MicroRNAs expression influence in ulcerative colitis and Crohn's disease: A pilot study for the identification of diagnostic biomarkers
Ana Elisa Valencise Quaglio, Felipe Jose Santaella, Maria Aparecida Marchesan Rodrigues, Ligia Yukie Sassaki,Luiz Claudio Di Stasi
Abstract
Key Words: Crohn’s disease; Ulcerative colitis; Inflammatory bowel disease; miRNA;Differential diagnosis; Biomarker
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC), the two main phenotypes of inflammatory bowel disease (IBD), are chronic, relapsing inflammatory disorders of the gastrointestinal (GI) tract whose etiologies remain unknown[1 ,2 ]. Although its pathophysiological mechanisms are unclear, IBD has been linked to an inappropriate and exacerbated immune response to commensal bacteria in genetically susceptible hosts, as well as with environmental factors, defects of the epithelial barrier, and dysregulation of the innate and adaptive immune responses at the level of the intestinal mucosa[1 ,2 ].
IBD affects 6 to 8 million people worldwide, with a prevalence of 84 .3 people (79 .2 -89 .9 ) per 100000 in 2017 and a mortality rate of 0 .51 [3 ]. The incremental increase in the incidence and prevalence of IBD globally is indicative of the need for further population-based genetic studies of those affected by these diseases. Differences in miRNA expression between these two diseases could lead to the identification and validation of diagnostic biomarkers, facilitating differential diagnosis and improving the understanding of IBD pathogenesis[4 ].
MiRNAs are a class of small (18 -25 nucleotides in length), endogenous, non-coding,single-stranded RNA molecules that can negatively regulate target gene expression at the post-transcriptional level through binding to the 3 ’untranslated regions of the target mRNAs and promoting mRNA degradation or translational repression[4 ].Overall, there is evidence that miRNAs contribute to the regulation of at least onethird of all protein-coding mRNAs, including those involved in the development,metabolism and cell cycle control[5 ]. Although studies have shown that miRNA plays an important role in both CD and UC and that they are differentially expressed in these disease states, there may have been potential confounding factors that were not considered. For example, the patients in these studies were treated with various medication classes, such as immunosuppressants, corticosteroids, and/or aminosalicylates[5 -8 ]. Therefore, the aim of the present study was to investigate miRNA expression patterns in the tissues of treatment-na?ve CD and UC patients to assess changes in miRNAs without the confounding influence of pharmacological agents.
MATERIALS AND METHODS
Ethics statement
The study was approved by the Botucatu Medical School Research Ethics Committee(protocol No. 71379417 .2 .0000 .5411 ).
Sample collection
A total of twenty formalin-fixed paraffin-embedded (FFPE) colonic samples were collected from patients in the Pathology Department of Botucatu Medical School at S?o Paulo State University (Unesp). IBD diagnosis was based on clinical, endoscopic,radiologic, and histological criteria[9 ]. After sample selection, the diagnoses of UC or CD were confirmed by histopathological analysis of the biopsied colonic samples collected from each patient during a diagnostic colonoscopy; therefore, none of the patients received any medication at the time of the examination.
RNA isolation, reverse transcription, pre-amplification, and qRT-PCR analyses
Total RNA from each colon biopsy was isolated using MagMAX? FFPE DNA/RNA Ultra Kit (Thermo Fisher Scientific, Massachusetts, EUA). For reverse transcription,Megaplex? Reverse Transcription Primer Pools and Taqman miRNA reverse transcription kits (Thermo Fisher Scientific) were used. Briefly, 3 μL of RNA (1 -350 ng) were reverse-transcribed by combining the Megaplex RT Primer (Thermo Fisher Scientific)with the TaqMan?miRNA Reverse Transcription Kit (Thermo Fisher Scientific). A volume of 2 .5 μL of the reverse transcription product was used for pre-amplification with Megaplex? PreAmp Primers (Thermo Fisher Scientific) and TaqMan?PreAmp Master Mix (Thermo Fisher Scientific). For qRT-PCR, 9 μL of the pre-amplified product was mixed with 441 μL of H2 O and 450 μL of TaqMan?Universal PCR Master Mix II without uracil-N-glycosylase (Thermo Fisher Scientific). Subsequently, 100 μL of each sample was loaded into each fill reservoir of the TaqMan microfluidic array cards.Real-time-PCR analysis was performed using a QuantStudio? Real-Time PCR System(with TaqMan?Array Block) (Thermo Fisher Scientific) with universal cycling conditions [95 °C/10 min, then (95 °C/15 s, 60 °C/60 s) for 40 cycles]. The TaqMan miRNA array output data (.sds files) were uploaded to the ThermoFisher Cloud App (https://www.thermofisher.com/mysso/LoginDisplay) and analyzedviathe relative quantification (ΔΔCt) method using defined threshold settings for each miRNA. The geometric means of the threshold cycle (Ct) values of three small nucleolar RNAs(snRNAs), RNU44 , RNU48 , and U6 , were used as the endogenous controls. Briefly, the change in quantification cycle (ΔCq) value was calculated as:Cq (miRNA of interest) - mean Cq (endogenous control).The ΔΔCq was subsequently calculated as:
ΔCq (miRNA of interest) - mean of ΔCq (miRNA of interest in the reference group -CD).
The relative quantification (Rq or gene expression fold-change) was calculated as 2-(ΔΔCq). Subsequently, the FC was calculated using the Rq and the FC values, andPvalues were log2 - and log10 -transformed, respectively, and plotted as a volcano plot,displaying the -log 10 (P value) vs the log2 (FC) for each target in the UC group relative to that in the CD group. The volcano plot was generated using an online server (https://paolo.shinyapps.io/ShinyVolcanoPlot/) after defining the statistical cutoffs as a log2 fold-change > 1 and a P value < 0 .05 . A heat map was created using a web tool (http://www.heatmapper.ca/expression/).
Target prediction and gene enrichment analysis
The targets of the DE miRNAs were predicted using miRNA Data Integration Portal(mirDIP) (http://ophid.utoronto.ca/mirDIP/index.jsp)[10 ,11 ] and the miRNA Target Interaction database (MirTarBase) (http://miRTarBase.mbc.nctu.edu.tw/)[12 ]. The combined gene list of each miRNA was uploaded to the Enrichr database (http://amp.pharm.mssm.edu/Enrichr/), a web server for comprehensive gene set enrichment analysis[13 ,14 ]. Duplicate susceptibility genes were excluded prior to analysis. The CD and UC susceptibility genes were identified from the National Human Genome Research Institute-European Bioinformatics Institute (NHGRI-EBI)Catalog of human genome-wide association studies (GWAS)[15 ].
Statistical analysis
The statistical methods of this study were reviewed by Ana E. V. Quaglio from the Laboratory of Phytomedicines, Pharmacology, and Biotechnology (PhytoPharmaTec),Department of Biophysics and Pharmacology, S?o Paulo State University (Unesp). All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA, United States). To determine if data is from a Gaussian distribution were performed D’Agostino-Person normality test. Parametric and non-parametric data were analyzed using unpaired and two-tailedt-tests and Mann-WhitneyUtests,respectively. Statistical significance was considered forP≤ 0 .05 .
RESULTS
This study included 20 patients with IBD (10 with UC and 10 with CD), with a mean age at the time of diagnosis of 36 .1 and 31 .6 years for those with UC and CD,respectively. In terms of sex, 40 % of the UC and 30 % of the CD patients were male,and 60 % and 70 % of the UC and CD patients were female, respectively. Other characteristics of the patients are presented in Table 1 .
In this study, 377 miRNAs and four controls were analyzed in each plate, totalizing 754 miRNAs of interest. Of these, 643 miRNAs were expressed in at least five patients diagnosed with UC or CD, whereas 111 miRNAs were not considered to be expressed in these patients. The expression levels of 27 miRNAs significantly differed between the CD and UC patients. Relative to the expression levels in CD patients, the five miRNAs that were downregulated in UC patients with a fold-change greater than 1 were selected for the subsequent enrichment analysis: miR-192 -3 p/5 p, miR-378 a-3 p/5 p, and miR-429 (Figure 1 ).
Target prediction and gene enrichment analysis of miRNAs
To assess the potential functions of miRNAs in UC or CD, the targets of the differentially expressed miRNAs (Supplementary Table 1 ) were predicted using an online database. Relative to the expression levels in CD patients, the miRNAs that were decreased in UC patients were those that were enriched in signaling pathways, such as forkhead box protein O (FOXO), transforming growth factor-beta (TGF-β), and mitogen-activated protein kinase (MAPK), as well as in pathways associated with cancer, particularly colorectal cancer (CRC) (Table 2 ).
Several of the miRNA gene targets have been previously flagged as CD or UC susceptibility genes. Using the IBD susceptibility gene list from the 2019 GWAS[17 ],the miRNA targets that had already been linked to one of the two diseases were identified. There are 619 and 474 genes that have been proposed as CD and UC susceptibility genes, respectively. Of the predicted targets of the miRNAs that were downregulated, 24 and 11 overlapped with the proposed CD and UC susceptibility genes, respectively. A total of 328 genes were common to both diseases, and 54 were common to the two diseases and the predicted targets (Figure 2 and Supplementary Table 2 ).
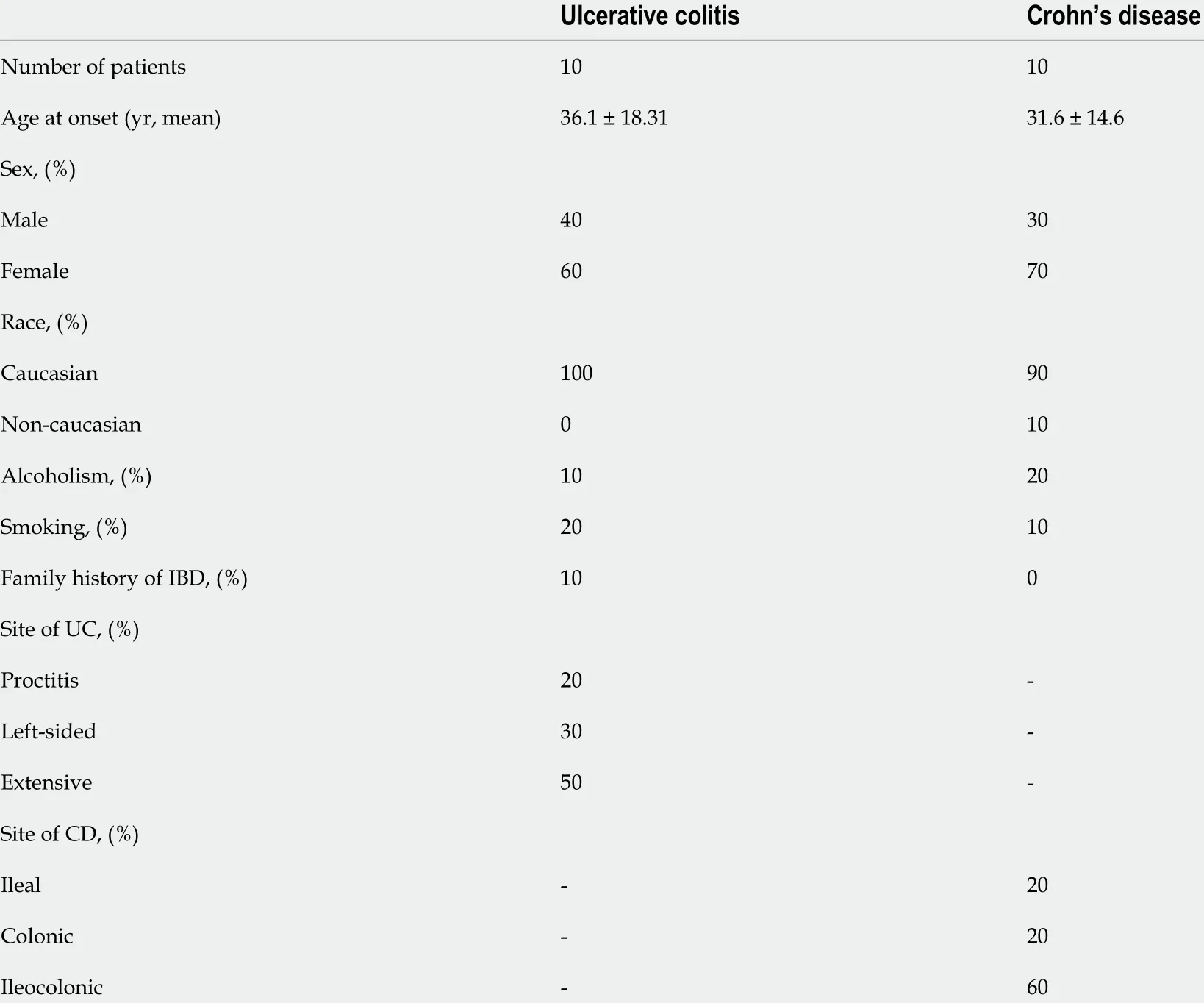
Table 1 Demographic characteristics and clinical features of inflammatory bowel disease patients
DISCUSSION
MiRNAs are short single-stranded, post-transcriptional regulatory RNAs that are involved in major cellular processes. The mature miRNA species may be derived from either the 5 ’ or 3 ’ arms of the precursor duplex and are referred to as the miRNA-5 p and -3 p species, respectively. Initially, it was believed that only one of these strands was functional and that the other strand, known as miRNA*, was destined for degradation[16 ]. However, recent reports have indicated that both the miRNA and miRNA* species often co-exist, and both are functional[16 ,17 ]. Based on this finding,the miRNA-5 p and -3 p nomenclature are used solely based on the 5 ’- or 3 ’-arm derivation of the miRNA species[16 ]. This study described, for the first time, the coexistence of miR-192 -3 p and miR-192 -5 p and, miR-378 a-3 p and miR-378 a-5 p in the colonic samples of IBD patients. In addition to miR-429 , the expression levels of both miRNAs were decreased in UC compared with CD patients.
Most of the altered pathways identified following the enrichment analysis are related to cancer processes, alterations in the extracellular matrix (ECM), inflammatory mediators like TGF-β, members of the heat shock protein (HSP) family, and MAPKs.The decrease in the expression of miRNAs related to all these pathways leads to dysregulation. Proteoglycans are important components of the ECM that have multiple functions depending on both their protein and carbohydrate constituents[18 ].Heparan sulphate proteoglycans on the cell membrane may play diverse roles in cancer-related processes, acting as either inhibitors or promoters of tumor progression depending on the tumor type and stage of progression[19 ]. In CRC, for example, the cell surface proteoglycan syndecan-2 (SDC2 gene) is upregulated, leading to increased cell migration[20 ]. Moreover, high SDC2 expression was shown to be related to tumorigenic behaviors mediated through the regulation of cell adhesion, proliferation,and migration[21 ]. Recently, chronic inflammatory hypoxia-mediated SD2 expression was reported to be correlated with CRC development[21 ]. In addition, acute inflammation could also induce SDC2 expression predominantly in the proximal colon,indicating its potential as a biomarker for acute colonic inflammation[22 ].
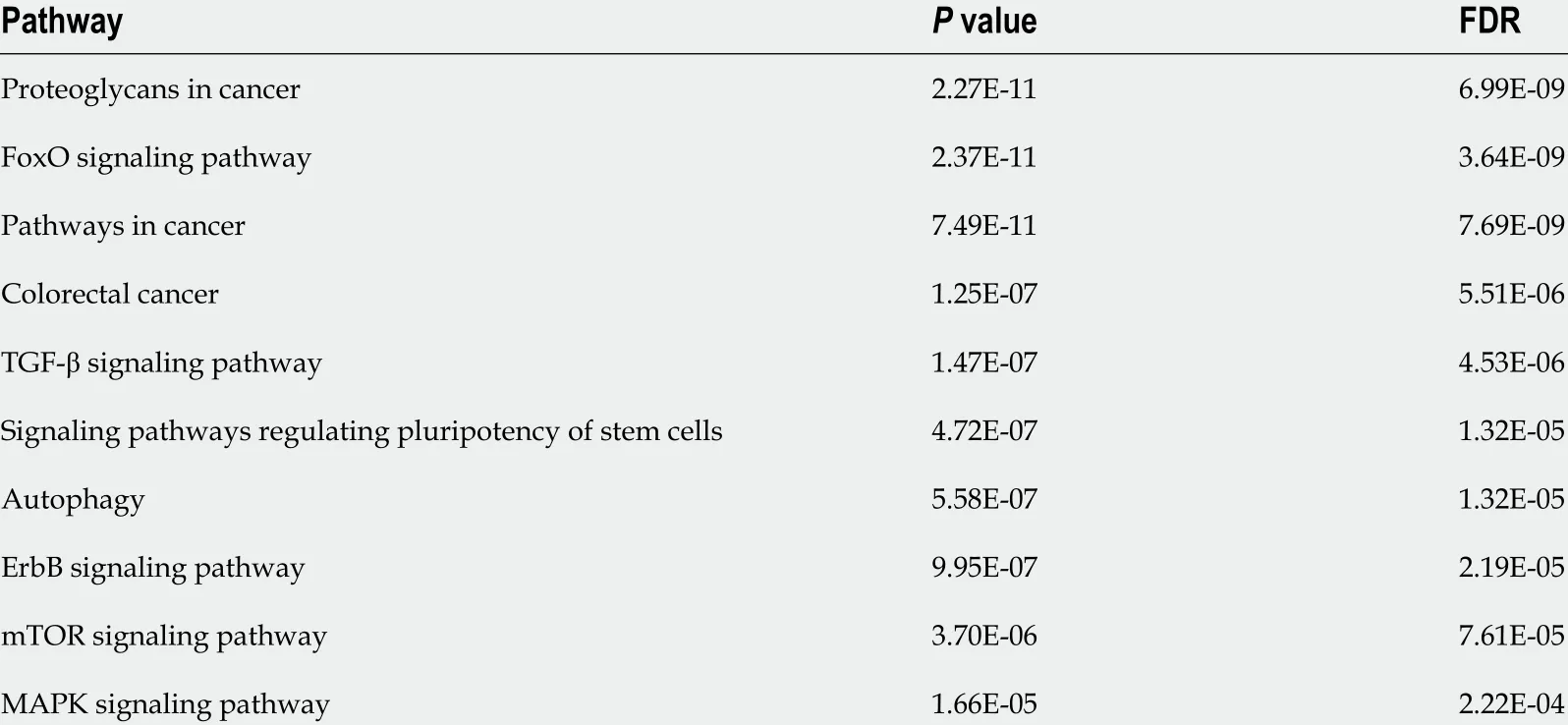
Table 2 Gene enrichment analysis of decreased microRNAs in ulcerative colitis patients compared with Crohn’s disease patients
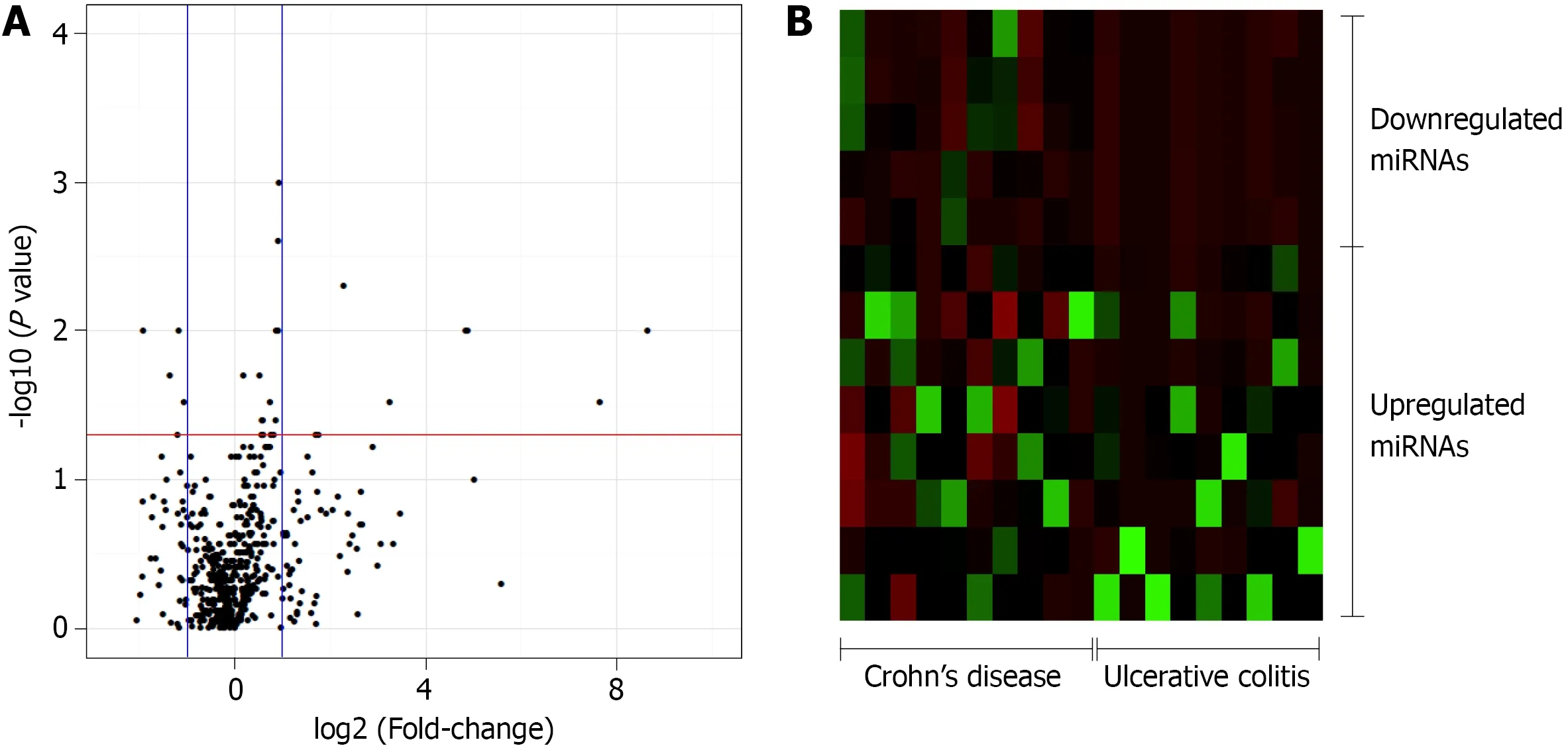
Figure 1 Volcano plot and heat map of the patients. A: Volcano plot of the 754 -microRNA (miRNA) analyzed in inflammatory bowel disease patients.Control group: Crohn’s disease (CD). Threshold x: fold change = 1 ; Threshold y: P ≤ 0 .05 ; B: Heat map of the 13 significantly different miRNA between the CD and ulcerative colitis patients with a fold-change in expression level greater than 1 relatively to the expression levels in CD patients (8 upregulated and 5 downregulated).miRNA: MicroRNA.
In the present study, SDC2 was predicted to be regulated by miR-429 , which was proven to be related to cancer progression and metastasis, likely due to dysregulated SDC2 expression, increasing cell migration and proliferation. It has been reported that miR-429 becomes downregulated in esophageal squamous carcinoma cells, and its expression could predict poor prognosis for patients[23 ]. Moreover, they showed that miR-429 inhibited cellular proliferation through the nuclear factor kappa B (NF-κB)pathway and inhibited cell migration-mediated epithelial-mesenchymal transition(EMT) processes[23 ].
In nasopharyngeal carcinoma, miR-429 functions as a tumor suppressor by downregulating talin-1 (TLN1 ), a protein that enhances the migration and invasion of various carcinomas[24 ]. This miRNA is also related to an antimetastatic function, as it can regulate the metastasis of hepatocellular carcinoma by directly targeting Crk-like protein, which is involved in processes related to EMT, as well as the progression,development, invasion, metastasis, and apoptosis of a variety of cancers[25 ]. Most functional studies have reported that miR-429 plays an oncogenic role in CRC, and its expression is downregulated as the disease progresses[26 ,27 ]. In studies involving a dextran sodium sulphate-induced model of intestinal inflammation, miR-429 was downregulated[28 ], corroborating the findings of the present study. Others have reported that miR-429 modulates mucin secretion in human CRC cells and mouse colon tissuesviaupregulation of myristoylated alanine-rich protein kinase C substrate expression confirming that miR-429 is a candidate for intestinal anti-inflammatory therapy in human UC[28 ].
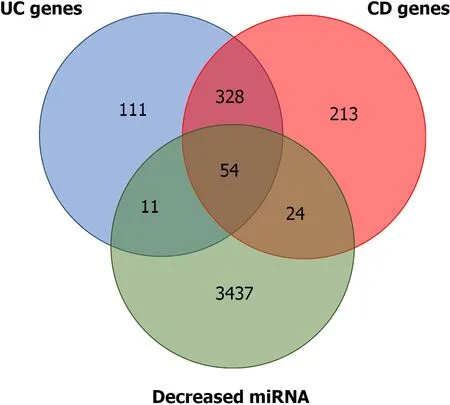
Figure 2 Overlap between predicted microRNA targets for microRNA differentially expressed and ulcerative colitis and Crohn’s disease susceptibility genes. Detailed information is provided in Supplementary material 1 . miRNA: MicroRNA; UC: Ulcerative colitis; CD: Crohn’s disease.
Another family of proteins with an important correlation with miRNA function is the HSP family. HSPs comprise several classes of constitutively active and/or stressinduced molecular chaperones that assist in proper polypeptide folding, the refolding of denatured proteins, protein transport, and stabilization of native protein structures,and numerous HSPs are overexpressed in inflamed tissues[29 ]. A recent review by Hoter & Naim (2019 )[30 ] that analyzed the roles of HSPs in IBD found accumulating evidence linking the upregulation of HSP90 and/or HSP70 expression to the pathogenesis of IBD in the intestinal mucosa of patients with UC at the time of diagnosis, with the expression levels decreasing following the initiation of pharmacological therapy. Similar results were reported in a pre-clinical model of intestinal inflammation in which the colonic expression of HSP70 increased following the induction of intestinal inflammation[31 ]. Several authors have reported that HSPs are subject to post-transcriptional regulation by miRNAs[32 ]. The selected downregulated miRNAs found in the UC patients in the present study are predicted to regulate several members of the HSP70 family, such as HSPA1 B and HSPA2 , as well as HSP90 B1 , a member of the HSP90 family that plays critical roles in the folding of proteins such as Toll-like receptors and integrins[33 ], suggesting that the decrease in these miRNAs may have been related to the increased levels of the altered HSPs observed in the UC patients. Dysregulation on HSP70 /90 Levels has also been related to intestinal inflammation associated with CRC. For example, Hoter & Naim (2019 )[30 ]found that HSP90 was highly expressed and was implicated in the progression from UC to UC-associated CRC. Furthermore, HSP90 inhibition has been actively investigated as a treatment for gastrointestinal and CRC arising from IBD progression.
MiR-378 a-3 p/5 p, two of the miRNAs correlated with HSP downregulation, have also been shown to be altered during intestinal inflammation[34 ]. The involvement of miR-378 in IBD patients has also been reported[35 -37 ], indicating an upregulation in UC or a downregulation in CD. These differences may have resulted from the small number of patients included in these previous studies or the use of pharmacological agents by the participants, which could reflect treatment rather than disease effects.The patients in the present study were treatment na?ve; therefore, the differences in expression resulted from the disease itself. A recent study conducted by Dubois-Camachoet al[38 ] in 2019 , corroborates the present findings, as they showed that a decrease in miR-378 a expression in UC patients was associated with higher levels of interleukin 33 and tumor necrosis factor-alpha (TNF-α), and that miR-378 a expression levels increased following treatment with an anti-TNF-α monoclonal antibody[38 ].
In CRC, the overexpression of miR-378 inhibits the proliferation of colon cancer cellsin vitroby inducing apoptosis and preventing migration and invasion[39 ]. For example, miR-378 a also alleviated the malignant phenotypes of colon cancer cells by inhibiting the Wnt/β-catenin signaling pathway[39 ]. In addition, CRC patients with low miR-378 a expression experienced a shorter survival time than those with high miR-378 a expression, indicating that miR-378 a may serve as an important diagnostic biomarker[40 ]. Collectively, it is possible to assume that the decrease in miR-378 a-3 p/5 p levels during inflammation and the later progression to CRC can increase HSP90 Levels. Thus, increased HSP90 and decreased miR-378 a-3 p/5 p levels could act as important biomarkers and potential targets of pharmacological interventions for UC and UC-related CRC.
Among the downregulated miRNAs observed in IBD patients, miR-192 -3 p/5 p,which was first cloned in 2003 [41 ], is a tumor-related miRNA, as its dysregulation has been reported in several types of cancer[42 ]. Overexpression of miR-192 has been shown to induce apoptosis in bladder cancer cells and arrest breast cancer cells'growth[42 ,43 ]. Moreover, in CRC, miR-192 regulates the enzyme dihydrofolate reductase and cellular proliferation through the p53 tumor suppressor network,decreasing cancer progression and metastases[42 ,43 ]. Decreased levels of miR-192 are also related to IBD. For example, Wuet al[6 ] reported a link between chronic IBD and the altered expression of certain miRNAs, demonstrating that miR-192 was downregulated in the colonic epithelial cells of active UC patients which corroborates the findings of the present study.
An inverse correlation exists between the expression of miR-192 and MPI-2 α, an epithelial cell-expressed chemokine previously implicated in IBD[6 ]. In addition, miR-192 was shown to be induced by TGF-β, suggesting that miR-192 plays a key role in inflammatory, fibrotic processes[6 ]. Moreover, other putative miR-192 targets identified in the enrichment analysis include mediators of inflammation and fibrosis,such as nucleotide-binding oligomerization domain-containing protein 2 , TNF receptor-associated factor-interacting protein 3 , 4 , and 5 , as well as matrix metalloproteinase 16 and 20 (Supplementary material 1 ). Changes in miR-192 expression have also been shown in a 2 ,4 ,6 -trinitrobenzenesulfonic acid-induced intestinal inflammation model[44 ]. In that elegant study, the EMT was activated as a result of intestinal inflammation, along with a simultaneous increase in the early growth response protein 1 and fibroblast growth factor 2 expression levels (based on mesenchymal markers); on the other hand, miR-192 expression was decreased[44 ].
Conversely, elevated levels of miR-192 are associated with tumor suppression and cell proliferation[45 -49 ]. Ji et al[45 ] demonstrated that miR-192 suppresses the growth of bladder cancer cellsviatargeting Yin Yang 1 , a transcription factor that plays an important role in regulating development and cell proliferation. Similarly, Flammanget al[48 ], in a study involving pancreatic ductal adenocarcinoma, described miR-192 as a marker with prognostic value, as increased miRNA expression exerted a suppressive effect. In CRC, miR-192 also acts as a tumor suppressor, inhibiting CRC invasion[46 ,47 ]. Furthermore, Huang et al[49 ] found that certain metabolites of normal intestinal microflora were capable of upregulating miR-192 expression, suppressing the proliferation of colon cancer cells through a decrease in bone morphogenic protein type 2 receptor levels; thus, cell proliferation, migration, and invasion ability are diminished,and the rate of cellular apoptosis is improved[49 ]. Collectively, these data indicate a potential role of miR-192 -3 p/5 p as a biomarker for UC activity and a target for pharmacological treatment.
These results provide information that may explain the differences in CRC and mortality rates between UC and CD patients. Two cohort studies from Olénet al[50 ,51 ](2020 a; 2020 b) compared CRC mortality and incidence in UC and CD patients. In the first study, which evaluated UC patients, the authors found that UC patients had a 36 % higher incidence of CRC than that of reference individuals [hazard ratio (HR) =1 .66 ][51 ]. Among CD patients, the incidence was 21 .9 % higher than that of the reference group (HR = 1 .4 )[50 ]. These findings demonstrated a tendency toward a higher probability of developing CRC in UC compared with CD, which was associated with higher mortality[50 ,51 ]. The lower CRC incidence in CD could be explained by the early removal of this portion of the intestine[52 ]. Nevertheless, there was differential expression of these selected miRNAs between those with CD and UC. The decreased expression levels of miR-378 a-3 p/5 p, miR-429 , and miR-192 -3 p/5 p in UC patients could represent a possible mechanism responsible for increasing their likelihood of developing CRC.
Although the overlap between DE-miRNAs targets and IBD susceptibility genes(Figure 2 ; Supplementary Table 1 ) suggested some involvement with the disease onset,progression and future cancer development, the data are preliminary and the sample size is small, so further studies are required to better comprehension of miRNA role in IBD pathogenesis. Future studies should aim to address some of the limitations by increasing the number of patients and investigating the potential links between these miRNAs and pathological UC and UC-associated CRC mechanisms.
CONCLUSION
In conclusion, this study highlighted the potential involvement of miR-192 -3 p, miR-192 -5 p, miR-378 a-3 p, miR-378 a-5 p and miR-429 as players in IBD pathology, UC differentiation and UC-associated CRC, indicating the potential use of these miRNAs as specific biomarkers for UC. Moreover, these miRNAs could be also useful as biomarkers for UC-associated CRC. Since samples of treatment-na?ve patients were used, the distinct expression found in this study was a result from the disease itself with no medication effect. This way, the DE-miRNAs may represent a new pharmacological target for UC treatment.
ARTICLE HIGHLIGHTS
ACKNOWLEDGEMENTS
The authors would like to acknowledge Professor dos Reis PP (PhD) for technical assistance.
 World Journal of Gastroenterology2021年45期
World Journal of Gastroenterology2021年45期
- World Journal of Gastroenterology的其它文章
- Diagnostic biomarkers for pancreatic cancer: An update
- Therapeutic potentials of fasudil in liver fibrosis
- Clinical presentation of gastric Burkitt lymphoma presenting with paraplegia and acute pancreatitis: A case report
- In-hospital mortality of hepatorenal syndrome in the United States: Nationwide inpatient sample
- Multimodality management of gallbladder cancer can lead to a better outcome: Experience from a tertiary care oncology centre in North India
- Gut microbiome in allogeneic hematopoietic stem cell transplantation and specific changes associated with acute graft vs host disease
