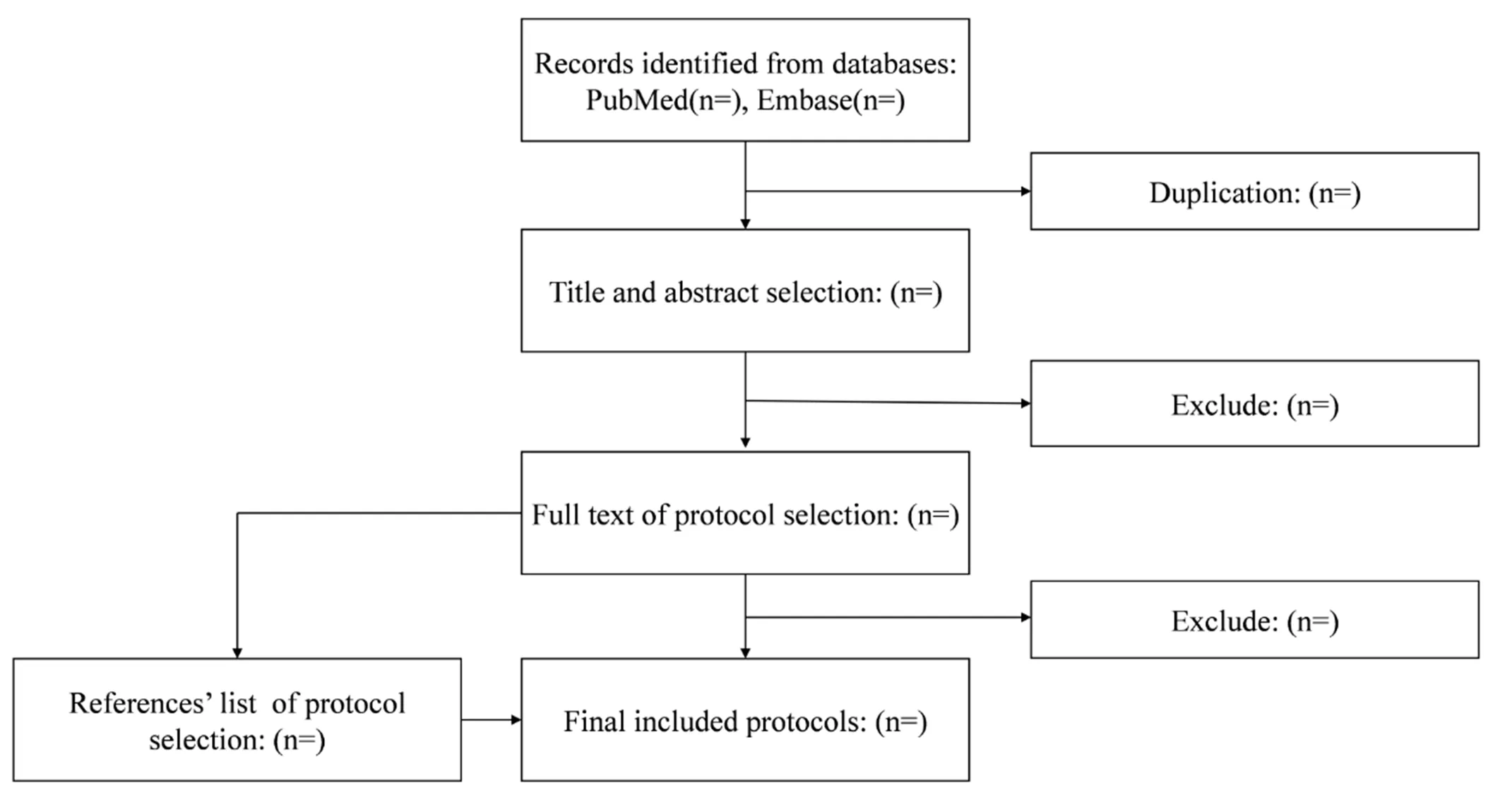
Figure 1 The flow chart of the present systematic review
Statistical analysis
The general information reported in the protocols of systematic reviews will be reported descriptively. The reporting quality of the included protocols assessed by the PRISMA-P checklist, we will report frequencies and percentages, then calculate total scores for each included protocol (Supplementary Table 1). Exploratory analyses will be conducted through univariable and multivariable linear regression analyses to explore the association between study characteristics and the reporting quality of the protocols [26]. These characteristics included, methodologist involvement (Yes vs. No), number of authors (≥ the median vs. < the median), international cooperation (Yes vs. No), PRISMA-P mentioned (Yes vs. No), registration website (International Prospective Register of Systematic Reviews vs. others, e.g., Open Science Framework website or International Platform of Registered Systematic Review and Meta-analysis Protocols), funding information (Yes vs. No + Not reported), and year of publication (before vs. after PRISMA-P 2015 published). All results will be presented using simple tables, radar charts, and forest plots. Excel 2019 (Microsoft, WA, USA) and Stata 13 (StataCorp, College Station, TX, USA) software will be used to manage and analyze the data.-values < 0.05 will be considered statistically significant.
Discussion
Osteoarthritis of the knee is a disease that is of considerable burden to healthcare systems globally. It is often the cause of knee dysfunction, pain, and other related adverse consequences, such as sleeping disorders, depression, and fatigue [1, 2]. Multiple protocols of systematic reviews and meta-analyses of interventions for knee osteoarthritis have been published [15–19], but no studies have yet been published that have evaluated the quality of reporting in these protocols. Herein, we designed the first study to systematically review the reporting quality of protocols of systematic reviews focusing on interventions for knee osteoarthritis. Additionally, we will use regression analyses to explore the factors with the potential to affect reporting quality. The results of this systematic review will reveal flaws in published protocols. These findings will be available to inform the development and reporting of protocols of systematic reviews of interventions for knee osteoarthritis, improving indirectly the quality of future systematic reviews for knee osteoarthritis. Ultimately, it will help patients with knee osteoarthritis benefit from high-quality research evidence.
However, the research is similar to other published systematic review publications, and we do acknowledge several limitations. Firstly, we will search only commonly used online databases (i.e., PubMed and Embase) and include only English-language publications. Secondly, the use of the PRISMA-P checklist to assess the reporting quality and calculation of scores for reporting quality also represents a limitation, but there are no superior tools available. Thirdly, the generalizability of the results will be limited as only published protocols will be included.
1. Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59.
2. Cui A, Li H, Wang D, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies.. 2020;29:100587.
3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017.. 2018;392(10159): 1789–1858.
4. Osani MC, Vaysbrot EE, Zhou M, et al. Duration of symptom relief and early trajectory of adverse Events for Oral Nonsteroidal Antiinflammatory drugs in knee osteoarthritis: a systematic review and meta-analysis.. 2020;72(5):641–651.
5. Si Y, Ma Y, Guo Y, et al. Efficacy and safety of Shaoyang Xibi decoction in patients with knee osteoarthritis: a multi-center, single-blind, randomized controlled trial.. 2018;38(5):733–739.
6. Helianthi DR, Simadibrata C, Srilestari A, et al. Pain reduction after laser acupuncture treatment in geriatric patients with knee osteoarthritis: a randomized controlled trial.. 2016;48(2):114–121.
7. Santoso MB, Wu L. Unicompartmental knee arthroplasty, is it superior to high tibial osteotomy in treating unicompartmental osteoarthritis? A meta-analysis and systemic review.. 2017;12(1):50.
8. Lu C, Li X, Yang K. Trends in shared decision-making studies from 2009 to 2018: a bibliometric analysis.. 2019; 7:384.
9. Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and metaanalysis and apply the results to patient care: users’ guides to the medical literature.. 2014;312(2):171–179.
10. Lu C, Lu T, Ge L, et al. Use of AMSTAR-2 in the methodological assessment of systematic reviews: protocol for a methodological study.. 2020;8(10):652.
11. Chang SM, Slutsky J. Debunking myths of protocol registration.. 2012;1:4.
12. Dos Santos MBF, Agostini BA, Bassani R, et al. Protocol registration improves reporting quality of systematic reviews in dentistry.. 2020;20(1):57.
13. Tsujimoto Y, Tsujimoto H, Kataoka Y, et al. Majority of systematic reviews published in high-impact journals neglected to register the protocols: a meta-epidemiological study.. 2017; 84:54–60.
14. Tawfik GM, Giang HTN, Ghozy S, et al. Protocol registration issues of systematic review and meta-analysis studies: a survey of global researchers.. 2020;20(1):213.
15. Zhang R, Li L, Chen B, et al. Acupotomy versus nonsteroidal anti-inflammatory drugs for knee osteoarthritis: Protocol for a systematic review and meta-analysis.. 2019;98(36): e17051.
16. Zhou X, Xiang K, Yuan X, et al. Chinese herbal medicine Wutou decoction for knee osteoarthritis: a protocol for systematic review and meta-analysis.. 2020;99(43):e22767.
17. Cheng S, Zhou J, Xu G, et al. Acupuncture and moxibustion for pain relief and quality of life improvement in patients with knee osteoarthritis: a protocol for systematic review and metaanalysis.. 2020;99(22):e20171.
18. Huang SC, Chen YF, Liu XD, et al. The efficacy and safety of opening-wedge high tibial osteotomy in treating unicompartmental knee osteoarthritis: protocol for a systematic review and meta-analysis.. 2019;98(12): e14927.
19. Goh EL, Lou WCN, Chidambaram S, et al. Joint distraction for knee osteoarthritis: protocol for a systematic review and meta-analysis.. 2018;7(1):162.
20. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation.. 2015;350: g7647.
21. Rainkie DC, Abedini ZS, Abdelkader NN. Reporting and methodological quality of systematic reviews and meta-analysis with protocols in diabetes mellitus type II: a systematic review.. 2020;15(12):e0243091.
22. Leclercq V, Beaudart C, Ajamieh S, et al. Meta-analyses indexed in PsycINFO had a better completeness of reporting when they mention PRISMA.. 2019;115:46–54.
23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.. 2009;339: b2535.
24. Chapman SJ, Drake TM, Bolton WS, et al. Longitudinal analysis of reporting and quality of systematic reviews in high-impact surgical journals.. 2017;104(3):198–204.
25. Sun X, Wang D, Wang M, et al. The reporting and methodological quality of systematic reviews and meta-analyses of nursing interventions for chronic obstructive pulmonary disease - a systematic review.. 2021;8(3): 1489–1500.
26. Croitoru DO, Huang Y, Kurdina A, et al. Quality of reporting in systematic reviews published in dermatology journals.. 2020; 182(6):1469–1476.
#Authors contributed equally to this article.
Corresponding to:Cun-Cun Lu. Lanzhou University, No. 222, Tianshui Road, Chengguan District, Gansu Province, China. E-mail: cuncunlu2017@163.com.
The authors did not receive any funding for this study.
:
PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-analysis Protocols.
:
The authors declare that they have no conflict of interest.
:
Li JY, Tang LY, Tang H, et al. Reporting quality of systematic review protocols of interventions for knee osteoarthritis: a systematic review protocol.. 2021;4(3):12. doi: 10.12032/MDM2021063008.
:Shan-Shan Lin.
:11 May 2021,
04 June 2021,
:22 June 2021
? 2021 By Authors. Published by TMR Publishing Group Limited. This is an open access article under the CC-BY license (http://creativecommons.org/licenses/BY/4.0/).
- Medical Data Mining的其它文章
- Internet public opinion monitoring in public health emergencies may benefit from artificial intelligence
- Data Mining Method for Exploring the Composition Law and Therapeutic Mechanism of Chinese medicine of macroscopic
- A meta-analysis of the efficacy of traditional Chinese medicine alone in the treatment of refractory gastroesophageal reflux disease
- A cross-sectional study on traditional Chinese medicine syndromes distribution for chronic atrophic gastritis based on data mining
- Study on pharmacodynamic mechanism of compound Shuanghuanglian in prevention and treatment of pneumonia based on network pharmacology and association analysis

