Diagnostic biomarkers for pancreatic cancer: An update
Ming Yang,Chun-Ye Zhang
Abstract Pancreatic ductal adenocarcinoma accounts for the primary type of pancreatic cancer (PC) with a 5 -year survival rate of only about 10 % in the United States.Early diagnosis will improve chances for curative treatment. To date, a broadly used serum marker for PC diagnosis is carbohydrate antigen 19 -9 , which is the only approved biomarker currently by the United States Food and Drug Administration. However, it has low specificity; therefore, development of novel biomarkers is urgently needed. Clinical trials are ongoing to evaluate candidate biomarkers for PC diagnosis, and the use of a multi-biomarker panel with current PC diagnostic biomarkers appears promising.
Key Words: Pancreatic ductal adenocarcinoma; Diagnosis; Biomarkers; Panel; Clinical trials
TO THE EDITOR
We read with great interest a review paper recently published by O'Neill and Stoita[1 ], reviewing diagnostic biomarkers currently applied in pancreatic cancer (PC). The biomarkers are from serum, urinary, pancreatic, salivary, biliary, and fecal sources and comprise many different types of molecules. For example, serum biomarkers include proteins of glycolipids, growth factors, cytokines, chemokines, adhesion molecules,non-coding RNAs (long non-coding RNAs and microRNAs), and liquid biopsy(exosomes, circulating tumor DNA or ctDNA, and circulating tumor cells or CTCs)[1 ].
Moreover, we agree with the authors' suggestion that early diagnosis of PC improves chances for curative treatment. PC comprises two main subtypes, including the more common exocrine cancers and less common endocrine cancers. Pancreatic ductal adenocarcinoma (PDAC) accounts for the primary type of PC, consisting of around 95 % in exocrine cancers and about 90 % in all PCs. The 5 -year survival rate of PC is relatively low and was only 10 % for all patients with PC in the United States from 2010 to 2016 [2 ]. To date, the only approved serum marker for PC diagnosis is carbohydrate antigen 19 -9 (CA 19 -9 ) in the United States, even though it has low specificity[3 ]. However, CA 19 -9 is a non-PC-specific marker, shown to increase in colorectal, liver, lung, and ovarian cancers, as well as desmoplastic fibroblastoma[4 ,5 ].Because of the low specificity of CA 19 -9 , a multi-marker panel that combines some of the currently investigated biomarkers (with CA 19 -9 ) can be used to improve the specificity and sensitivity of PC diagnosis. For example, a multi-biomarker panel with enzyme-linked immunosorbent assay using three potential biomarkers, leucine-rich alpha-2 -glycoprotein 1 , transthyretin, and CA 19 -9 , improved the diagnosis of PDAC in normal pancreas and benign pancreatic disease and other tumors[6 ]. Although a multi-biomarker panel provides a better approach for early PC diagnosis, some limitations, including cost, the requirement for large sample volumes, good technique and analytical performance, and practical feasibility, may impact their broad application[3 ,7 ,8 ].
In addition, many of the biomarkers discussed in the abovementioned paper,including extracellular matrix-associated proteins such as matrix metalloproteinase and tissue inhibitor of metalloproteinase 1 , profibrotic factors such as transforming growth factor-beta, growth factors such as vascular endothelial growth factor, cell-cell interacting protein such as intercellular adhesion molecule 1 , and microRNAs such as mi-R21 , are not specific markers implicated in many other cancers and diseases[9 -12 ].Furthermore, germline mutations in genes such as cyclin-dependent kinase inhibitor 2 A, tumor protein p53 , serine/threonine kinase ATM, MutL homolog 1 , and breast cancer 1 and 2 have been significantly associated with PC[13 ]. The authors also mentioned genetic factors associated with PC, such asKRASin ctDNA andKRASmutation in CTCs. Therefore, genetic mutation or inherited factors may be a predisposing factor for PC and should be considered during the diagnosis.
Finally, this letter summarizes the actively recruiting and completed clinical trials to evaluate diagnostic methods or biomarkers for PC (Table 1 ). The data were collected from the website https://clinicaltrials.gov (accessed on July 18 , 2021 ) using the keywords biomarkers and PC. Overall, the specificity and sensitivity of PC diagnosis can be increased by using multiple marker panels in combination with CA 19 -9 or with novel screened biomarkers. In addition, accuracy, cost-effectiveness, and ease of application together will ensure the broad application of any new diagnostic method.
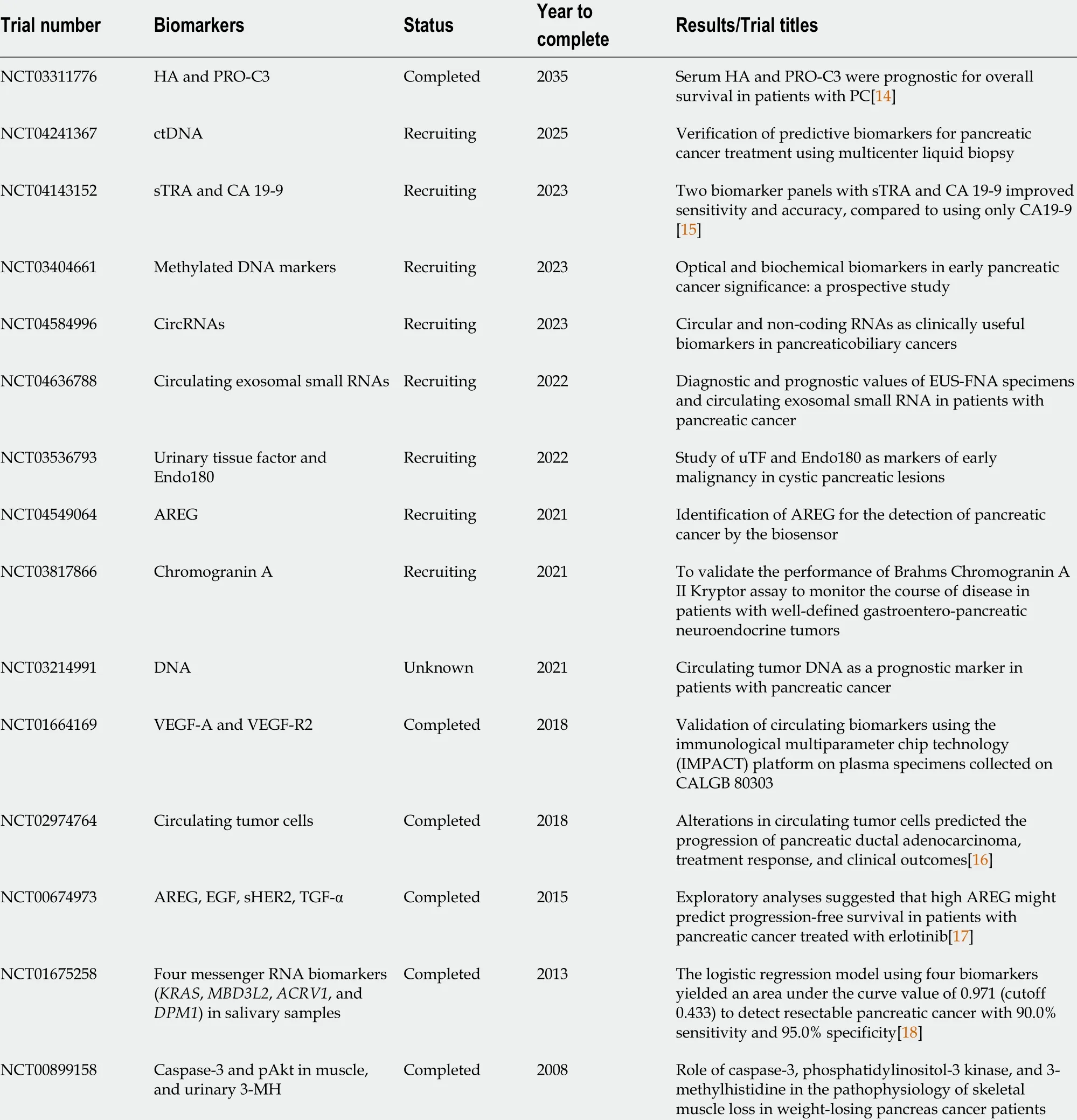
Table 1 Clinical trials for pancreatic cancer with representative diagnostic biomarkers
 World Journal of Gastroenterology2021年45期
World Journal of Gastroenterology2021年45期
- World Journal of Gastroenterology的其它文章
- Therapeutic potentials of fasudil in liver fibrosis
- Clinical presentation of gastric Burkitt lymphoma presenting with paraplegia and acute pancreatitis: A case report
- In-hospital mortality of hepatorenal syndrome in the United States: Nationwide inpatient sample
- Multimodality management of gallbladder cancer can lead to a better outcome: Experience from a tertiary care oncology centre in North India
- MicroRNAs expression influence in ulcerative colitis and Crohn's disease: A pilot study for the identification of diagnostic biomarkers
- Gut microbiome in allogeneic hematopoietic stem cell transplantation and specific changes associated with acute graft vs host disease
