Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival
INTRODUCTION
Despite the great medical and scientific achievements attained over the last decades in the fields of cancer understanding,early detection,and care,cancer continues to be a majorly threatening disease worldwide.Amongst the many pathologies gathered under this term,colorectal cancer (CRC) accounts for 9.4% of overall cancer deaths,ranking second just after lung cancer[1].CRC treatments vary depending on tumour location and stage of diagnosis;standard colectomy (along with lymphadenectomy)without adjuvant therapy is the usual treatment in early stages I and II,while most patients in advanced stages III and IV follow with chemo-and/or radiotherapy to reduce the risk of recurrence[2].However,a large proportion of these patients present with (synchronous;15%-25%) or will develop (metachronous;40%-75%) metastases,mainly in the liver[3],which constitutes the major cause of deaths[4].Therefore,a 5-year relative survival rate is reduced from 90% in early-stage detection to 12% in advanced cases[2].Thus,finding robust markers before surgery to predict patient outcomes constitutes a safe strategy in order to stratify those groups with a high risk of recurrence and design personalised pre-and postoperative therapies.
A wide variety of factors,mainly based on clinical and pathological features,have been tested as prognostic markers for CRC development,such as:weight loss,haemoglobin levels,tumour-nodes-metastasis classification (TNM) staging and tumour differentiation,mismatch-repair proficiency,lymph node involvement,or response to (neo-) adjuvant therapies[5-7].Moreover,since a clear distinction between the behaviour of right-sided CRC (RCRC) and left-sided CRC (LCRC) patients is well established,much effort has been put into categorising putative prognostic markers according to their respective characteristics,though still with controversial results[8].
Currently,an increasing number of research and clinical trials are supporting evidence of the influence of the systemic inflammatory response in cancer progression[5].A measure of this response has been assessed by combining the number of peripheral circulating leukocytes:lymphocyte-to-monocyte ratio (LMR),neutrophil-tolymphocytes ratio (NLR),and platelet-to-lymphocyte ratio (PLR).These analyses have shown interesting prognostic associations in several cancer types including urothelial,nasopharyngeal,osteosarcoma,lung carcinomas[9-12],and CRC[13-16].Nevertheless,few studies have been directed towards the prognostic value of intertwined relationships across circulating and tumour-infiltrated populations of leucocytes on solid tumour progression[17-19].
too long, the Vizier would threaten laughingly to tell the Chaliphess the subject of the discussion carried on one night outside the door of Princess Screech Owl
Herein,we aimed to delve deep into the prognostic value of leukocyte distribution ratios,in both blood and tumour tissues,for CRC patient outcomes after surgery.We hypothesised that stronger indexes than circulating (blood) leukocyte ratios to predict patient outcome will result from combining both circulating and infiltrated(tumour/peritumoural tissues) concentrations of leukocytes.We show six robust predictors for RCRC overall survival (CD8
,CD4CD8
,LMR
,CD8MR
,LMR
/LMR
,LMR
/CD8MR
),three for RCRC recurrence-free survival (LMR
,CD8MR
,LMR
/LMR
,LMR
/CD8MR
),and another one for LCRC overall survival (LMR
/CD4MR
),all these being based on the ratios between blood and peritumoural tissue concentration of lymphocytes and monocytes.Moreover,we highlight the importance of these variables in designing
surgical strategies,due to the ease with which surgeons can build a protocol by taking samples of peripheral blood and peritumoural tissue during a preoperative colonoscopy.
This has become such a habit with me that I don t think I could break myself of it; and, of course, if I got rid of the guitar I could never bring her back to life again
MATERIALS AND METHODS
Patient selection
Sixty-five patients diagnosed with colon adenocarcinoma,with no records of previous neo-adjuvant therapy,were recruited at the Digestive Surgery Service of La Paz University Hospital (Madrid,Spain) from January 2017 to September 2019.They were surgically treated according to each patient’s condition for right (caecum,ascending,or transverse colon) or left (descending or sigmoid colon) hemicolectomies followed by anastomosis,with partial hepatectomy if synchronous metastasis was present.Patients’ clinical records were followed-up until March 2021.Overall survival (OS)was then defined as the length of time since surgery until
or the end of the study,whilst recurrence-free survival (RFS) was considered the interval from surgery until relapse,either from disease-free or (synchronous/metachronous) metastases-free statuses.All patients signed written consent,in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and the Ethics Committee for Clinical Research of La Paz University Hospital (PI-1958),for further uses of blood samples and surgically resected organs for research purposes.
Exclusion criteria
Only patients with adenomas or rectum adenocarcinoma were excluded from the study.
Blood tests
Venous blood samples were collected in 10 mL EDTA-tubes in the hospital room,24 h prior to surgery and routinely tested for white blood cell,lymphocyte (L),monocyte(M),neutrophil (N) and platelet (P) counts at the Central Laboratory (CORE) of the La Paz University Hospital.Preoperative blood LMR (LMR
),NLR (NLR
),and PLR(PLR
) were then calculated for each patient by dividing the absolute counts of the respective populations in the peripheral blood (Table 1).
Tissue preparation
He crossed the frontier, and soon found himself on the banks of a river; and before him, in the middle of the stream, a beautiful girl sat chained to a rock and weeping bitterly
Organ samples were washed with PBS solution containing 56 μg/mL gentamicin(Braun,Melsungen,Germany;636159),2.5 μg/mL fungizome/anphotericin-B (Gibco,Amarillo,TX,United States;15290-018),and 1% penicillin/streptomycin (Sigma-Aldrich,Saint Louis,MO,United States;P4333-100mL) and gently shaken for 30 min at room temperature.Then they were fixed in 4% paraformaldehyde for 16 h,washed with PBS for 24 h,and paraffin-embedded by standard procedures.
Tissue microarrays (TMA) recipient paraffin-blocks (24 mm × 2.0 mm) were prepared with a TMA builder kit (Histopathology Ltd.,Baranya,7632,Hungary;20010.2) and filled with properly matched samples of previous patients’ blocks,following manufacturer’s protocol.
Immunohistochemistry and image analysis
Thin sections (5 μm thick) of TMAs were cut with a Leica (RM2255) ultrathinmicrotome and allowed to completely adhere to slides for 30 min at 60°C,before staining with commercially available antibodies against assessed surface markers was performed by standardised protocols (see Supplementary Table 1 for a complete list of primary and secondary antibodies used).Briefly,sections were deparaffinised with xylene,rehydrated through graded (100% to 70%) ethanol,and blocked for endogenous peroxidase by immersion in 97% methanol.Next,sections were immersed in heated sodium citrate buffer (10 mmol/L,pH 6.0) for antigenicity recovery and then incubated in unspecific-binding blocking solution [TBS solution containing 1% BSA,1% Triton X-100 (Thermo Scientific;Waltham,MA,United States,85111) and 2.5%horse serum (Gibco;Amarillo,TX,United States,26050088)].Primary antibodies were then added at recommended dilutions and incubated overnight at 4°C in a humid chamber.After washing slides with TBS,matched HRP-secondary antibodies were added and incubated for 1 h at room temperature.Then,DAB chromogen (DAB substrate kit,Cell Marque;Rocklin,CA,United States,1-957D-30) was added for a few seconds until colour change and gently washed with TBS and distilled water.Finally,sections were counterstained by immersion in haematoxylin,dehydrated through graded (70% to 100%) ethanol,and mounted with DPX medium (Sigma-Aldrich;Saint louis,MO,United States,06522).
An average of four photographs per sample (in order to cover the whole field for each sample on the TMA sections) were taken with an Olympus BX-41 microscope and blind-analysed by two independent observers with ImageJ (v1.52p),for the calculus of the relative areas to each antibody corresponding surface marker expression (CD4,CD8,and CD14).For a detailed description of the image processing see Supplementary Figure 1A.A percentage of the total tissue area (A) for the three surface markers,in each patient’s tumour and peritumour samples,was reported as the mean of all their relative areas per field.
Visiting for the Thanksgiving holiday, he finished unloading the luggage and took it to the guestroom downstairs. After driving all day from Salt Lake to Ft. Collins, his temper showed. That one finger rule may work with the twins, but it ll never work with Hannah, he insisted.
Total tumour and peritumour LMRs (respectively,LMR
and LMR
) were calculated by dividing the sum of the areas for CD4 and CD8 by the area for CD14,
,LMR
=(A(CD4
)+A(CD8
))/A(CD14
).Individual subpopulation ratios were also analysed for both tumour and peritumour samples (CD4MR
,CD8MR
and CD4MR
,CD8MR
,respectively),
,CD4MR
=A(CD4
)/A(CD14
).Then,blood-to-tissue ratios for all previous tumour and peritumour subpopulation ratios (LMR
/LMR
,LMR
/CD4MR
,LMR
/CD8MR
and LMR
/LMR
,LMR
/CD4MR
,LMR
/CD8MR
,respectively)were also reported for each patient.
Nomogram construction and validation
The segment of the large intestine proximal to the splenic flexure,
the right colon(comprising caecum,ascending colon,and proximal two-thirds of the transverse colon),derives from the embryonic midgut;whereas the left colon (comprising the distal third part of the transverse colon and descending and sigmoid colon) derives from the embryonic hindgut[21].Distinct embryologic origin of right and left sides of the colon markedly determines important physiological differences,mainly:cell motility,vasculature,lymphatic drainage,extrinsic innervation,development of the endocrine components,and the expression and patterns of epigenetic marks of crucial molecular factors for cell development[21,22].
Statistical analysis
Data are represented as mean ± standard deviation.Student’s
test was used for pairwise comparisons.Mann-Whitney
analysis was applied for equal standard deviations,otherwise Welch’s correction was used.The distribution of the variables was assessed by a nonparametric test.Spearman
correlations were used to evaluate the association between the variables and ratios with the OS and RFS observed in our patients.Survival and population ratio relationships were analysed using Cox proportional hazard ratios;statistically significant variables in univariate analysis were further evaluated with the Cox multivariate step-by-step backward method to identify those with independent prognostic value.The Kaplan-Meier method was used to calculate the differences in OS and RFS rates for RCRC and LCRC over time (months),and significance was compared using the log-rank (Mantel-Cox) test;median time(months) survival proportions and P accuracy were reported.We calculated the receiver-operating characteristic (ROC) curve and the area under the curve (AUC) to determine whether the different variables and ratios could be used to predict OS and RFS in our cohort.We indicated the sensitivity,the specificity,the positive and negative predictive values,and 95% confidence interval for AUC and P accuracy.Optimal cutoff values,as determined with Youden’s index,Harrell’s C-index,and P accuracy,were calculated with R software.
values of 0.05 or less were considered indicative of statistical significance,and all these were two-sided.All statistics were performed in either Prism 6.0 (GraphPad,San Diego,CA,United States) or SPSS version 23 (IBM,NY,United States) software.
RESULTS
Cohort baseline characteristics
The cohort included in this study was exclusively recruited by one team of surgeons,from their assigned patients for surgically treated disorders of the digestive tract,thus only a fraction is constituted of the whole figure of CRC patients attended at La Paz University Hospital during the period of recruitment.Detailed clinicopathological characterisation of patients is shown in Table 1.
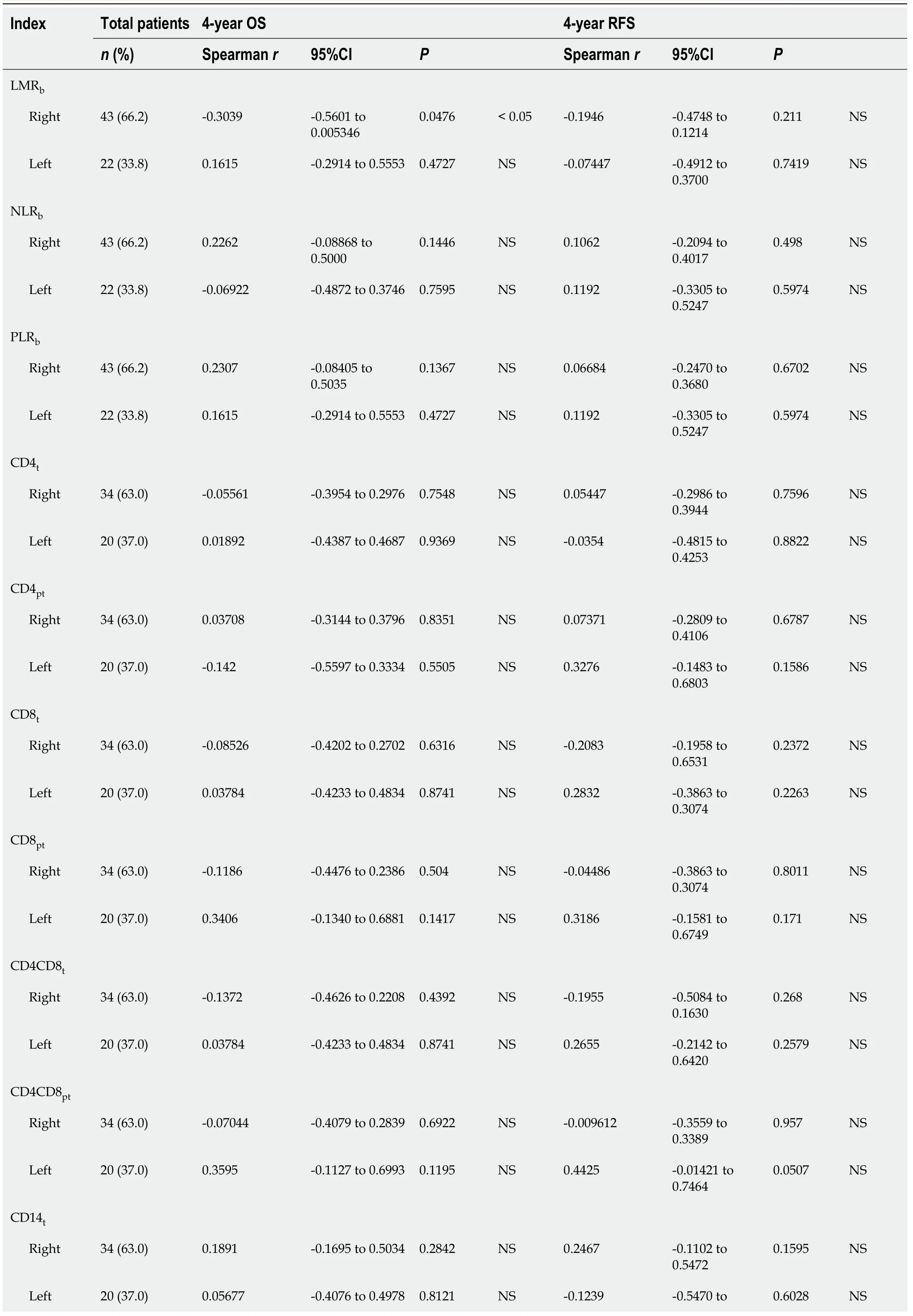
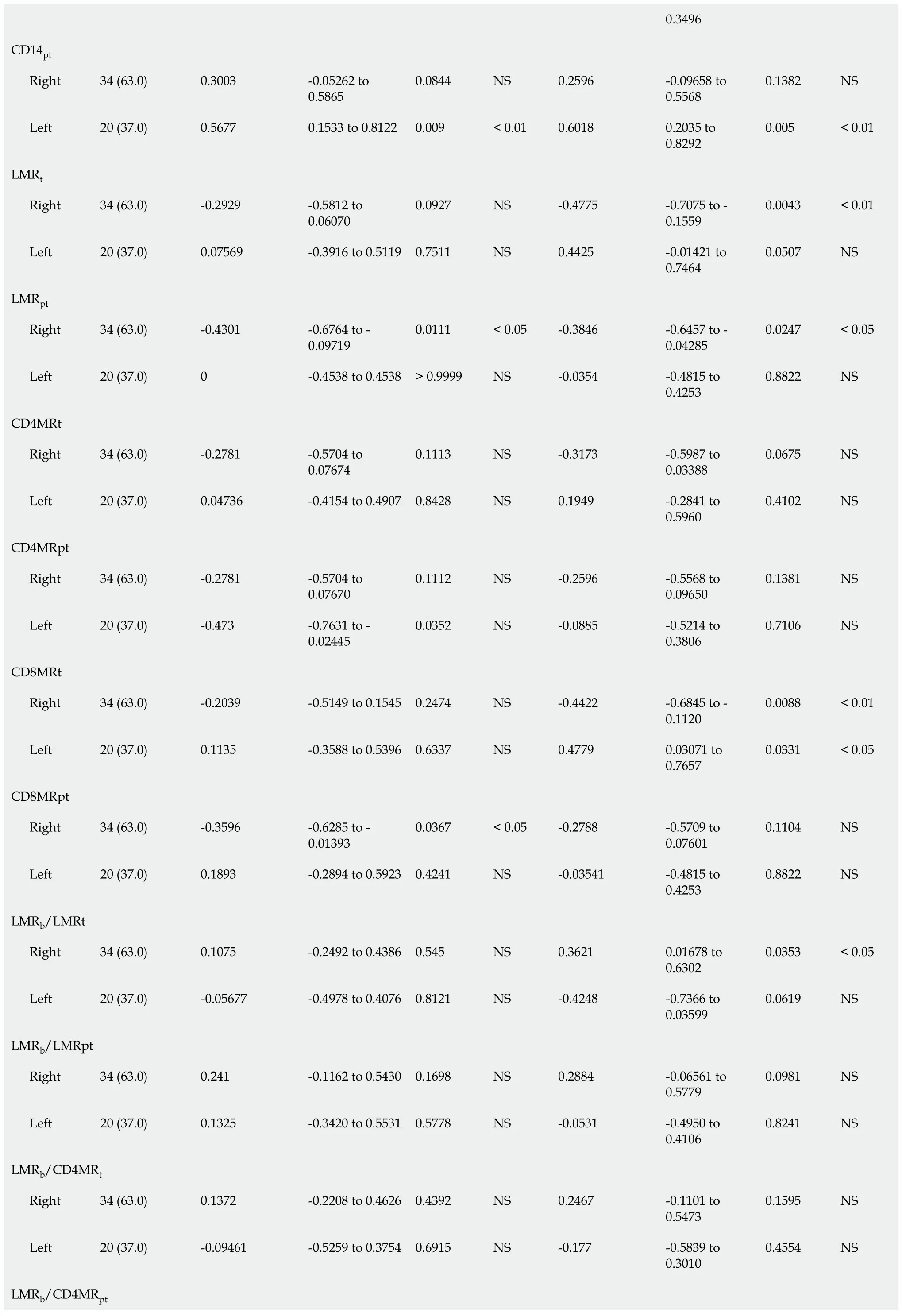
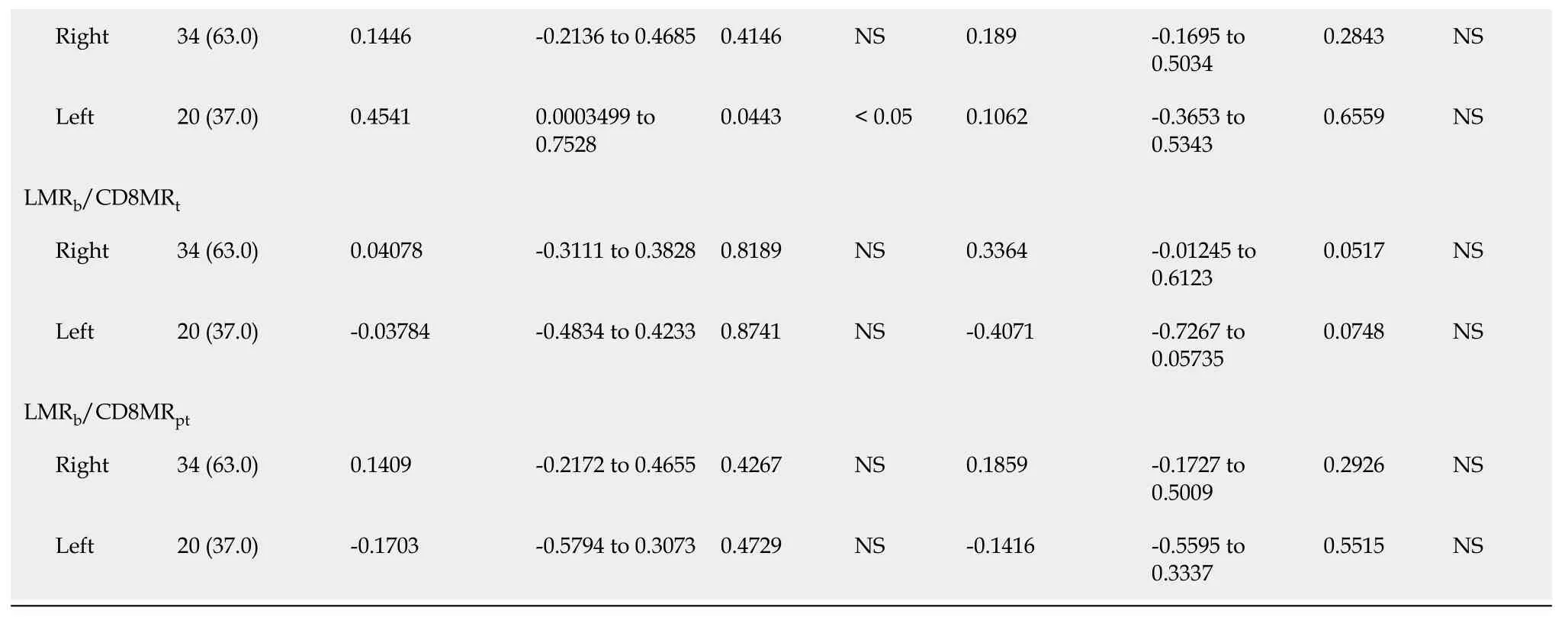
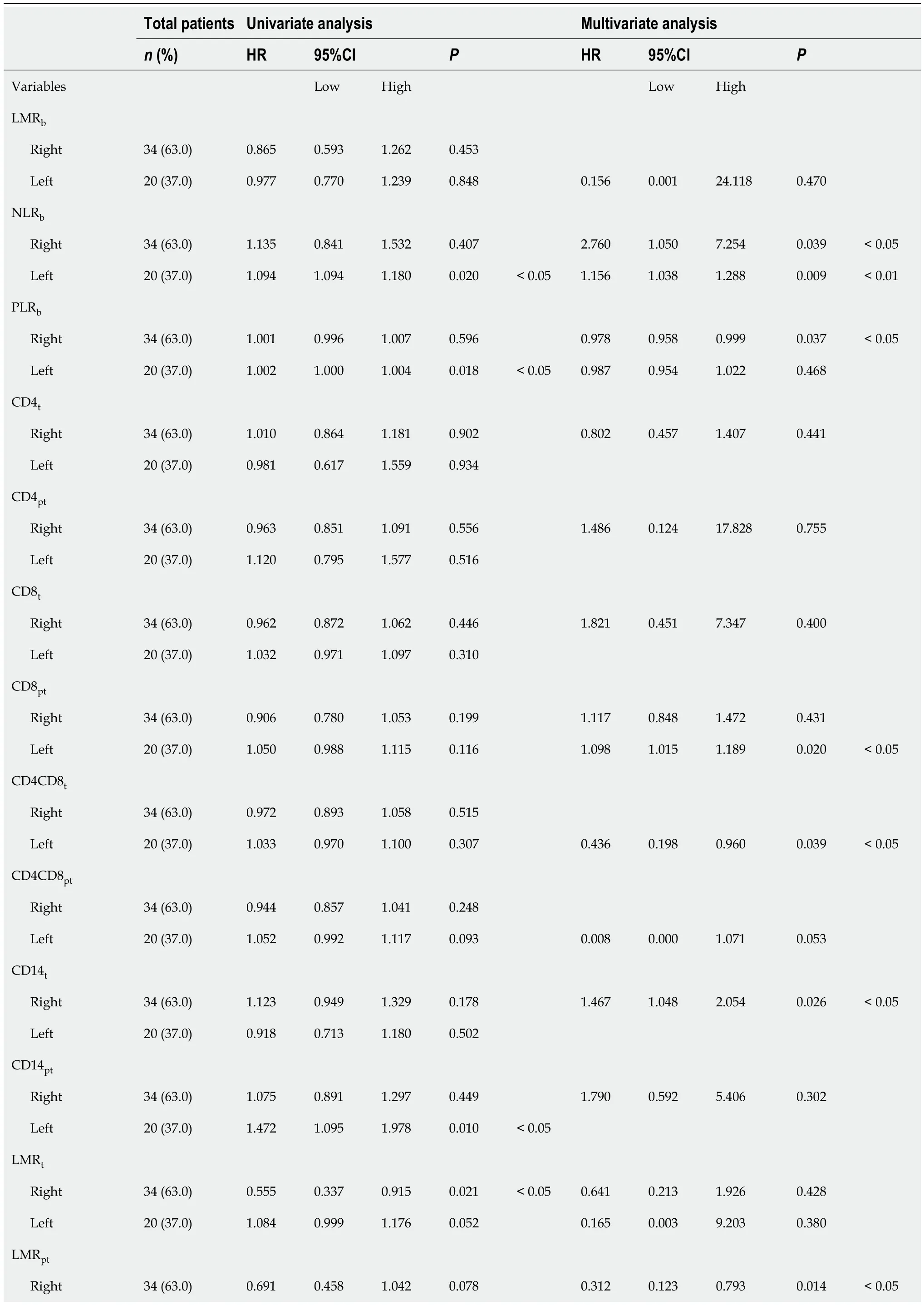
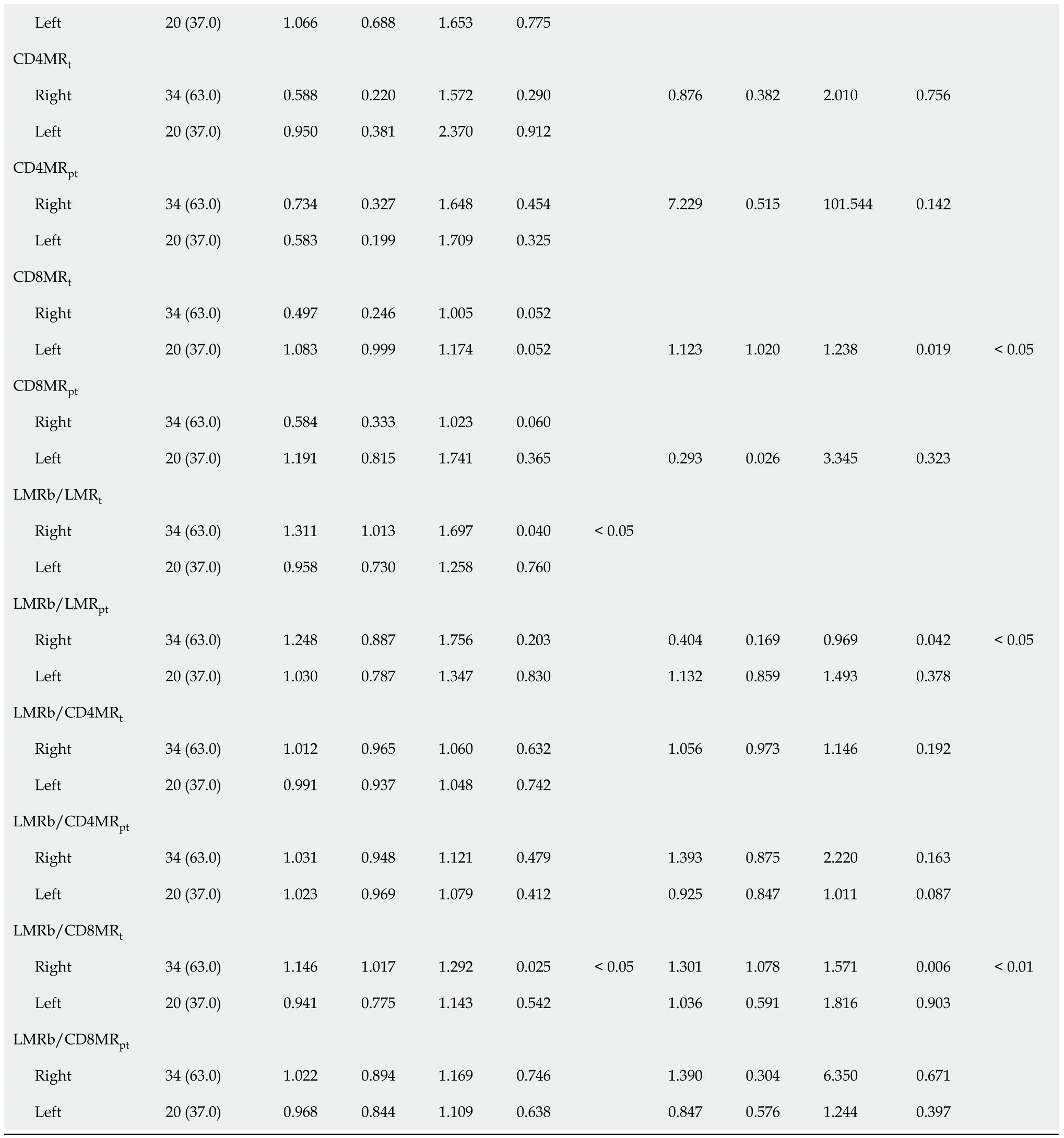
Regarding RFS (Table 4),the univariate analysis showed that in addition to previously found LMR
(
=0.021) and LMR
/LMR
(
=0.040) in RCRC,LMR
/CD8MR
(
=0.025) also significantly correlated with RFS,and CD8MR
(
=0.052)showed a trend towards being associated.In addition to previously found CD14
(
=0.010),NLR
(
=0.020) and PLR
(
=0.018) were also significantly correlated with RFS in LCRC patients.After the multivariate analysis,several variables emerged as independent prognostic factors for RFS in RCRC patients:NLR
(
=0.039),PLR
(
=0.037),CD14
(
=0.026),LMR
(
=0.014),LMR
/LMR
(
=0.042),and LMR
/CD8MR
(
=0.006).In LCRC patients,NLR
(
=0.009),CD8
(
=0.020),CD4CD8
(
=0.039),and CD8MR
(
=0.019) were found,together with a trend observed for CD4 CD8
(
=0.053).
Patient progression follow-up
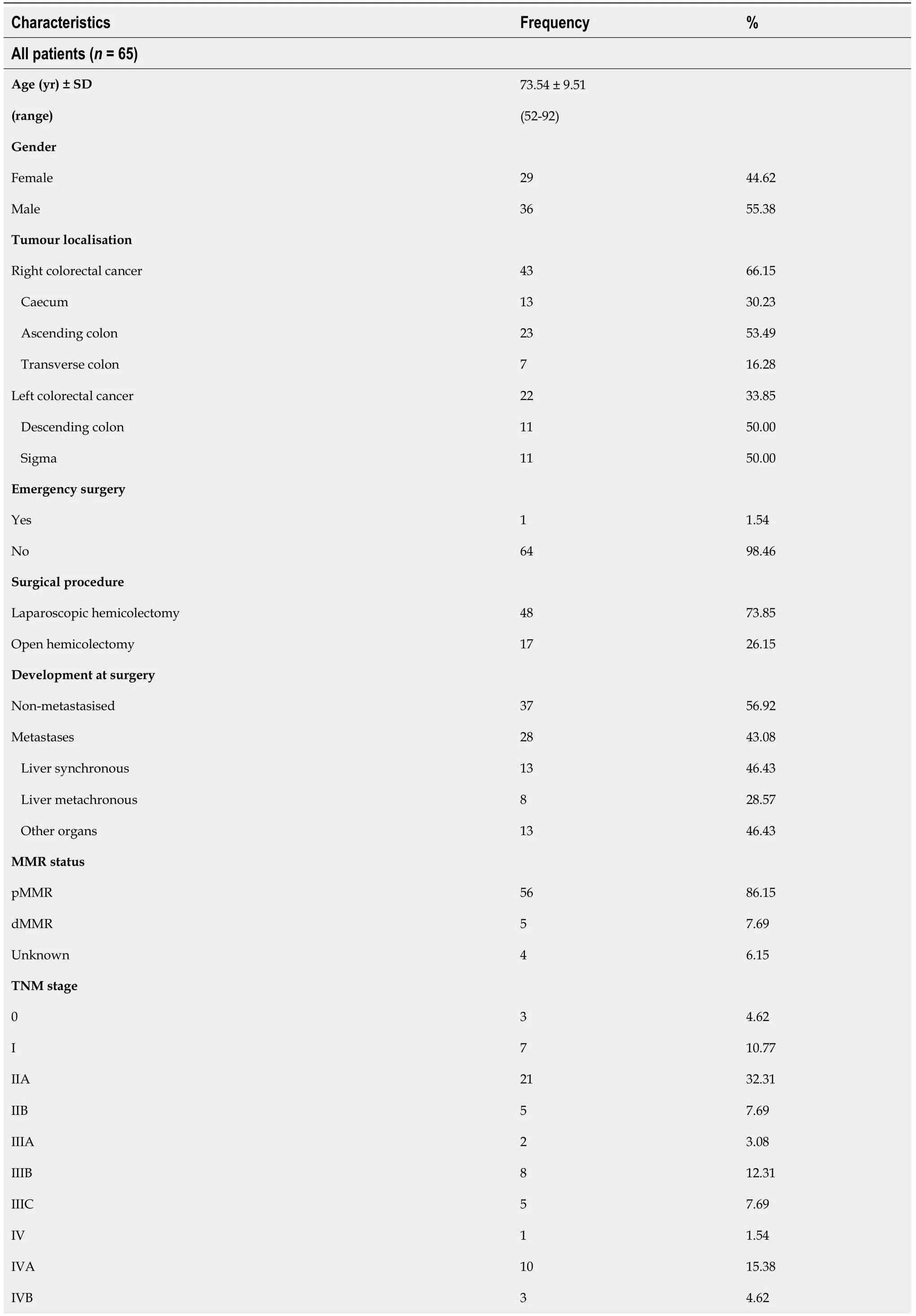
The survival analysis,with a median follow-up of 26 mo,showed no differences for OS between RCRC and LCRC patients (Figure 1A) but a trend towards poorer outcome for the latter (74.5%
40.8%,
=0.1875).However,in the analysis of RFS(Figure 1B),we observed significantly better outcomes for RCRC compared to LCRC patients (60.4%
19.1%,
=0.0036).
Leukocyte counts and ratios
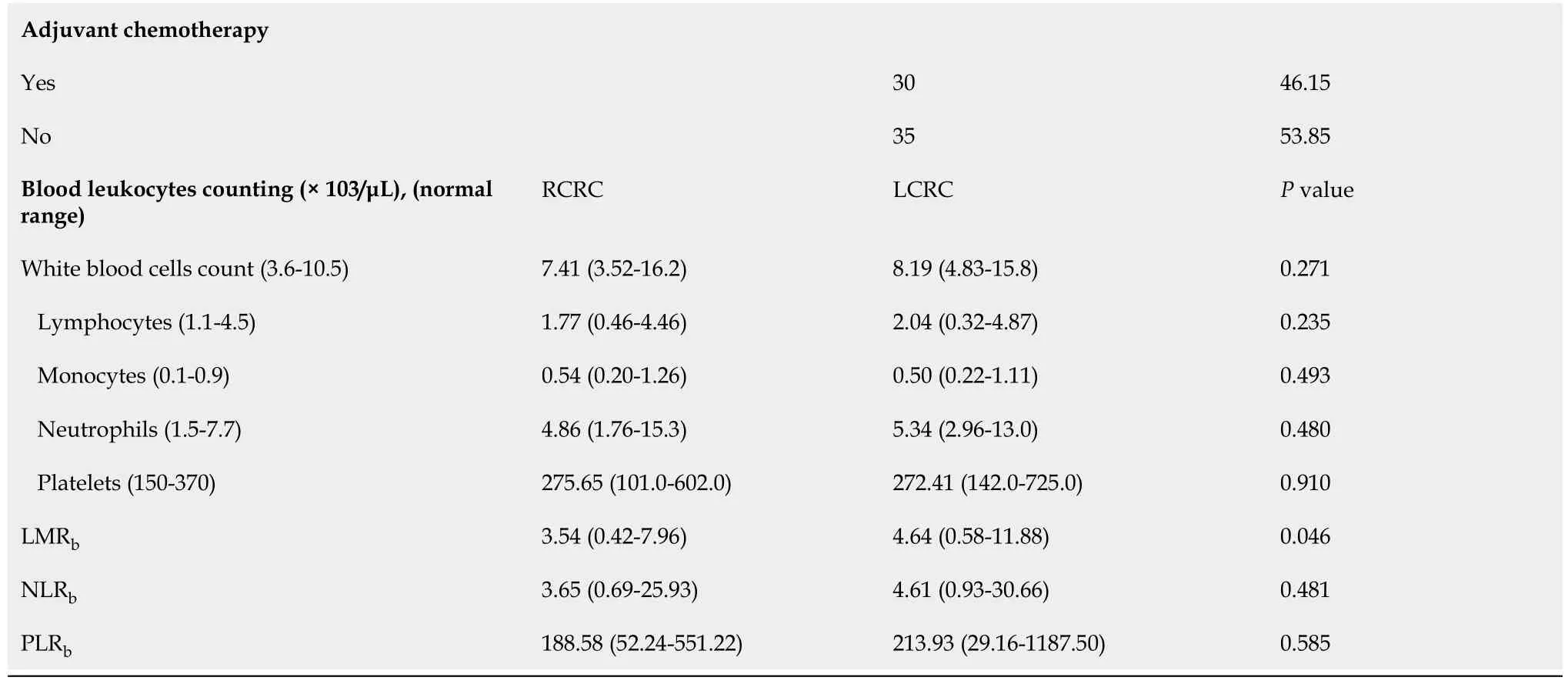
We found no differences (Table 1) in total leukocyte counts nor in individual populations of circulating lymphocytes,monocytes,neutrophils,or platelets between RCRC and LCRC patient peripheral blood.However,though all mean counts for both groups were within the normal physiological ranges,RCRC patients showed a trend towards low circulating lymphocytes.Thus,their LMR
was lower (
=0.0462) than LCRC patients (Figure 2A).Neither NLR
nor PLR
showed differences between RCRC and LCRC patients (Figure 2B and C).
Tissues from 54 out of the total 65 patients included in the study,34 from RCRC patients (63%) and 20 from LCRC patients (37%),could be assessed for leukocyte infiltration analyses.This fact was mainly due to the morphological characteristics of 11 tumours,which made it impossible to separate pieces for research purposes without affecting the global diagnostics by pathologists.
Since seminal contributions by Bufill
[23],an increasing number of studies have supported the hypothesis that these differences in origin may explain why RCRC and LCRC constitute two distinct clinical entities,which arise through different pathogenetic mechanisms[22,24,25].Thus,differential aspects such as incidence,presentation,microbiome composition,genetic burden,or immunogenicity could be explained on these grounds[26-31].In a large study with more than 17000 CRC patients,Benedix
[32] showed that RCRC represents a more distinct tumour entity than LCRC,mainly because of its higher incidence in women and older people,poor differentiation,locally advanced carcinomas,a distinct pattern of metastatic spread,and worse outcome.
Take the bird which hangs over the Princess s head, and which by its song sang her into this enchanted sleep--a song which it has had to continue ever since; take it and kill it, and cut its little heart out and burn it to a powder, and then put it into the Princess s mouth; then she will instantly awaken31, and will bestow32 on you her heart and hand, her kingdom and castle, and all her treasures
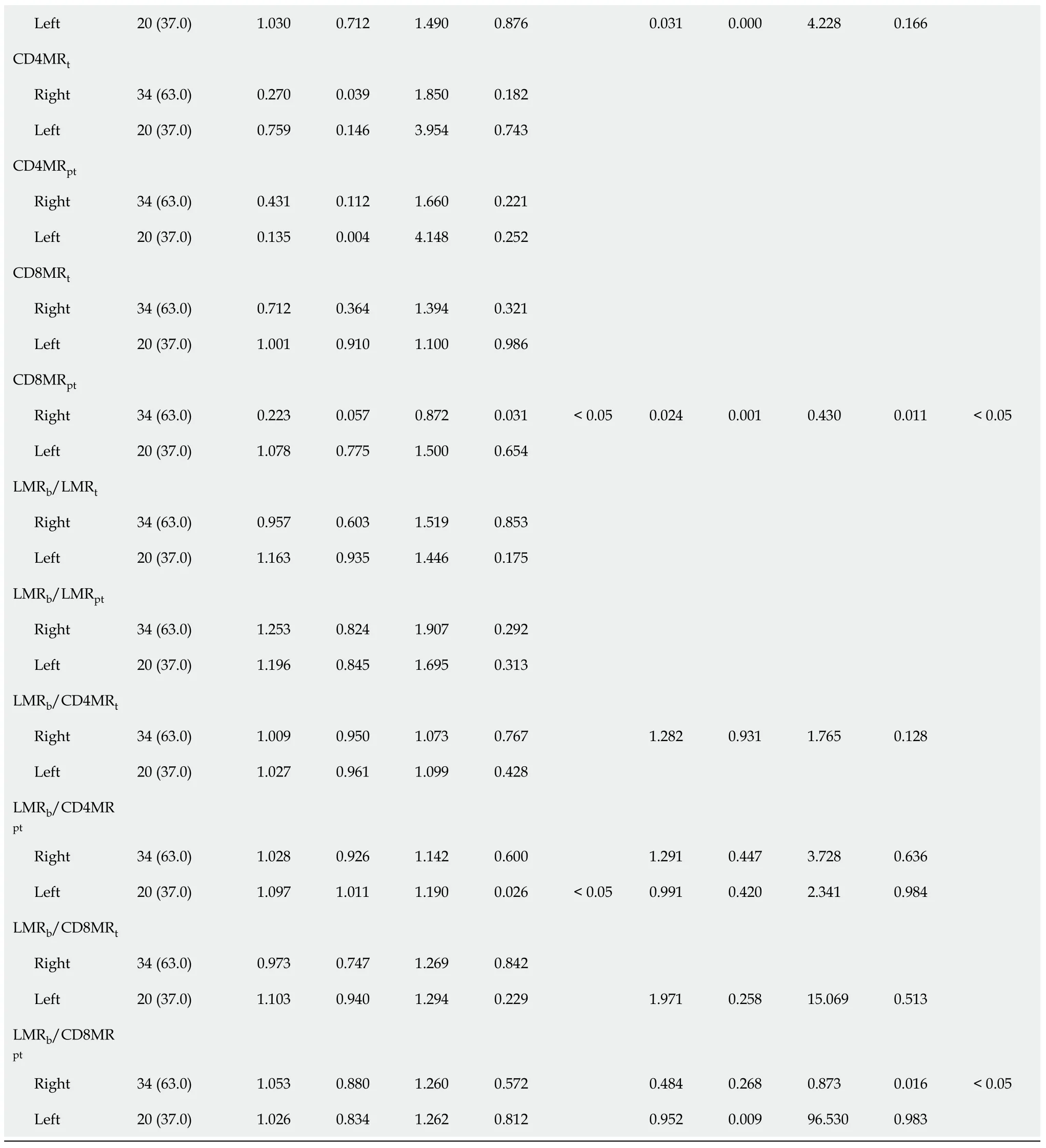
The analysis of resulting ratios for lymphocyte and monocyte counts in tumour (t)and peritumoural (pt) tissues showed a higher LMR
with respect to LMR
(4.128 ±1.363
2.022 ± 0.3432,
=0.0023) beside higher CD8MR
than CD8MR
(4.121 ± 1.374
1.218 ± 0.3297,
=0.0001) in LCRC patients (Figure 2D-F).No differences were detected for these ratios amongst RCRC respective tissues.Consequently,the analysis of blood-to-tissue ratios (Figure 2G-I) showed LCRC patients exhibited lower LMR
/LMR
than LMR
/LMR
(2.104 ± 0.601
2.900 ± 0.5061,
=0.0282) as well as lower LMR
/CD8MR
than LMR
/CD8MR
(3.381 ± 1.083
5.898 ± 1.138,
=0.0033).There were no differences in these ratios for RCRC respective tissues.
Association of leukocyte balance and patient’s outcome
27. King s son: In many fairy stories the sleeping heroine is woken by the kiss of a man (eg Briar Rose / Sleeping Beauty ). In this case it is his love and devotion that (indirectly) cause her awakening. In either case this symbolises the fact that a girl must be awakened to womanhood by a man. IRReturn to place in story.
Next,the effect of these variables on survival was assessed by Cox proportional hazards regression.For OS (Table 3),the univariate analysis revealed that besides previously found LMR
(
=0.043),LMR
(
=0.024),and CD8MR
(
=0.031) in RCRC patients,NLR
(
=0.038) also significantly correlated with OS;LMR
/CD4MR
(
=0.026) was also confirmed to be significantly correlated with OS of LCRC patients.After adjusting for confounding variables through the multivariate analysis,NLR
(
=0.038),CD8MR
(
=0.011),and LMR
/CD8MR
(
=0.016) resulted in a significant association with OS of RCRC patients;CD8
(
=0.058) also showed a trend towards being associated.
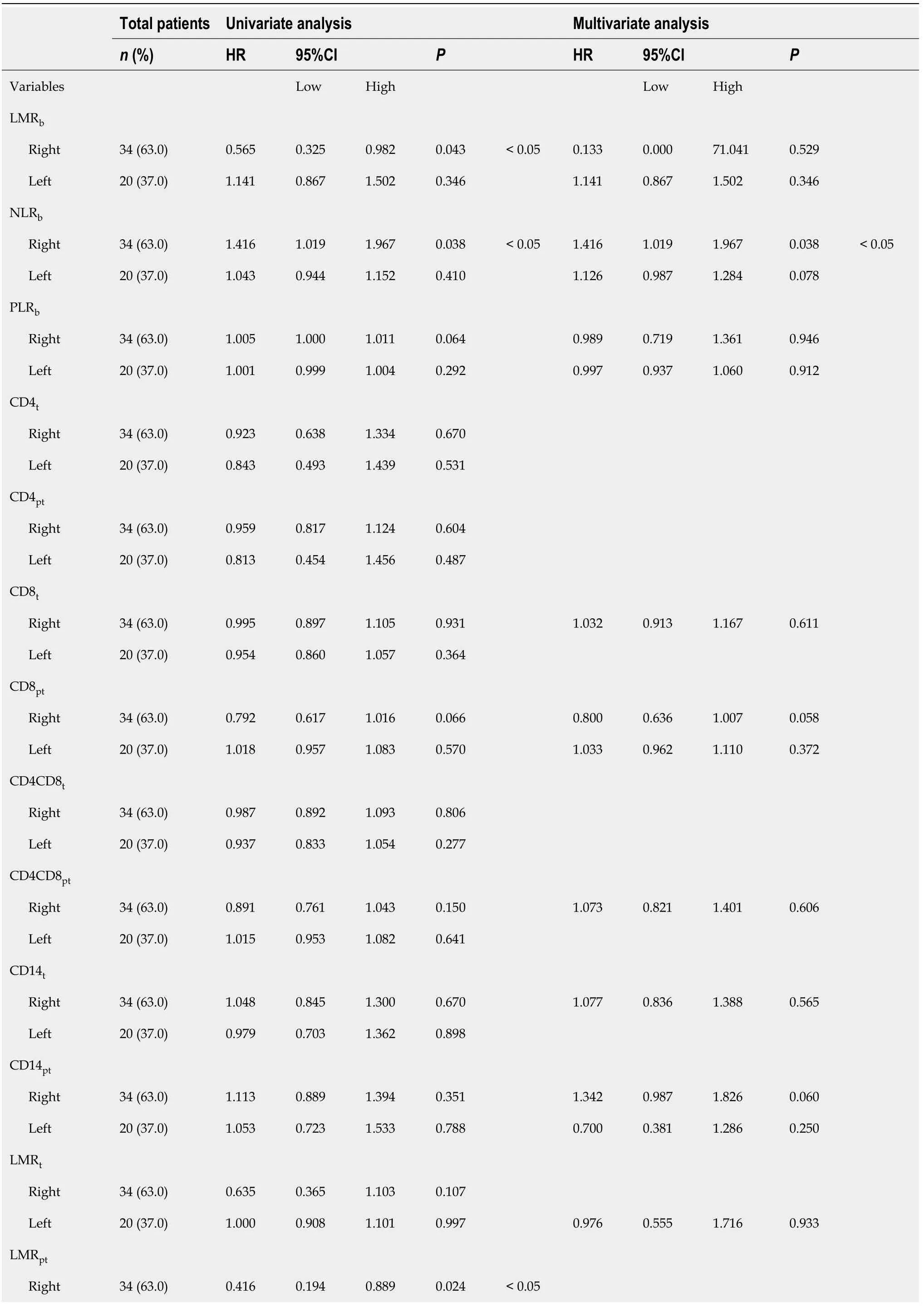
A total of 65 patients with a mean age of 73.5 years,of whom 43 (66.1%) presented with RCRC and 22 (33.8%) with LCRC,were finally enrolled.Of these,29 (44.6%) were women and 36 (55.4%) were men.With the exception of one case,all had been programmed for surgery without an emergency condition.Forty-eight (73.8%) were hemicolectomised by minimally invasive laparoscopic procedure.They ranged from stages 0 to IV,based on TNM classification;28 (43.1%) were presenting metastasis(either synchronous or metachronous at the time of surgery),and 30 (46.1%) received adjuvant therapy after surgery.Fifty-six (86.1%) of the tumours were found proficient for the mismatch-repair machinery at the histological level.
Survival prognostic value of the studied variables and ratios
Taking into account all previous correlations,we then calculated the optimal cutoff values by ROC analyses for those variables significantly correlated with OS or RFS,using respectively cancer-specific death or relapse as the endpoints for both RCRC(Figures 4 and 6) and LCRC (Figure 5) patients after surgical intervention.
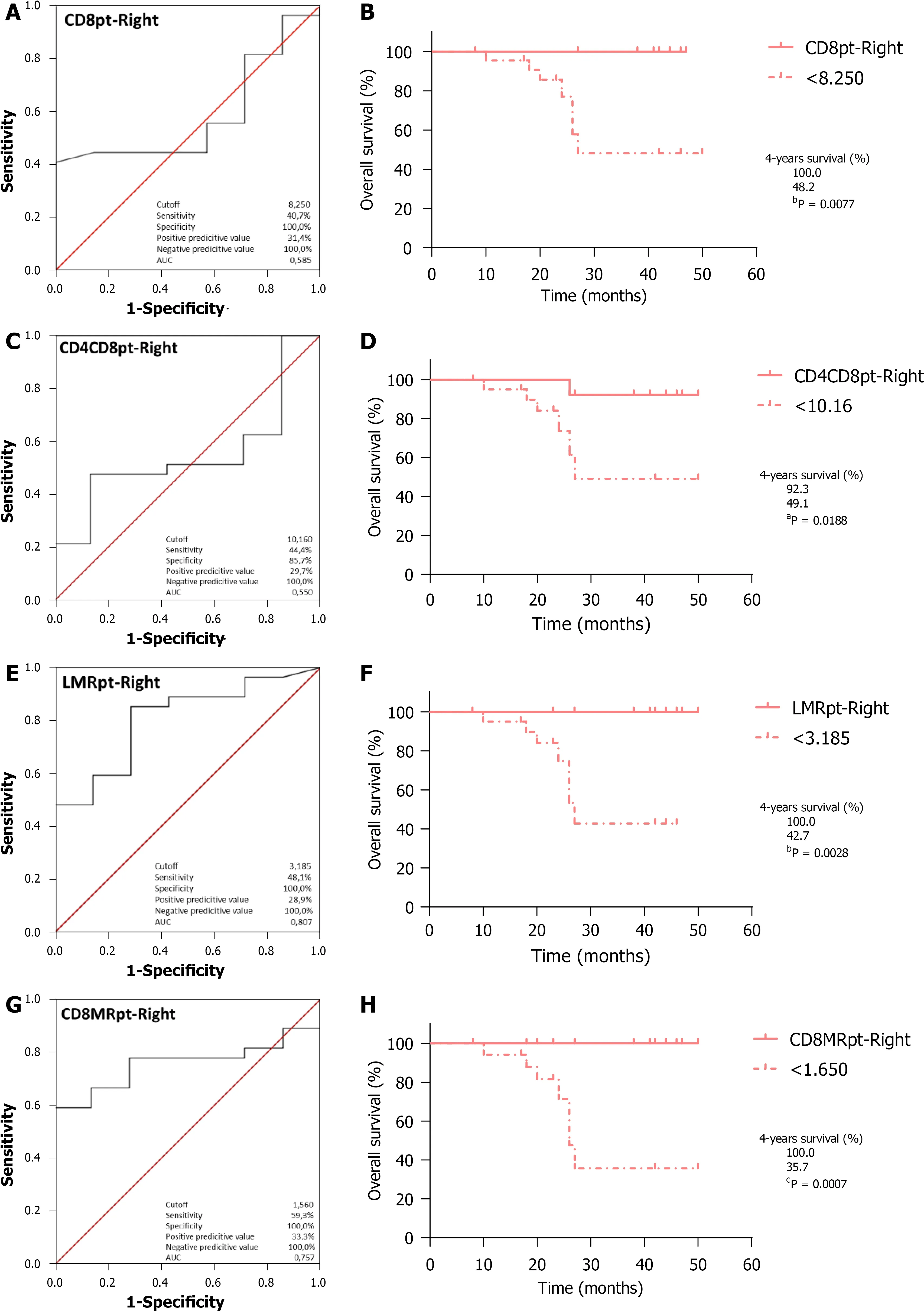
Regarding OS,ROC curve analysis of CD8
(Figure 4A;AUC=0.585,95%CI:0.376-0.793,
=0.496) identified the optimal cutoff point at 8.250,which entails significantly worse outcomes for RCRC patients ranking below this (Figure 4B;100%
48.2%,
=0.0077).CD4CD8
analysis (Figure 4C;AUC=0.550,95%CI:0.334-0.766,
=0.686)identified 10.16 as the optimal cutoff,with worse outcomes for RCRC patients ranking below this (Figure 4D;92.3%
49.1%,
=0.0188).LMR
analysis (Figure 4E;AUC=0.807,95%CI:0.641-0.973,
=0.013) identified 3.185 as the optimal cutoff,with worse outcomes for RCRC patients ranking below this (Figure 4F;100%
42.7%,
=0.0028).CD8MR
analysis (Figure 4G;AUC=0.757,95%CI:0.600-0.914,
=0.039) identified 1.650 as the optimal cutoff,with worse outcomes for RCRC patients ranking below this(Figure 4H;100%
35.7%,
=0.0007).LMR
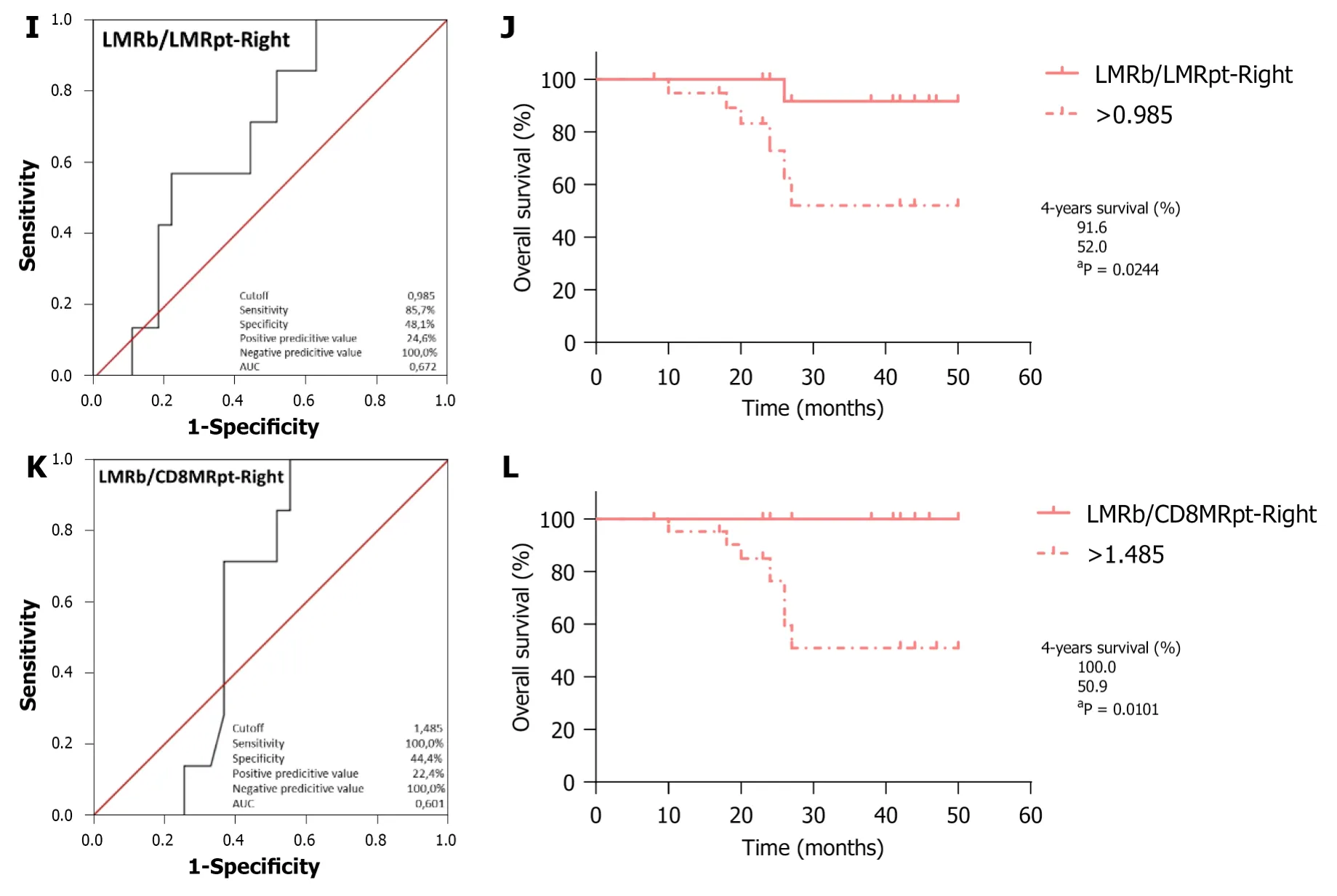
/LMR
analysis (Figure 4I;AUC=0.672,95%CI:0.479-0.865,
=0.166) identified 0.985 as the optimal cutoff,with worse outcomes for RCRC patients ranking above this (Figure 4J;91.6%
52.0%,
=0.0244).LMR
/CD8MR
analysis (Figure 4K;AUC=0.601,95%CI:0.419-0.782,
=0.418)identified 1.485 as the optimal cutoff,with worse outcomes for RCRC patients ranking above this (Figure 4L;100%
50.9%,
=0.0101).Finally,LMR
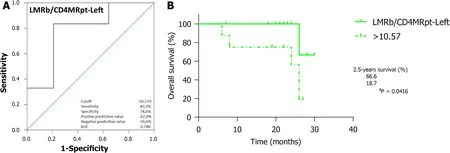
/CD4MR
analysis(Figure 5A;AUC=0.786,95%CI:0.564-1.000,
=0.048) identified 10.57 as the optimal cutoff,with worse outcomes for LCRC patients ranking above this (Figure 5B;66.6%
18.7%,
=0.0416).In addition,ROC curve analyses (Supplementary Figure 3) of CD4CD8
,PLR
,CD4CD8
,and CD8MR
,though they showed significant AUC (0.524,0.619,0.726 and 0.571,respectively),rendered optimal cutoff values with no significant differences for LCRC survival.
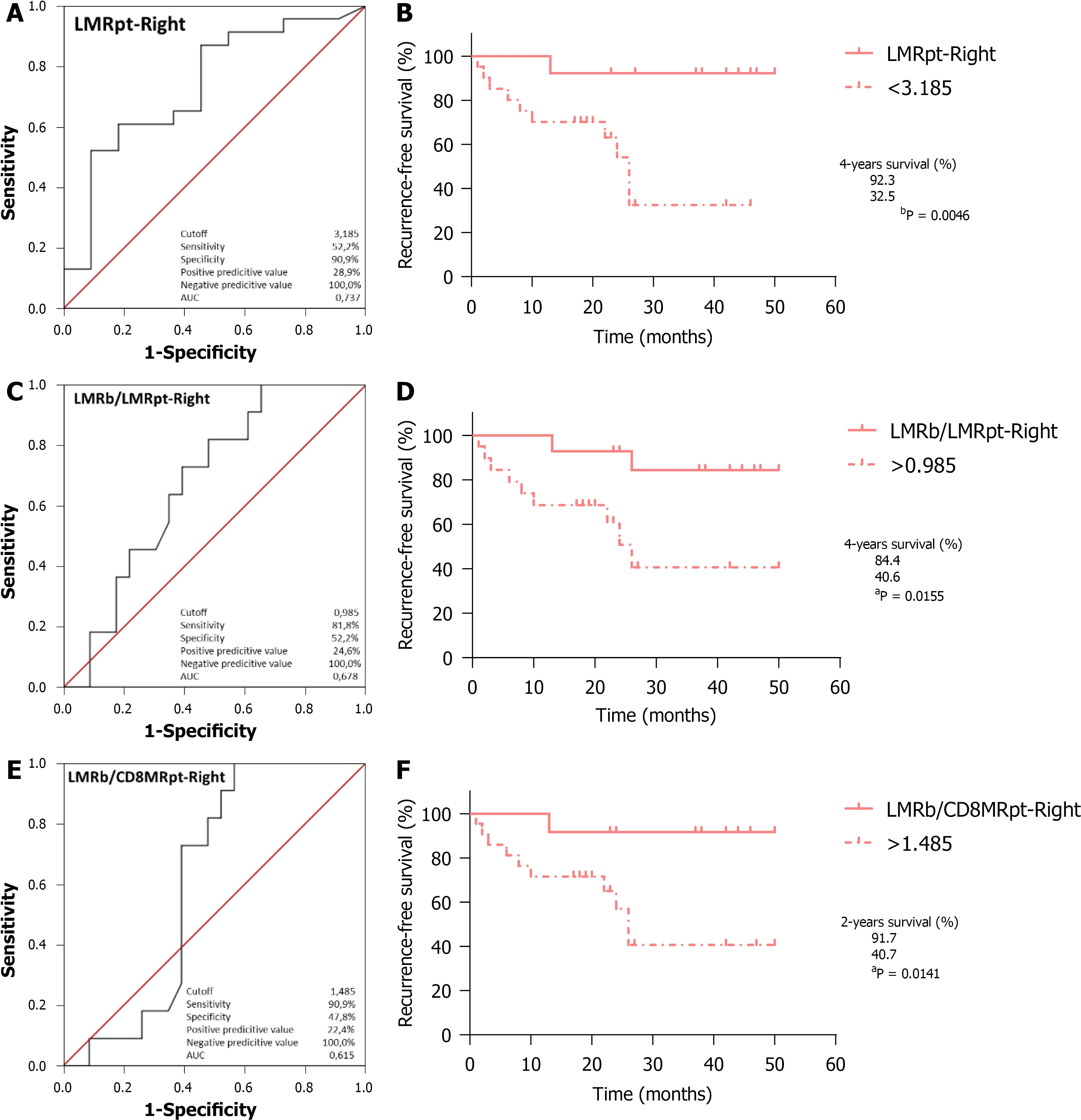
With respect to RFS,ROC curve analysis of LMR
(Figure 6A;AUC=0.737,95%CI:0.554-0.920,
=0.027) identified 3.185 as the optimal cutoff,with worse outcomes for RCRC patients ranking below this (Figure 6B;92.3%
32.5%,
=0.0046).LMR
/LMR
analysis (Figure 6C;AUC=0.678,95%CI:0.499-0.857,
=0.098) identified 0.985 as the optimal cutoff,with worse outcomes for RCRC patients ranking above this(Figure 6D;84.4%
40.6%,
=0.0155).LMR
/CD8MR
analysis (Figure 6E;AUC=0.615,95%CI:0.427-0.802,
=0.286) identified 1.485 as the optimal cutoff,with worse outcomes for RCRC patients ranking above this (Figure 6F;91.7%
40.7%,
=0.0141).The ROC analyses in RCRC patients of CD8MR
,CD8
,and CD4CD8
(Supplementary Figure 4),though they showed significant AUC (0.672,0.528 and 0.506,respectively) did not render optimal cutoff values that significantly prognosticated the RCRC patient RFS.Similarly,ROC analyses of CD4CD8
,PLR
,CD4CD8
,CD8MR
,and LMR
/CD4MR
(Supplementary Figure 5) with significant AUC (0.656,0.635,0.760,0.781 and 0.563,respectively) did not provide optimal cutoff values with significant prognostication in LCRC patient RFS.
Samples from the middle part (avoiding both the epicentre and the edge) of the tumours,5 cm-adjacent peritumoural (non-neoplastic),and liver (in case of synchronous metastases) tissues were taken at the time of surgery,upon
evaluation of morphological characteristics by pathologists.Histological types and grades were based on microscopic features.Microsatellite stability analyses were performed as previously described[20].
Nomograms modelling and validation
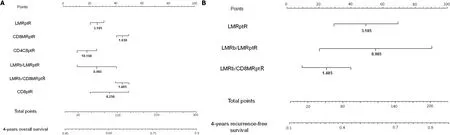
In order to avoid conflicts in handling the different values of the predictive indexes for RCRC patients,clinician-friendly nomograms were developed for both OS (Figure 7A)and RFS (Figure 7B) of these patients.The six significant predictive variables found for OS and the three found for RFS were used to construct the respective nomograms,with data from the training set of RCRC patients.The calibration of these nomograms revealed C-indexes of 0.600 (95%CI:0.561-0.639) and 0.605 (95%CI:0.579-0.631),respectively (Supplementary Figure 6A-B).Moreover,the reliability of the nomograms was evaluated with the validation set of RCRC patients,showing a moderate accuracy,with C-indexes of 0.500 (95%CI:0.475-0.525) and 0.570 (95%CI:0.541-0.599) for OS and RFS,respectively (Supplementary Figure 6C-D).
DISCUSSION
All RCRC patients from the cohort were randomly divided into training (60%) and validation (40%) sets to establish and validate the clinician-friendly nomograms.For each nomogram to predict the probability of OS or RFS,the six or the three respectively significant predictive factors found early were used to formulate the nomograms with several R packages.The discriminatory ability of the nomogram was assessed by calculating the Harrell’s concordance index (C-index).
In order to assess the degree to which leukocyte balance (
both the concentration and ratios of leukocytes for blood,tumour,and peritumours described above) was associated with RCRC and LCRC patient OS and RFS,we first conducted a Spearman correlation analysis (Table 2).We found that for RCRC LMR
(
=-0.3039,
=0.0476),LMR
(
=-0.4301,
=0.0111),and CD8MR
(
=-0.3596,
=0.0367) were negatively correlated with OS;LMR
(
=-0.4775,
=0.0043),LMR
(
=-0.3846,
=0.0247),and CD8MRt (
=-0.4422,
=0.0088) negatively correlated,but LMR
/LMR
(
=0.3621,
=0.0363) positively correlated with RFS.LMR
/CD8MR
(
=0.3364,
=0.0517) also showed a trend towards being positively correlated.For LCRC,CD14
(
=0.5677,
=0.009) and LMR
/CD4MR
(
=0.4541,
=0.0443) positively correlated,but CD4MR
(
=-0.473,
=0.0352) negatively correlated with OS,whilst both CD14
(
=0.6018,
=0.005) and CD8MR
(
=0.4779,
=0.331) positively correlated with RFS,and CD4CD8
(
=0.4425,
=0.0507) also showed a trend towards being positively correlated.
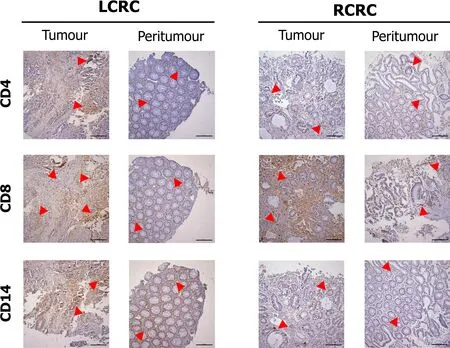
Figure 3 shows the staining pattern for CD4,CD8,and CD14 cells in tumour and peritumour samples from two representative patients of LCRC and RCRC.The distribution of total (CD4
plus CD8
) lymphocytes,CD4
lymphocytes,CD8
lymphocytes,and CD14
monocytes,in all analysed tissues,is shown in Supplementary Figure 2.Higher total lymphocyte content in tumours than peritumours from LCRC patients(13.06 ± 2.123
7.57 ± 1.794,
=0.0095) seemed due to the proportional increase of CD8
lymphocytes (11.19 ± 2.158
5.13 ± 1.757,
=0.0020),as we detected no differences amongst infiltrated CD4
lymphocytes in these tissues.No differences were found for lymphocyte infiltration in right tumours with respect to right peritumoural tissues.Moreover,infiltrated-leukocyte content in right tumours showed no differences to right peritumours.
When Friday arrived, we could hardly contain ourselves. After lunch, when we saw the delivery truck coming, we told “our family” about the surprise. It was like watching ecstatic children on Christmas morning.
They divided the apple of life and ate it together, and her heart grew full of love for him, so they lived together to a great age in undisturbed happiness
Likewise,survival after surgical intervention to remove the tumour should constitute a prominent feature to differentiate both pathologies.In this line,controversial results arise throughout the literature.Thereby,some studies support RCRC patients having poorer overall and disease-free survival rates[8],whilst others call attention to the stage of the disease,with better rates for RCRC being limited to stage II and better rates for LCRC being limited to stage III[33].In our cohort,perhaps due to the stage’s heterogeneity of the patients,both OS and RFS were found side-dependent,with better outcomes in RCRC patients,reinforcing the idea that prognostic markers for the two pathologies should be studied separately.
A number of studies have stressed the importance of the systemic inflammatory response in CRC development and the search for variables involving its components as a valuable tool to drive prognosis[15,34].Important prognostic records have been obtained in several research works[16,35],which avail the use of blood leukocyte ratios as predictors in CRC progression after surgery.However,some studies have highlighted inherent failures to these analyses.Thus,Zhang
[36] warn against the impact of the use of distinct factors,within different studies,to adjust possible confounders for multivariate hazard ratio determination,which can make the latter at risk of bias and heterogeneity,in turn making LMR fail to reach significance in survival.Likewise,sample size,race heterogeneity,and most of all the pre/postoperative dynamic changes in circulating leukocyte population can dramatically affect the observable effects of these variables in the multivariate models for survival progression[37].In our correlative analyses,though all preoperative blood leukocyte ratios significantly rose at different stages,in the end we were unable to establish a predictor value for any of them,neither for RCRC nor for LCRC survival,perhaps due to a conjunction of previously discussed handicaps.Nonetheless,we do not discard the possibility for them to emerge as good predictors in the putative case those handicaps could be solved,thus improving the multivariate analyses.
Notably,we report tissue leukocyte ratios,both alone and combined with preoperative blood LMR
,as six variables with a strong predictor value for RCRC overall survival (CD8
,CD4CD8
,LMR
,CD8MR
,LMR
/LMR
,LMR
/CD8MR
),three variables for recurrence-free survival (LMR
,CD8MR
,LMR
/LMR
,LMR
/CD8MR
),and another robust variable to predict LCRC overall survival (LMR
/CD4MR
).In addition,to avoid conflicts when interpreting the different survival predictors of RCRC,physician-friendly nomograms are proposed for both OS and RFS.Albeit much effort has been made in describing and associating the leukocyte content of tumour tissues with CRC survival[38],most studies have been performed on disaggregated tumour and peritumour samples,and only a few of them have attempted to measure leukocyte expression in fixed samples of these tissues to associate them with circulating ratios[19] or to correlate them with patient survival[18,39].Hence,this could be the first study in which leukocyte measures in both blood and fixed tissues are put together into predictor indexes for CRC survival.
It is worth noting that,in addition to the well-established predictor value of blood leukocyte ratios,the 10 indexes involve leukocyte concentrations in peritumoural zones of the bowel but not in the tumour mass.A peritumour constitutes an easily obtainable tissue during a preoperative exploration of the patient (this could be the colonoscopy),which might be safely biopsied without affecting the tumour environment in an adenoma-like surgical extraction protocol.Therefore,on a routine basis,surgeons might access both preoperative peripheral blood parameters as well as non-neoplastic peritumoural tissue (without disturbing the tumour itself) and make use of the described ratios and nomograms to predict the patient’s outcome after surgery.Thus,
surgical strategies can be designed to allow physicians to continue with surgery as programmed or delay the intervention until better scores are achieved after personalised treatments to correct the leukocyte levels in the patient.
Altogether,these indexes could be implemented in the first line of prognosis,making it easier to predict the outcome of patients after surgery depending on the tumour location and leukocyte distribution in both peripheral blood and biopsies of the peritumoural region.
Then we tried waiting by the light. We d go over and knock on the window and the driver would roll it down, looking at us kind of leery, and I d say, Hi. Since today is Thanksgiving, we d like to know if you would be willing to drive us to Harlem so we can feed some people. Every time the driver would look away quickly, furiously roll up the window and pull away without saying anything.
Limitations
Our study is mainly limited by the cohort size.It might be expected that the extension of these variables to a greater cohort would reinforce our conclusions or even make foregoing unobserved interactions surface.
My mommy says that you lost your daughter and you re very, very sad with a broken heart. Susie held her hand out shyly. In it was a Band-Aid. This is for your broken heart. Mrs. Smith gasped5, choking back her tears. She knelt down and hugged Susie. Through her tears she said, Thank you, darling girl, this will help a lot.
CONCLUSION
Herein we present important remarks on the value of combining circulating leukocyte ratios and tissue infiltrated leukocyte ratios on the sustaining of valuable prognosis tools for physicians in order to stratify patients regarding their putative outcome.In the era of personalised medicine,such indexes will provide benefits to improving both resources and well-being of CRC patients after surgery.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer (CRC) points to 9.4% of cancer deaths worldwide,ranking second after lung cancer.Despite the wide variety of factors tested to predict their outcome,most patients with similar variables show big differences in survival.Moreover,rightsided CRC (RCRC) and left-sided CRC (LCRC) patients exhibit large differences in outcome after surgical intervention as assessed by preoperative blood leukocyte ratios[today,the most extended parameters used to assess a patient’s overall survival (OS)and recurrence-free survival (RFS) after surgery].However,few efforts have been made to link tumour infiltrated leukocyte ratios to patient outcomes.
Research motivation
To determine whether both RCRC and LCRC patient outcomes could be accurately predicted based on the counting of infiltrated leukocytes in tumour and peritumoural tissues.
Research objectives
The aim of this study was to find stronger indexes than circulating (blood) leukocyte ratios to predict RCRC and LCRC patient outcomes.
After 19 years of marriage, Steve Benson unsettled his wife, Jean, when he announced three years ago that he would no longer eat meat, for ethical12 reasons
It was crowded at the golf store. I like it when it’s that way. The salespeople1 are too busy to pester2 you, and you can play with the putters all day long. I have won many imaginary tournaments on that little carpeted green.
Research methods
A prospective study was performed with CRC patients who had undergone surgical intervention to resect the tumours.Leukocyte concentrations in peripheral blood,tumour,and non-neoplastic peritumoural tissues were determined.Ratios of these parameters were evaluated as predictors for OS and RFS using Spearman correlations,Cox univariate and multivariate proportional hazards regression followed by the calculation of the receiver-operating characteristic and area under the curve (AUC)and the determination of Youden’s optimal cutoff values for those variables that significantly correlated with either RCRC or LCRC patient outcomes.Clinicianfriendly nomograms were developed to predict OS and RFS from the prediction indexes.The accuracy of the model was evaluated using calibration and validation analyses.
Research results
We obtained six robust predictors of worse OS in RCRC:CD8
lymphocyte content in peritumour (CD8
,AUC=0.585,cutoff<8.250,
=0.0077),total lymphocyte content in peritumour (CD4CD8
,AUC=0.550,cutoff<10.160,
=0.0188),lymphocyte-tomonocyte ratio in peritumour (LMR
,AUC=0.807,cutoff<3.185,
=0.0028),CD8
LMR in peritumour (CD8MR
,AUC=0.757,cutoff<1.650,
=0.0007),the ratio of blood LMR to LMR in peritumour (LMR
/LMR
,AUC=0.672,cutoff>0.985,
=0.0244),and the ratio of blood LMR to CD8
LMR in peritumour (LMR
/CD8MR
,AUC=0.601,cutoff>1.485,
=0.0101).In addition,three robust predictors of worse RFS in RCRC were found:LMR
(AUC=0.737,cutoff<3.185,
=0.0046),LMR
/LMR
(AUC=0.678,cutoff>0.985,
=0.0155),and LMR
/CD8MR
(AUC=0.615,cutoff>1.485,
=0.0141).Furthermore,the ratio of blood LMR to CD4
LMR in peritumour (LMR
/CD4MR
,AUC=0.786,cutoff>10.570,
=0.0416) was found to robustly predict poorer OS in LCRC patients.The developed nomograms to predict OS and RFS of RCRC patients showed C-indexes of 0.600 (95% confidence interval:0.561-0.639) and 0.605 (95% confidence interval:0.579-0.631),respectively.
Research conclusions
Easily obtainable variables at preoperative consultation,defining the status of leukocyte balances between peripheral blood and peritumoural tissue,have been shown to render indexes that accurately predict OS and RFS of CRC patients after surgical ablation of the tumours.
This ring was still more useful than the box, because when one wished oneself at any place one was there directly, without even the trouble of flying to it through the air
Research perspectives
We hope these indexes could be implemented in the first line of prognosis,making it easier to predict the outcome of patients after surgery depending on the tumour location and leukocyte distribution in both peripheral blood and biopsies of the peritumoural region.
We thank Dr.María Teresa Vallejo-Cremades and Onys Camps-Ortega from the Service of Image and Immunohistochemistry of IdiPAZ for helpful assistance,and Dr.Jair A.Tenorio for software support.
1 Sung H,Ferlay J,Siegel RL,Laversanne M,Soerjomataram I,Jemal A,Bray F.Global Cancer Statistics 2020:GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.
2021;71:209-249 [PMID:33538338 DOI:10.3322/caac.21660]
2 Miller KD,Nogueira L,Mariotto AB,Rowland JH,Yabroff KR,Alfano CM,Jemal A,Kramer JL,Siegel RL.Cancer treatment and survivorship statistics,2019.
2019;69:363-385[PMID:31184787 DOI:10.3322/caac.21565]
3 Augestad KM,Merok MA,Ignatovic D.Tailored Treatment of Colorectal Cancer:Surgical,Molecular,and Genetic Considerations.
2017;11:1179554917690766[PMID:28469509 DOI:10.1177/1179554917690766]
4 Forster S,Radpour R.Molecular Immunotherapy:Promising Approach to Treat Metastatic Colorectal Cancer by Targeting Resistant Cancer Cells or Cancer Stem Cells.
2020;10:569017 [PMID:33240813 DOI:10.3389/fonc.2020.569017]
5 Tan D,Fu Y,Tong W,Li F.Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer:A meta-analysis.
2018;55:128-138 [PMID:29807167 DOI:10.1016/j.ijsu.2018.05.030]
6 Mao Y,Chen D,Duan S,Zhao Y,Wu C,Zhu F,Chen C,Chen Y.Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers:a meta-analysis.
2018;18:201 [PMID:30534002 DOI:10.1186/s12935-018-0698-5]
7 Peng J,Li H,Ou Q,Lin J,Wu X,Lu Z,Yuan Y,Wan D,Fang Y,Pan Z.Preoperative lymphocyteto-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival.
2017;10:3789-3799 [PMID:28794643 DOI:10.2147/OTT.S140872]
8 Guo D,Li X,Xie A,Cao Q,Zhang J,Zhang F,Li W,Chen J.Differences in oncological outcomes and inflammatory biomarkers between right-sided and left-sided stage I-III colorectal adenocarcinoma.
2020;34:e23132 [PMID:31755593 DOI:10.1002/jcla.23132]
9 Altan M,Haberal HB,Akdo?an B,?zen H.A critical prognostic analysis of neutrophil-lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma.
2017;22:964-971 [PMID:28600686 DOI:10.1007/s10147-017-1150-x]
10 Lu A,Li H,Zheng Y,Tang M,Li J,Wu H,Zhong W,Gao J,Ou N,Cai Y.Prognostic Significance of Neutrophil to Lymphocyte Ratio,Lymphocyte to Monocyte Ratio,and Platelet to Lymphocyte Ratio in Patients with Nasopharyngeal Carcinoma.
2017;2017:3047802 [PMID:28321405 DOI:10.1155/2017/3047802]
11 Liu B,Huang Y,Sun Y,Zhang J,Yao Y,Shen Z,Xiang D,He A.Prognostic value of inflammationbased scores in patients with osteosarcoma.
2016;6:39862 [PMID:28008988 DOI:10.1038/srep39862]
12 Biswas T,Kang KH,Gawdi R,Bajor D,Machtay M,Jindal C,Efird JT.Using the Systemic Immune-Inflammation Index (SII) as a Mid-Treatment Marker for Survival among Patients with Stage-III Locally Advanced Non-Small Cell Lung Cancer (NSCLC).
2020;17[PMID:33143164 DOI:10.3390/ijerph17217995]
13 Ying HQ,Liao YC,Sun F,Peng HX,Cheng XX.The Role of Cancer-Elicited Inflammatory Biomarkers in Predicting Early Recurrence Within Stage II-III Colorectal Cancer Patients After Curable Resection.
2021;14:115-129 [PMID:33500648 DOI:10.2147/JIR.S285129]
14 Dell'Aquila E,Cremolini C,Zeppola T,Lonardi S,Bergamo F,Masi G,Stellato M,Marmorino F,Schirripa M,Urbano F,Ronzoni M,Tomasello G,Zaniboni A,Racca P,Buonadonna A,Allegrini G,Fea E,Di Donato S,Chiara S,Tonini G,Tomcikova D,Boni L,Falcone A,Santini D.Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer:a retrospective analysis of the TRIBE study by GONO.
2018;29:924-930 [PMID:29324972 DOI:10.1093/annonc/mdy004]
15 Clarke SJ,Burge M,Feeney K,Gibbs P,Jones K,Marx G,Molloy MP,Price T,Reece WHH,Segelov E,Tebbutt NC.The prognostic role of inflammatory markers in patients with metastatic colorectal cancer treated with bevacizumab:A translational study [ASCENT].
2020;15:e0229900 [PMID:32142532 DOI:10.1371/journal.pone.0229900]
16 Ying HQ,Deng QW,He BS,Pan YQ,Wang F,Sun HL,Chen J,Liu X,Wang SK.The prognostic value of preoperative NLR,d-NLR,PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients.
2014;31:305 [PMID:25355641 DOI:10.1007/s12032-014-0305-0]
17 Wu Y,Ye S,Goswami S,Pei X,Xiang L,Zhang X,Yang H.Clinical significance of peripheral blood and tumor tissue lymphocyte subsets in cervical cancer patients.
2020;20:173 [PMID:32131750 DOI:10.1186/s12885-020-6633-x]
18 Hamada T,Ishizaki H,Haruyama Y,Hamada R,Yano K,Kondo K,Kataoka H,Nanashima A.Neutrophil-to-Lymphocyte Ratio and Intratumoral CD45RO-Positive T Cells as Predictive Factors for Longer Survival of Patients with Colorectal Liver Metastasis after Hepatectomy.
2020;251:303-311 [PMID:32779620 DOI:10.1620/tjem.251.303]
19 Guo G,Wang Y,Zhou Y,Quan Q,Zhang Y,Wang H,Zhang B,Xia L.Immune cell concentrations among the primary tumor microenvironment in colorectal cancer patients predicted by clinicopathologic characteristics and blood indexes.
2019;7:179 [PMID:31300050 DOI:10.1186/s40425-019-0656-3]
20 Cantero-Cid R,Casas-Martin J,Hernández-Jiménez E,Cubillos-Zapata C,Varela-Serrano A,Avenda?o-Ortiz J,Casarrubios M,Montalbán-Hernández K,Villaca?as-Gil I,Guerra-Pastrián L,Peinado B,Marcano C,Aguirre LA,López-Collazo E.PD-L1/PD-1 crosstalk in colorectal cancer:are we targeting the right cells?
2018;18:945 [PMID:30285662 DOI:10.1186/s12885-018-4853-0]
21 Wilson DJ,Bordoni B.Embryology,Bowel.Treasure Island (FL):StatPearls,2021.Available from:https://www.ncbi.nlm.nih.gov/pubmed/31424831/
22 Kostouros A,Koliarakis I,Natsis K,Spandidos DA,Tsatsakis A,Tsiaoussis J.Large intestine embryogenesis:Molecular pathways and related disorders (Review).
2020;46:27-57[PMID:32319546 DOI:10.3892/ijmm.2020.4583]
23 Bufill JA.Colorectal cancer:evidence for distinct genetic categories based on proximal or distal tumor location.
1990;113:779-788 [PMID:2240880 DOI:10.7326/0003-4819-113-10-779]
24 Kwak HD,Ju JK.Immunological Differences Between Right-Sided and Left-Sided Colorectal Cancers:A Comparison of Embryologic Midgut and Hindgut.
2019;35:342-346[PMID:31937074 DOI:10.3393/ac.2019.03.17.1]
25 Lee GH,Malietzis G,Askari A,Bernardo D,Al-Hassi HO,Clark SK.Is right-sided colon cancer different to left-sided colorectal cancer?
2015;41:300-308 [PMID:25468456 DOI:10.1016/j.ejso.2014.11.001]
26 álvaro E,Cano JM,García JL,Brandáriz L,Olmedillas-López S,Arriba M,Rueda D,Rodríguez Y,Ca?ete á,Arribas J,Inglada-Pérez L,Pérez J,Gómez C,García-Arranz M,García-Olmo D,Goel A,Urioste M,González-Sarmiento R,Perea J.Clinical and Molecular Comparative Study of Colorectal Cancer Based on Age-of-onset and Tumor Location:Two Main Criteria for Subclassifying Colorectal Cancer.
2019;20 [PMID:30813366 DOI:10.3390/ijms20040968]
27 Baek SK.Laterality:Right-Sided and Left-Sided Colon Cancer.
2017;33:205-206[PMID:29354601 DOI:10.3393/ac.2017.33.6.205]
28 Baran B,Mert Ozupek N,Yerli Tetik N,Acar E,Bekcioglu O,Baskin Y.Difference Between Left-Sided and Right-Sided Colorectal Cancer:A Focused Review of Literature.
2018;11:264-273 [PMID:30116425 DOI:10.14740/gr1062w]
29 Ghidini M,Petrelli F,Tomasello G.Right Versus Left Colon Cancer:Resectable and Metastatic Disease.
2018;19:31 [PMID:29796712 DOI:10.1007/s11864-018-0544-y]
30 Jung MK,Shin US,Ki YJ,Kim YB,Moon SM,Sung SJ.Is the Location of the Tumor Another Prognostic Factor for Patients With Colon Cancer?
2017;33:210-218 [PMID:29354603 DOI:10.3393/ac.2017.33.6.210]
31 Stintzing S,Tejpar S,Gibbs P,Thiebach L,Lenz HJ.Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes.
2017;84:69-80 [PMID:28787661 DOI:10.1016/j.ejca.2017.07.016]
32 Benedix F,Kube R,Meyer F,Schmidt U,Gastinger I,Lippert H;Colon/Rectum Carcinomas(Primary Tumor) Study Group.Comparison of 17,641 patients with right-and left-sided colon cancer:differences in epidemiology,perioperative course,histology,and survival.
2010;53:57-64 [PMID:20010352 DOI:10.1007/DCR.0b013e3181c703a4]
33 Huang ZS,Wu JW,Li Y,Lin YH,Li XY.Effect of sidedness on survival among patients with earlystage colon cancer:a SEER-based propensity score matching analysis.
2021;19:127 [PMID:33874958 DOI:10.1186/s12957-021-02240-3]
34 Patel M,McSorley ST,Park JH,Roxburgh CSD,Edwards J,Horgan PG,McMillan DC.The relationship between right-sided tumour location,tumour microenvironment,systemic inflammation,adjuvant therapy and survival in patients undergoing surgery for colon and rectal cancer.
2018;118:705-712 [PMID:29337962 DOI:10.1038/bjc.2017.441]
35 Guo G,Chen X,He W,Wang H,Wang Y,Hu P,Rong Y,Fan L,Xia L.Establishment of inflammation biomarkers-based nomograms to predict prognosis of advanced colorectal cancer patients based on real world data.
2018;13:e0208547 [PMID:30513126 DOI:10.1371/journal.pone.0208547]
36 Zhang J,Chen L,Zhou R,Sun H,Liao Y,Liao W.Pretreatment Lymphocyte Monocyte Ratio Predicts Long-Term Outcomes in Patients with Digestive System Tumor:A Meta-Analysis.
2016;2016:9801063 [PMID:27594882 DOI:10.1155/2016/9801063]
37 Li Z,Zhao R,Cui Y,Zhou Y,Wu X.The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer.
2018;8:9453 [PMID:29930287 DOI:10.1038/s41598-018-27896-y]
38 Idos GE,Kwok J,Bonthala N,Kysh L,Gruber SB,Qu C.The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer:A Systematic Review and Meta-Analysis.
2020;10:3360 [PMID:32099066 DOI:10.1038/s41598-020-60255-4]
39 Forssell J,Oberg A,Henriksson ML,Stenling R,Jung A,Palmqvist R.High macrophage infiltration along the tumor front correlates with improved survival in colon cancer.
2007;13:1472-1479 [PMID:17332291 DOI:10.1158/1078-0432.CCR-06-2073]
 World Journal of Gastrointestinal Oncology2022年1期
World Journal of Gastrointestinal Oncology2022年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis”
- Liquid biopsy:Precise diagnosis and therapy for cholangiocarcinoma
- Increased risk of colorectal neoplasia in inflammatory bowel disease patients with post-inflammatory polyps:A systematic review and meta-analysis
- Exosomes as potential diagnosis and treatment for liver cancer
- Effects of cognitive behavior therapy combined with Baduanjin in patients with colorectal cancer
- Digestive cancer incidence and mortality among young adults worldwide in 2020:A population-based study
