Gut microbiome in allogeneic hematopoietic stem cell transplantation and specific changes associated with acute graft vs host disease
Quentin Le Bastard,Patrice Chevallier, Emmanuel Montassier
Abstract Allogeneic hematopoietic stem cell transplantation (aHSCT) is a standard validated therapy for patients suffering from malignant and nonmalignant hematological diseases. However, aHSCT procedures are limited by potentially life-threatening complications, and one of the most serious complications is acute graft-versus-host disease (GVHD). During the last decades, DNA sequencing technologies were used to investigate relationship between composition or function of the gut microbiome and disease states. Even if it remains unclear whether these microbiome alterations are causative or secondary to the presence of the disease, they may be useful for diagnosis, prevention and therapy in aHSCT recipients. Here, we summarized the most recent findings of the association between human gut microbiome changes and acute GVHD in patients receiving aHSCT.
Key Words: Gut microbiome; DNA sequencing technologies; Allogeneic hematopoietic stem cell transplantation; Transplants; Acute graft vs host disease; Biomarkers; Composition; Function
INTRODUCTION
In human health, DNA sequencing technologies, including 16 S rRNA gene-based amplicon sequence analysis and whole genome shotgun metagenomic analysis were used to discover how exogenous and intrinsic host factors influence gut microbiome composition[1 ,2 ]. In large cohorts, scientists investigated the impact of factors such as lifestyle, dietary information, anthropometrics and drugs on the gut microbiome communities[3 ,4 ]. They found that age, gender, dietary factors and intrinsic parameters were highly correlated with composition and function of the gut microbiome.They also observed that several drug categories, such as antibiotics, proton-pump,metformin, statins, and laxatives, had a strong effect on the gut microbiome. On the other hand, gut microbiome can affect the bioavailability of oral drugs[5 ]. Thus, microorganisms can impact drug absorption and metabolism, that may explain, in part,inter-individual heterogeneity in drug response and disposition[6 ].
In addition, DNA sequencing technologies were used to investigate associations between composition or function of the gut microbiome and various disease states.Studies performed cross sectional comparisons between subjects with and without disease, and reported changes in the gut microbiome of patients with inflammatory bowel diseases, colorectal cancer, diabetes, obesity and metabolic disease, or autoimmune diseases compared to controls[7 ]. Even if it remains unclear whether these microbiome alterations are causative or secondary to the presence of the disease, gut microbiome could be viewed as a diagnostic biomarker[8 ].
Allogeneic hematopoietic stem cell transplantation (aHSCT) is a standard validated therapy, which every year allows an increasing number of patients suffering from malignant and nonmalignant hematological diseases, to benefit from an HSC transplant. The donor can be related (called geno-identical if HLA matched or haploidentical if sharing only one haplotype) or unrelated (called pheno-identical if HLA matched and including cord blood). However, aHSCT procedures are limited by potentially life-threatening complications, and one of the most serious complications is acute graft-versus-host disease (GVHD). GVHD occurs when immune competent T cells in the donated tissue recognize the recipient as foreign[9 ], that induces tissue damages in target organs of the host[10 ], including skin, liver and the gut, and typically occurs 3 to 6 wk after transplantation in nearly 60 % of related donor transplants and 80 % of unrelated donor transplants[11 ]. Hahn et al[12 ] assessed acute GVHD risk factors in 1960 adults after HLA-identical sibling myeloablative transplant in 226 centers worldwide. They found that cyclophosphamide and total-body irradiation, blood cellvsbone marrow grafts in patients age 18 to 39 years, recipient age 40 and older, chronic myeloid leukemia, Karnofsky performance score less than 90 , and recipient/donor cytomegalovirus-seronegative were independent factors significantly associated with grade 2 to 4 acute GVHD[12 ].
Based on preclinical data demonstrating reduced acute GVHD severity in germ-free or antibiotic-treated mice (i.e., gut microbiome with reduced diversity and richness),the gut microbiome was suggested to play a pivotal role in the pathogenesis of acute GVHD following aHSCT[13 ]. These findings are in contradiction with the common paradigm that loss of microbiome diversity is associated with diseases[14 ]. Most recent studies, based on microbiome sequencing, however, reported that higher diversity of intestinal microbiota at the time of neutrophil engraftment was associated with lower mortality in patients undergoing allogeneic hematopoietic-cell transplantation[15 ].
Here, we summarized the most recent findings of the association between human gut microbiome changes and graftvshost disease of the intestinal tract in patients receiving aHSCT.
ALTERATIONS OF THE GUT MICROBIOME DURING aHSCT
Several studies reported the profound alteration of the gut microbiota during the aHSCT procedure, as summarized in Table 1 . Following various conditioning regimens, both myeloablative or of reduced-intensity, a recent 16 S rRNA gene-based amplicon sequence analysis that profiled 8767 fecal samples from 1362 patients undergoing aHSCT at four different centers observed a significant gut microbiota disruption characterized by loss of diversity and domination by single taxa[15 ],defined as occupation of at least 30 % of the gut microbiota by a single predominating bacterial taxon. They also identified an association between lower intestinal diversity and higher risks of transplantation-related death. Moreover, samples collected prior transplantation also showed evidence of microbiome disruption, and lower diversity before transplantation was associated with poor survival. In another study, mortality outcomes were significantly worse in patients with lower intestinal diversity, with an overall survival at 3 years of 36 % in low gut microbiome diversity patients compared to 67 % in high diversity groups. Overall, low diversity showed a strong effect on mortality after multivariate adjustment for other clinical predictors. Furthermore, in subjects with lower diversity, the gut microbiota was generally dominated by a single bacterial genus, includingEnterococcus,Streptococcus, Enterobacteriaceae (EscherichiaandKluyvera), andLactobacillus[16 ].
Tauret al[16 ] analyzed a total of 439 fecal specimens obtained from 94 patients during their transplant hospitalization and reported that over the course of aHSCT,gut microbiome diversity index decreased and remained low until the end of the observation period (Day 35 post-transplant). Patients also demonstrated significant changes in gut microbiome composition, and in most patients, the microbial composition became dominated by a single bacterial taxon:Enterococcus(40 % of the patients), Streptococcus (37 %) and the phylum Proteobacteria (13 %). Interestingly,they demonstrated that patients with enterococcal domination in the gut had a 9 -fold increased risk of Vancomycin ResistantEnterococcusbacteremia, and intestinal domination by Proteobacteria increased the risk of bacteremia with aerobic gramnegative bacilli 5 -fold[17 ]. In a recent study using metagenomic shotgun, able to achieve bacterial identification to species and strain level, Ilettet al[10 ] reported that, in addition to a significant loss of gut microbiota diversity, post-aHSCT samples were enriched inStaphylococcus,Eggerthella,Streptococcus,EnterococcusandLactobacilluscompared to pre-aHSCT samples, and several samples were dominated by a single micro-organism [Enterococcus(n= 41 of 112 ) or Streptococcus (n = 10 of 112 )]. At species level, they observed an enrichment ofEnterococcus faecium,Lactobacillus delbrieckii,Staphylococcus epidermidis, andStreptococcus thermophilusin the post-aHSCT samples compared to pre-aHSCT samples[10 ].
In patients receiving intensive chemotherapy regimen used as myeloablative conditioning treatment to prepare patients for aHSCT, gut microbiota after chemotherapy exhibited significant decrease in Firmicutes and Actinobacteria, and significant increase in Proteobacteria compared to samples collected before chemotherapy. At the genus level, gut microbiota after chemotherapy was significantly depleted inRuminococcus,Oscillospira,Blautia,Lachnospira,Roseburia,Dorea,Coprococcus,Anaerostipes,Clostridium,Collinsella,AdlercreutziaandBifidobacterium, and with significant increase inCitrobacter,Klebsiella,Enterococcus,MegasphaeraandParabacteroidescompared with samples collected before chemotherapy. In addition, functional composition assessed using PICRUSt[18 ] revealed that following conditioning regimen, patients had reduced capacity for nucleotide metabolism, energy metabolism,metabolism of cofactors and vitamins, and increased capacity for glycan metabolism,signal transduction and xenobiotics biodegradation[19 ].
Thus, overall, aHSCT procedure is associated with a loss of gut microbiota diversity,a decrease in micro-organisms associated with health-promoting effects[20 ], and with a significant increase or domination by potentially pathobionts (Figure 1 ).
ALTERATIONS OF THE DIVERSITY OF THE GUT MICROBIOME AND ACUTE GVHD
Studies reported that decreased gut microbiota diversity and richness was associated with the onset of acute intestinal GVHD. Jenqet al[21 ], in 2015 , reported in a cohort of 115 patients receiving aHSCT, that increased bacterial diversity was associated with reduced GVHD-related mortality[21 ]. Moreover, in their recent 16 S rRNA gene-based amplicon sequence analysis, Peledet al[15 ] found that higher intestinal diversity wasassociated with decreased risk of deaths attributable to GVHD (17 GVHD-related deaths among 244 patients in the higher-diversity group vs 26 such deaths among 184 patients in the lower-diversity group; hazard ratio, 0 .49 ; 95 %CI: 0 .26 -0 .90 )[15 ].
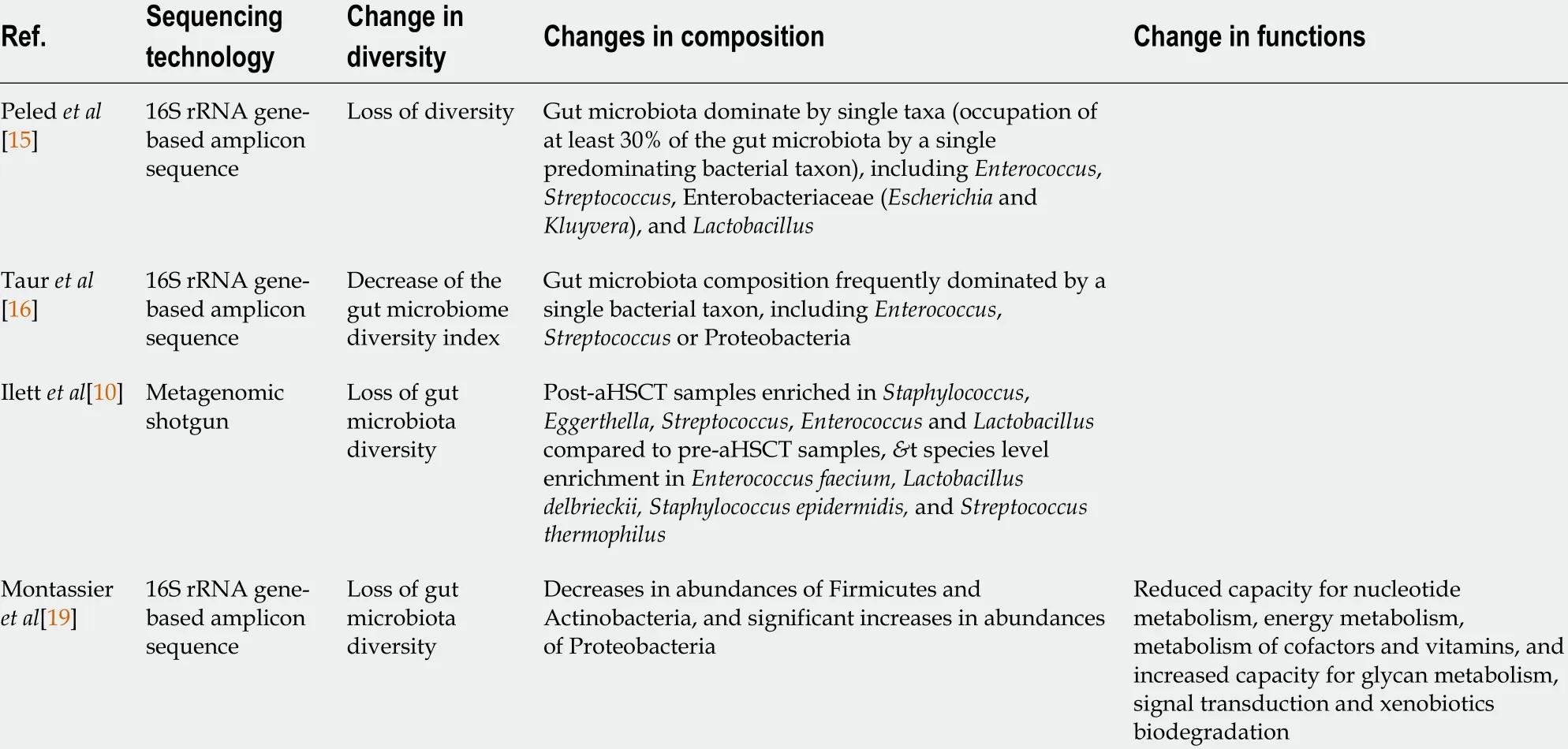
Table 1 Gut microbiome diversity, composition and function changes in gut microbiota during allogeneic hematopoietic stem cell transplantation procedure
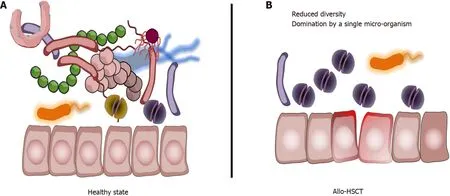
Figure 1 Gut microbiota changes during the allogeneic hematopoietic stem cell transplantation procedure. A: Healthy state with diverse and rich gut microbiota; B: Following conditioning regimen, gut microbiome alterations are marked by a drastic loss of diversity, a significant decrease in micro-organisms associated with health-promoting effects, and with a significant increase or domination, defined as occupation of at least 30 % of the microbiota by a single predominating bacterial taxon, by potentially pathobionts. Allo-HSCT: Allogeneic hematopoietic stem cell transplantation.
In a cohort of 44 patients, in which 16 (36 %) experienced acute intestinal GVHD(median time to diagnosis: 53 d), Galloway-Pe?a et al[22 ] found that lower Shannon diversity index, popular diversity index in the ecological literature, of fecal samples collected at the time of engraftment was significantly associated with increased incidence of acute GVHD[22 ]. Golob et al[23 ] also found that diversity was statistically significantly lower in patients with acute GVHD when compared to those with no acute GVHD[23 ]. In another cohort of 57 patients, Liu et al[24 ] reported that decreased gut microbiota recipients’ diversity was not associated with decreased risk of aGVHD.However, they found that high gut microbiota donor diversity was associated with decreased risk of acute GVHD[24 ]. In a cohort of 70 patients, Payen et al[25 ] reported that patients with severe aGVHD had reduced gut microbiota diversity at disease onset, whereas patients with mild aGVHD had gut microbiota diversity more similar to those of controls[25 ].
Ilettet al[10 ] applied shotgun metagenomic sequencing to study a large cohort of adults (n= 150 ) undergoing aHSCT. Among them, 36 developed acute GVHD (median time to development: 34 d (interquartile range, 26 -50 d post-aHSCT). They did not find significant association between diversity measures and acute GVHD in samples collected from the pre-aHSCT period. However, in samples collected in the early postaHSCT period, patients who later developed acute GVHD had a significantly lower gene richness compared with those who did not develop acute GVHD[10 ].
Altogether, these findings clearly associate decreased diversity and richness of the gut microbiota, known to be associated with enhanced inflammation and impaired immunity[26 ], to onset of acute GVHD (Table 2 ).
COMPOSITION CHANGES IN GUT MICROBIOME AND ACUTE GVHD
Using a 16 S rRNA-based sequencing analysis, Jenq et al[21 ] reported that genusBlautiawere most significantly associated with reduced GVHD-related mortality, whereas genusVeillonella, was associated with increased GVHD-related mortality[21 ].
Holleret al[27 ] reported that post-transplant samples were increased in enterococci,and that the increase was more pronounced in patients developing acute GVHD compared to patients who did not develop aGVDH. They confirmed this trend using enterococcal polymerase chain reaction (PCR) and observed a predominance ofEnterococcus faeciumandEnterococcus faecalisin samples collected in patients who developed acute GVHD. Moreover, post-transplant samples collected in patients with acute GVHD were significantly depleted in Clostridia andEubacterium rectale[27 ]. Stein-Thoeringeret al[28 ] also reported that fecal domination byEnterococcus(i.e., relative genus abundance ≥ 30 % in samples collected during early post-transplant period) was associated with increased risk of acute GVHD, and increased GVHD-related mortality[28 ]. Importantly, in a in gnotobiotic model, the authors demonstrated thatEnterococcusgrowth is dependent on disaccharide lactose, and that dietary lactose depletion attenuatesEnterococcusoutgrowth and reduces the severity of GVHD[28 ].
In their cohort of 44 patients, Galloway-Pe?a et al[22 ] reported that only one taxon at the time of engraftment,Coriobacteriia, a class of Gram-positive bacteria within the Actinobacteria phylum, was negatively correlated with the incidence of aGVHD[22 ].In another cohort of 107 patients, Doki et al[29 ] reported patients who developed acute GVHD exhibited a significantly higher abundance of phylum Firmicutes than patients who did not develop aGVHD[29 ].
Golobet al[23 ] found in a cohort of 66 patients, that in samples collected at neutrophil recovery post-HCT, the presence of Actinobacteria and Firmicutes was positively correlated with subsequent acute GVHD, whereas Lachnospiraceae were negatively correlated. In detail,Butyricicoccus,Bacteroides luti,Bacteroides thetaiotaomicron,Bacteroides ovatus, andBacteroides caccaewere negatively correlated with subsequent acute severe GVHD, whileRothia mucilaginosa,Solobacterium moorei,Veillonella parvula, andBacteroides doreiwere positively correlated[23 ]. Payen et al[25 ]reported thatLachnoclostridium,Blautia,Sellimonas,Anaerostipes,Faecalibacterium,Flavonifractor,ErysipelatoclostridiumandLactococcuswere negatively associated with subsequent acute severe GVHD, whereasPrevotellaandStenotrophomonaswere considered positive biomarkers of severe aGVHD. Moreover, using qPCR, they observed a significant depletion of theBlautia coccoidesgroup (cluster XIVa) in patients with aGVHD compared with controls and patients with no aGVHD[25 ].
Based on a shotgun metagenomic sequencing analysis in 150 patients, Ilett et al[10 ]found that no bacteria were associated with acute GVHD in samples collected during the pre-aHSCT period. In samples collected during the early post-aHSCT period, they found thatBlautia,Akkermansia, andCampylobacter, as well as the specific speciesAkkermansia muciniphila,Blautia obeum,Blautia hydrogenotropica, andBlautia hanseniiwere all significantly associated with reduced risk of a GVHD[10 ]. Still using shotgun metagenomic sequencing analysis, Turner assessed samples collected in nine patients with aGVHD and treated with standard-of-care high-dose steroids. Three of these patients were steroid-refractory, whereas six had a response. They showed thatDorea longicatenawas associated with response to high-dose steroids treatment whereasAkkermansia muciniphilawas associated with refractoriness. They also reported that maintenance of a stableDorea/Akkermansiaratio predicted steroid response, whereas adecline in this ratio preceded refractory disease. Importantly, Shonoet al[30 ] previously demonstrated in a mouse model an increased inAkkermansia muciniphila, a commensal bacterium with mucus-degrading capabilities, raising the possibility that mucus degradation may contribute to murine GVHD[30 ].
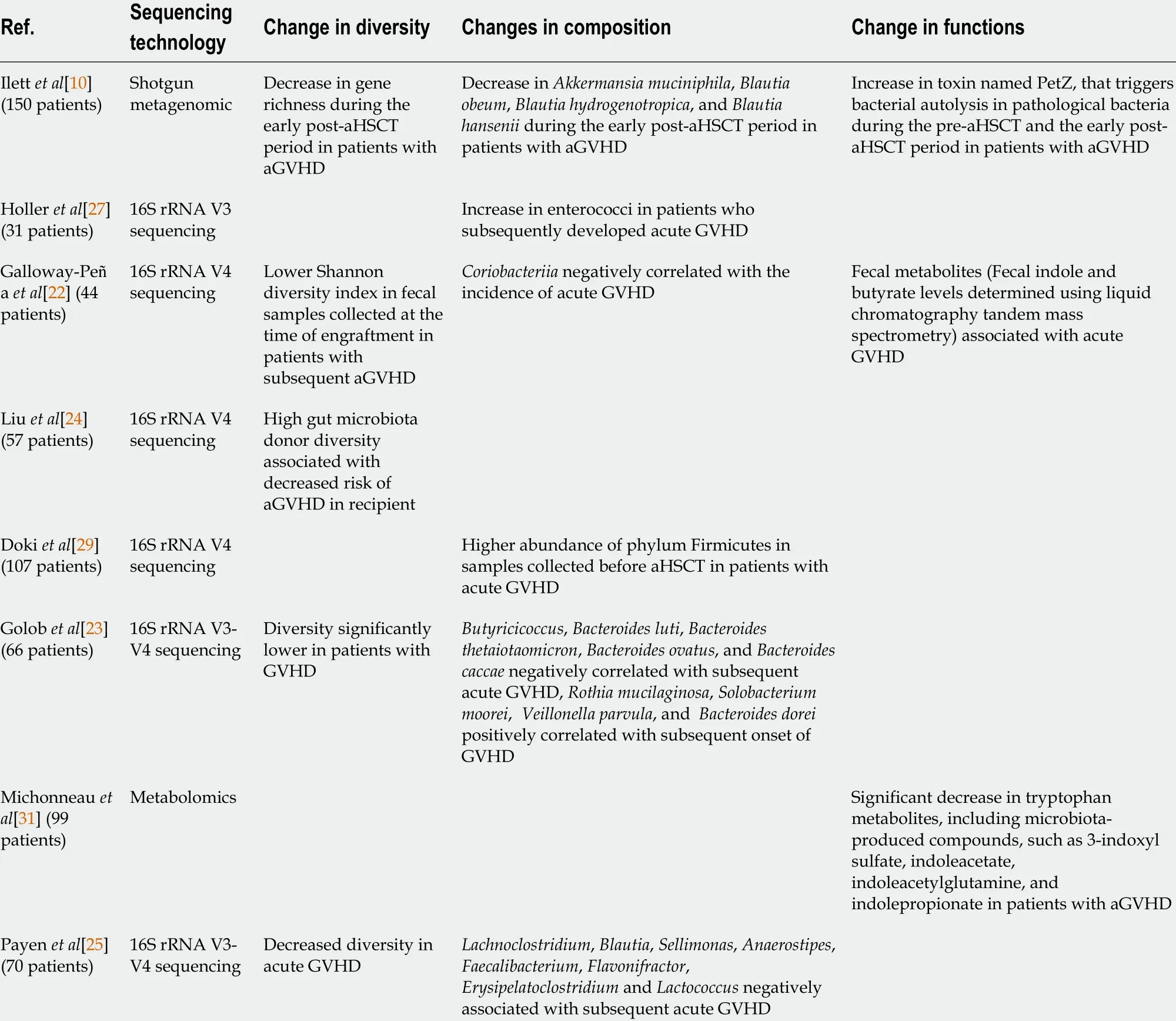
Table 2 Gut microbiome diversity, composition and function changes in patients receiving allogeneic hematopoietic stem cell transplantation procedure and developing acute graft versus host disease
Further studies are needed to confirm or not the controversial role ofAkkermansiain acute GVHD. Moreover, some species of the genusBlautiashould be investigated as a potential biomarker: High relative abundance at the time of engraftment being protective against GVHD, while low relative abundance could be considered a risk factor for secondary development of GVHD.
FUNCTION CHANGES IN GUT MICROBIOME AND ACUTE GVHD
The functional alterations of the intestinal microbiome in patients receiving aHSCT are currently poorly described because the majority of studies have used 16 S ribosomal RNA gene-based technics, which boil down to providing bacterial taxonomy only at the genus level, but are ineffective in obtaining functional information. In a cohort of 44 patients, Galloway-Pe?a et al[22 ] reported that fecal metabolites (fecal indole and butyrate levels) were not associated with aGVHD[22 ]. In another study, Michonneauet al[31 ] reported that aGVHD was characterized by specific metabolomics changes in two cohorts of patients (n= 99 ). They found that bile acids, plasmalogens, tryptophan,and arginine metabolites were the main contributors involved, especially the aryl hydrocarbon receptor ligand 3 -indoxyl sulfate. In addition to host-derived metabolites,they also identified significant variation in microbiota-derived indole compounds,especially in aryl hydrocarbon receptor ligands. The authors suggested that allogeneic immune response during aGVHD might be influenced by bile acids and by the decreased production of aryl hydrocarbon receptor ligands by gut microbiome that could limit indoleamine 2 ,3 -dioxygenase induction and influence allogeneic T cell reactivity[31 ]. To assess if altered composition of the gut microbiota may result in an altered metabolome, which potentially disrupts functionalities at the onset of aGVHD,Payenet al[25 ] quantified the Short Chain Fatty Acids (SCFA) content in fecal samples with measurement of total SCFAs and acetate, propionate, and butyrate. They found that the fecal amount of all SCFAs was drastically diminished at aGVHD onset. In detail, they observed that total SCFAs, acetate and butyrate respectively decreased by 80 %, 75 % and 95 % in severe aGVHD patients as compared with controls[25 ].Importantly, in a mouse model, Mathewsonet al[32 ] demonstrated that butyrate restoration improved intestinal epithelial cells junctional integrity and mitigated aGVHD[32 ].
Based on a shotgun metagenomic sequencing analysis, Ilettet al[10 ] found that a total of 1267 and 1289 genes were present in significantly different amounts among those who developed aGVHDvsthose who did not develop aGVHD during pre-HSCT and early post-aHSCT, respectively. Of these genes, 24 overlapped between the 2 time periods, all being significantly higher in abundance among those who did not develop aGVHD. They pointed out that 1 gene (O2 .CD1 -0 -PT_GL0039283 ) had a 3 -log foldchange in both periods and is known to function as a toxin named Petz, that triggers bacterial autolysis in pathological bacteria[10 ].
CONCLUSION
The investigations described in this mini review provide an understanding of the role of the gut microbiome in the pathophysiology of aGVHD in patients receiving aHSCT.Observational studies have shown that a decrease in diversity of the gut microbiome and specific species or metabolic pathways were associated with aGVHD. These specific changes could serve as biomarkers for diagnosis and prevention in patients receiving aHSCT. Moreover, functional alterations of the gut microbiome during aHSCT should be more investigated so that modulations of the gut microbiome could be tested to prevent this potentially life-threatening complication.
 World Journal of Gastroenterology2021年45期
World Journal of Gastroenterology2021年45期
- World Journal of Gastroenterology的其它文章
- Diagnostic biomarkers for pancreatic cancer: An update
- Therapeutic potentials of fasudil in liver fibrosis
- Clinical presentation of gastric Burkitt lymphoma presenting with paraplegia and acute pancreatitis: A case report
- In-hospital mortality of hepatorenal syndrome in the United States: Nationwide inpatient sample
- Multimodality management of gallbladder cancer can lead to a better outcome: Experience from a tertiary care oncology centre in North India
- MicroRNAs expression influence in ulcerative colitis and Crohn's disease: A pilot study for the identification of diagnostic biomarkers
