Association of time-varying changes in physical activity with cardiac death and all-cause mortality after ICD or CRT-D implantation
Xue-Rong SUN, Chen-Di CHENG, Bin ZHOU?, Shuang ZHAO, Ke-Ping CHEN,Wei HUA, Yan-Gang SU, Wei XU, Fang WANG, Xiao-Han FAN, Yan DAI, Zhi-Min LIU,Shu ZHANG,?
1. Arrhythmia Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; 2. Department of Cardiology, Laboratory of Heart Center, Zhujiang Hospital, Southern Medical University, Guangzhou, China; 3. Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University, Shanghai, China;4. Department of Cardiology, Nanjing Drum Tower Hospital, Nanjing, China; 5. Department of Cardiology, Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
ABSTRACT
Physical activity (PA), which reflects the individual health status, is a strong independent predictor of cardiovascular diseases,hospitalizations for heart failure, atrial arrhythmia events, cardiovascular death, and all-cause mortality.[1?5]However, in most studies, PA was only accessed at baseline using a self-reported questionnaire over the preceding months or an accelerometer recording during the first 30?60 days after device implantation.[6]Time-varying changes in PA could reflect the longitudinal changes in respiratory and cardiovascular functions, muscle mass and strength,health-related quality of life, and lifestyle modification.[6?8]
In patients after implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) implantation, PA changes were indicated associated with CRT response or heart-focused anxiety after defibrillation implantation.[9?11]Promoting PA during the early stages after implantation is encouraged to improve cardiovascular rehabilitation.[12]Cardiac death remains as the leading cause in ICD/CRTD recipients.[13?16]However,it is controversial about the association of increasing PA post device implantation with cardiac death and all-casue mortality.[9]Lack of evidence supported that increasing PA after ICD/CRT-D implantation was beneficial to the long-term clinical outcomes without additional risks of ventricular arrhythmias(VAs) and ICD shocks.[9,17]
In this cohort study, patients received ICD or CRT-D implantation, which was equipped with a remote home monitoring system capable of providing continuous PA recording.[18]We collected the changes in PA and aimed: (1) to demonstrate the PA changes over 12 months after ICD or CRT-D implantation;(2) to determine whether increasing PA could reduce the risks of cardiac death and all-cause mortality without inducing further VAs or ICD shocks;and (3) to explore the effects of increasing PA on long-term outcomes in patients with different levels of baseline PA.
METHODS
Study Design and Participants
A retrospective cohort study was conducted using the archived home monitoring data from the Study of Home Monitoring System Safety and Efficacy in Cardiac Implantable Electronic Device-implanted Patients (SUMMIT) registry. The present study adhered to the principles of the Declaration of Helsinki, and it was approved by the ethics committees of Fuwai Hospital (the chief institute) and all other participating organizations. All patients were enrolled after providing written informed consent.
Patients undergoing ICD or CRT-D (Biotronik,Germany) implantation between May 2010 and May 2014 were included when the following criteria were met: (1) ICD or CRT-D were implanted per the guideline’s recommendations; (2) continuous home monitoring system was set-up and immediately transmitted after implantation was initiated;(3) PA recording data were complete at least for the first year after implantation; and (4) life expectancy after implantation was more than one year. Patients were excluded if they were less than 18 years old at the time of implantation, got lost to follow-up,were diagnosed with a malignant tumor, or were scheduled for heart transplantation.
PA Measurement and Data Collection
PA was measured and recorded using Biotronik accelerometers. The patients were monitored continuously during all the activities. Daily PA was expressed as the percentage of the duration period when the motion sensors detected any acceleration above 0.473 m/s2over 24 h. For example, 30 min of PA over 24 h was approximately 2.1%. The accuracy of the PA measurement was evaluated using treadmill tests.[19]Raw data were downloaded from the Biotronik home monitoring service center, saved as.csv files, and further processed.[3,4]PA changes during the early stages after ICD or CRT-D implantation were evaluated for each patient based on PA at baseline, 6 months, and 12 months after implantation.Baseline PA, PA at 6 months, and PA at 12 months were defined as the average PA recorded during the first 30-60 days, the sixth month, and the twelfth month after implantation, respectively. This processing method for PA data has been used in previous studies.[3,4]Other baseline characteristics data,including demographic and echocardiographic characteristics, comorbidities, and medications,were collected from the medical records.
Home Monitoring and Device Programming
Home monitoring data were transmitted continuously to the service center, including daily PA, heart rate, atrial and/or ventricular pacing, recorded supraventricular episodes (atrial fibrillation (AF), atrial flutter, and sinus tachycardia), VAs, anti-tachycardia pacing (ATP), shock therapy, etc. If the transmission was interrupted, the research coordinator contacted the patients or family members to immediately confirm their health conditions. Routine follow-ups were also conducted via clinic visits or telephone interviews.
All patients received ventricular fibrillation (VF)and ventricular tachycardia (VT) monitor zones programmed independently of the device type. VT was detected at rates of ≥ 140 beats/min, and VF was detected at rates of ≥ 200 beats/min. Additionally, the ICD was equipped with the Biotronik SMART algorithm, which can distinguish VT/VF from SVT episodes after analyzing the waveform and frequency of electrocardiograms.
Grouping and Study Endpoints
Based on the changes in PA over 12 months after implantation, the patients were divided into three groups: reduced or unchanged PA group, improved PA lasting for ≤ 30 min (PA ≤ 2.1%) group, and improved PA lasting for > 30 min (PA > 2.1%) group.Additionally, based on the PA tertiles at baseline,the patients were allocated to the low (tertile 1: median = 5.7%; interquartile range (IQR) = 4.0%?7.2%),moderate (tertile 2: median = 10.6%; IQR = 9.4%?11.7%),and high (tertile 3: median = 16.2%; IQR = 14.6%?18.7%) baseline PA groups.
The primary endpoints were cardiac death (ICD-10: I00?I09, I11, I20?I51) and death from all causes.The cause of death and the date of death were identified based on the death certificates supplied by family members. The secondary endpoints were the first VA event and the first appropriate ICD shock.The first VA event was defined as the first VF or VT episode requiring ATP and/or ICD shock therapies post device implantation. The VA episodes and ICD therapy were obtained from the device monitoring recordings, which were reviewed by two physicians.
Statistical Analysis
Continuous variables are presented as mean ±SD, and categorical variables are presented as frequencies and percentages. One-way analyses of variance were performed to assess the differences between the continuous variables, and chi-squared tests were used for the categorical variables. The clinical outcomes of the groups were compared using the chi-squared test.
Kaplan-Meier survival curves with log-rank tests were generated to compare the accumulative incidences of cardiac death and all-cause mortality between the different groups of PA changes. Univariable and multivariable Cox proportional hazard models were used to evaluate the effects on cardiac death and all-cause mortality. The multivariable regression model was adjusted for the variables withP-value < 0.05 in the univariable analysis and considerable meaningful cofounders mentioned in the previous studies.[3?8]
Subgroup analysis was conducted to evaluate the effects of PA changes on cardiac death and all-cause mortality in patients with different levels of baseline PA. HRs and 95% CIs were calculated to determine their impact. Statistical significance was set atP<0.05, and all tests were two-sided. The statistical analyses were conducted using SPSS Statistics (version 23.0; IBM Corp., Armonk, NY) and GraphPad Prism software (version 8.0; GraphPad Software, La Jolla, CA, USA).
Results
Baseline Characteristics
Of 1015 patients who received ICD or CRT-D implantation, 705 patients were included in this retrospective analysis. Some patients were excluded due to unavailability of home monitoring (n= 229), incomplete PA data (n= 31), life expectancy < 1 year after implantation (n= 49), and being lost to followup (n= 1).
In this cohort study, the mean age at implantation was 60.4 years, and there were more males (74.6%).ICD implantation was performed in 518 (73.5%) patients, with a mean left ventricular ejection fraction(LVEF) of 42.9%. Higher baseline PA and lower PA levels at 6 and 12 months after implantation were observed in the reduced/unchanged PA group than in the improved PA lasting for ≤ 30 min and improved PA lasting for > 30 min groups (12.39%vs.10.16%vs.10.53%,P< 0.001). Additionally, there were significant differences in sex (male, 80.7%vs.76.9%vs.67.4%,P< 0.001), diabetes mellitus (DM)(13.9%vs.12.7%vs.4.8%,P= 0.001), and pre-implant syncope (14.3%vs.26.6%vs.23.1%,P= 0.004)across the three groups. Table 1 shows the comparison of the baseline characteristics.

Table 1 Baseline characteristics.
PA Performance Over 12 Months
The PA changing curves from baseline to 6 months and 12 months after ICD implantation are shown in Figure 1. The improved PA lasting for > 30 min group showed a significant increasing trend; the improved PA lasting for ≤ 30 min group gradually increased and maintained at a level; and the reduced/unchanged PA tended to decline continuously. The distribution of PA changes from different levels of baseline PA over 6 months after ICD or CRT-D implantation was comparable to that of the PA changes over 12 months. The majority of patients (69.3% in low baseline PA levels, 65.9% in moderate baseline PA levels, and 54.5% in high baseline PA levels)had improved PA (lasting for ≤ 30 min and > 30 min) over 12 months from different levels of baseline PA. However, the percentage of patients with reduced/unchanged PA consequently increased from 30.6% in low baseline PA levels to 34.0% in moderate PA levels to 45.5% in high baseline PA levels.
Clinical Outcomes
Over a mean follow-up duration of 61.5 ± 19.9 months, 99 events of cardiac death (14.0%) and 153 events of all-cause mortality (21.7%) were observed.Higher incidences of cardiac death events (17.8%vs.13.9%vs.10.6%) and all-cause mortality (27.4%vs.19.1%vs.17.9%) were observed in patients with reduced or unchanged PA. Figure 2 shows the distribution of the incidences of cardiac death and allcause mortality based on different levels of baseline PA and different PA changes over 12 months post device implantation.
Regarding the secondary endpoints, VAs occurred in 419 patients (59.4%) and ICD shock occurred in 287 patients (40.7%). However, no significant differences in the first VA event (60.6%vs.58.4%vs.59.0%,P= 0.881) and the first ICD shock(39.8%vs.46.2%vs.40.7%,P= 0.216) were observed across the reduced/unchanged PA, improved PA lasting for ≤ 30 min, and improved PA lasting for > 30 min groups.
Kaplan-Meier survival analysis was used to compare the accumulated incidences of cardiac death,all-cause mortality, the first VA event, and the first ICD shock for the groups of PA changes over 12 months in Figure 3.
Effects of PA Changes on Cardiac Death
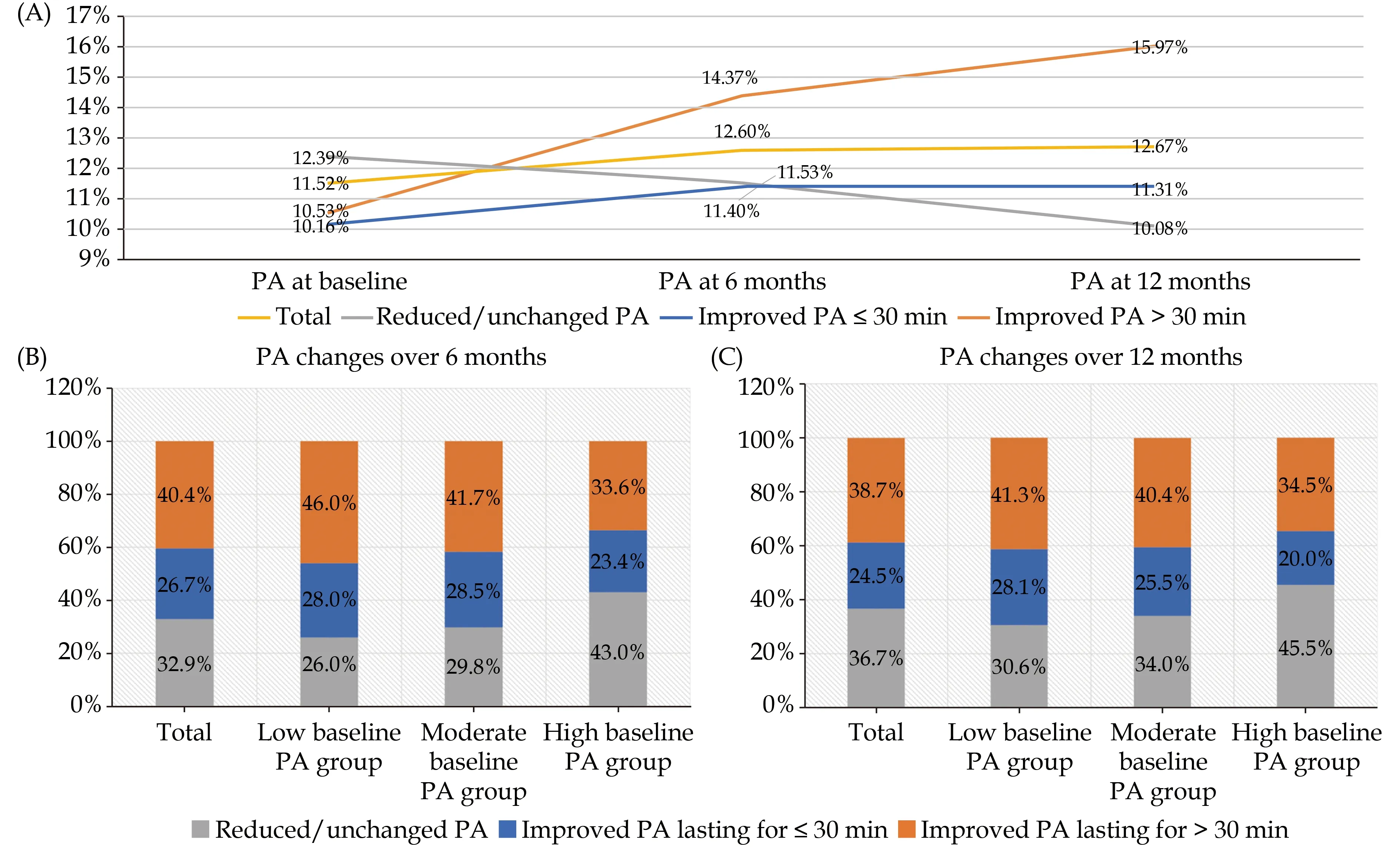
Figure 1 The curves (A) and distribution of time-varying PA changes after implantation (B & C). PA: physical activity.
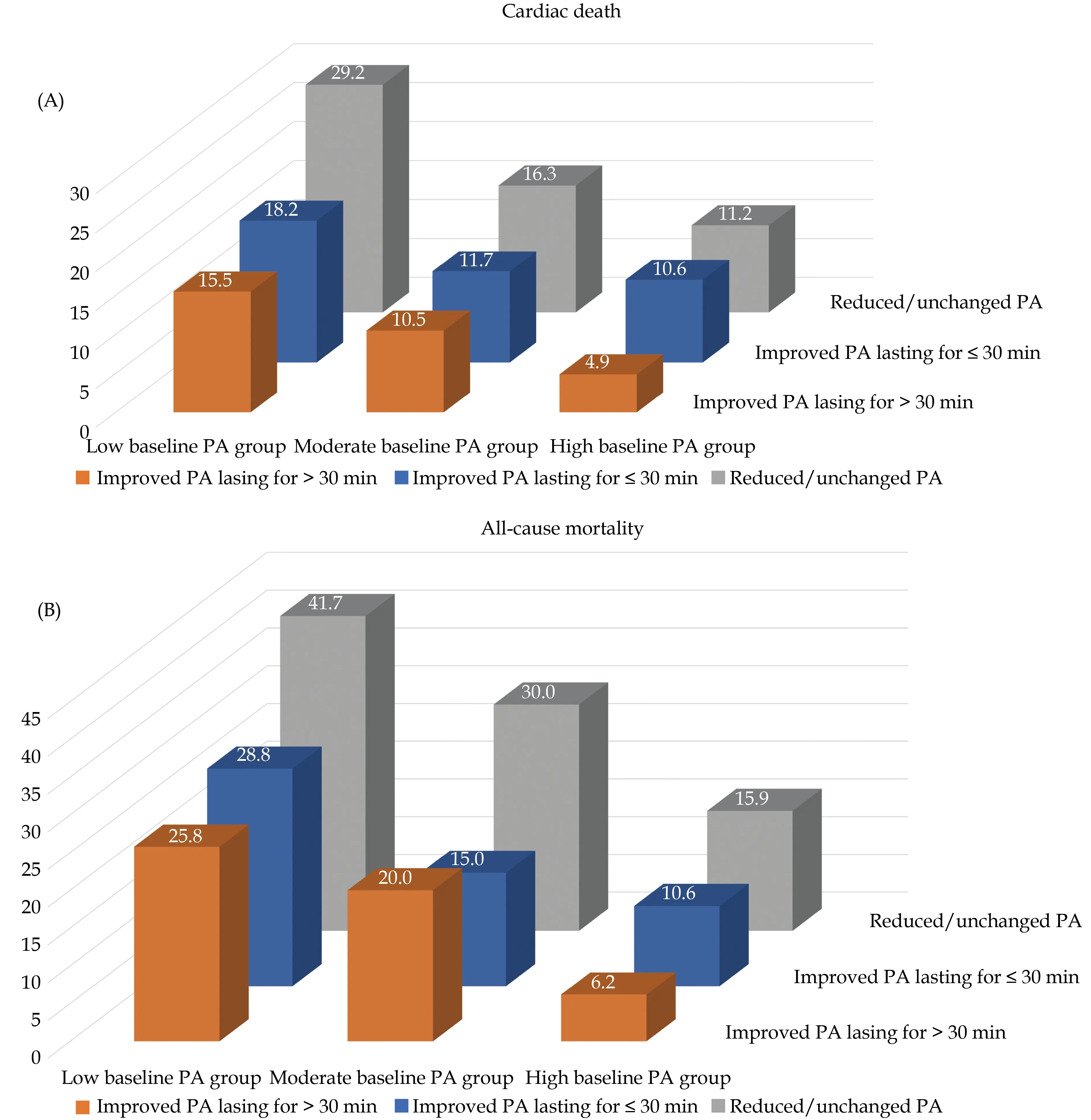
Figure 2 The incidences of cardiac death (A) and all-cause mortality (B) among different groups. PA: physical activity.
Univariable Cox proportional hazard regression analysis showed that increasing baseline PA (HR =0.914, 95% CI: 0.877-0.951,P< 0.001) and PA changes over 12 months after ICD or CRT-D implantation (HR = 0.936, 95% CI: 0.892?0.982,P=0.007) were inversely associated with the risk of cardiac death (Table 2). The multivariable Cox proportional hazard regression model was adjusted for PA at baseline, PA changes over 12 months, age at implantation, sex, body mass index (BMI), device type,NYHA class III-IV, LVEF, left ventricular end-diastolic dimension (LVEDD), hypertension (HP), DM,dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), ischemic cardiomyopathy(ICM), prior myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), prior AF, usage of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARBs), aldosterone antagonists, and loop diuretics. Lower baseline PA (HR = 0.898, 95% CI: 0.856?0.942,P< 0.001), reduced PA changes over 12 months (HR = 0.886, 95%CI: 0.834?0.940,P< 0.001), and larger LVEDD (HR =1.041, 95% CI: 1.023?1.059,P< 0.001), as continuous variables, were independent risks factor of cardiac death. Compared to the low baseline PA group, the incidence of cardiac death was reduced by 46.6% and 54.1% in the moderate (HR = 0.534,95% CI: 0.326?0.876,P= 0.013) and high (HR =0.359, 95% CI: 0.204?0.632,P< 0.001) baseline PA groups, respectively. Patients with improved PA lasting for ≤ 30 min (HR = 0.494, 95% CI: 0.288?0.848,P= 0.010) and improved PA lasting for > 30 min (HR = 0.390, 95% CI: 0.235?0.648,P< 0.001) had 50.6% and 61.0% lower risks of cardiac death than those with reduced or unchanged PA over 12 months after implantation.
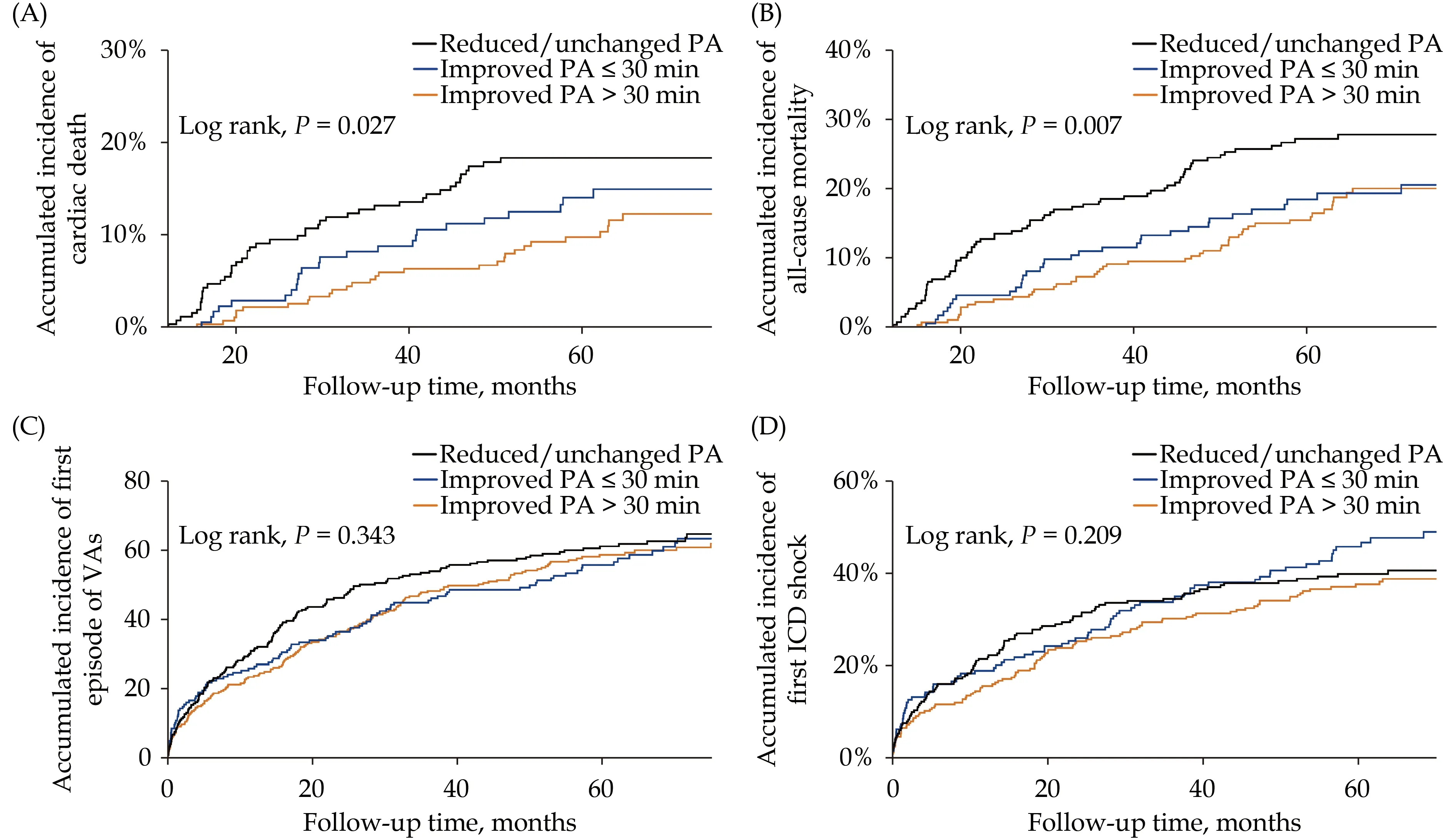
Figure 3 Kaplan-Meier survival analysis of clinical outcomes. (A): Cardiac death; (B): all-cause mortality; (C): first episode of VAs;(D): first ICD shock. ICD: implantable cardioverter-defibrillator; PA: physical activity; VA: ventricular arrthymia.
Effects of PA Changes on All-cause Mortality
The multivariable Cox proportional hazards regression analysis adjusted for PA at baseline, PA changes over 12 months, age at implantation, sex,BMI, device type, NYHA class III-IV, LVEF, LVEDD,HP, DM, DCM, HCM, ICM, prior MI, PCI, CABG,prior AF, usage of ACEI/ARBs, aldosterone antagonists, and loop diuretics showed that increased PA at baseline (HR = 0.902, 95% CI: 0.868?0.938,P<0.001), increased PA changes (HR = 0.897, 95% CI:0.855?0.942,P< 0.001) and BMI (HR = 0.932, 95%CI: 0.878?0.989,P= 0.020) were significant protective factors against all-cause mortality, while larger LVEDD (HR = 1.023, 95% CI: 1.008?1.038,P= 0.003)was an independent risk factor. In contrast with the incidence of the low baseline PA group, lower incidences of 36.4% and 68.1% of all-cause mortality were reported for the moderate (HR = 0.636, 95%CI: 0.433?0.933,P= 0.021) and high (HR = 0.319,95% CI: 0.198?0.515,P< 0.001) baseline PA groups,respectively. Patients with improved PA lasting for ≤30 min (HR = 0.467, 95% CI: 0.299?0.728,P= 0.001)and improved PA lasting for > 30 min (HR = 0.451,95% CI: 0.304?0.669,P< 0.001) over 12 months showed 53.3% and 54.9% reduced risks of all-cause mortality compared to those with reduced/unchanged PA (Table 2).
Subgroup Analysis on the Effects of PA Changes
In ICD/CRT-D recipients, the effects of PA changes on cardiac death and all-cause mortality were evaluated for the different levels of baseline PA using a multivariable Cox regression model. PA changes over 12 months, as a continuous variable,can predict the risk of cardiac death (HR = 0.850,95% CI: 0.764?0.946,P= 0.003) only in the low baseline PA group (Table 3). Compared to the re-duced/unchanged PA, the improved PA contributed to a 59.5% reduction in the risk of cardiac death. Regarding all-cause mortality, increasing PA over 12 months after implantation was associated with siginificantly lower risks of all-cause mortality in the three baseline PA groups. Improving PA reduced the risk of all-cause mortality by 56.7%, 57.4%,and 62.3% in the low (HR = 0.433, 95% CI: 0.254?0.739, P = 0.002), moderate (HR = 0.426, 95% CI:0.229?0.795, P = 0.007), and high (HR = 0.377, 95%CI: 0.156?0.911, P = 0.030) baseline PA groups, respectively, compared to the reduced/unchanged PA.
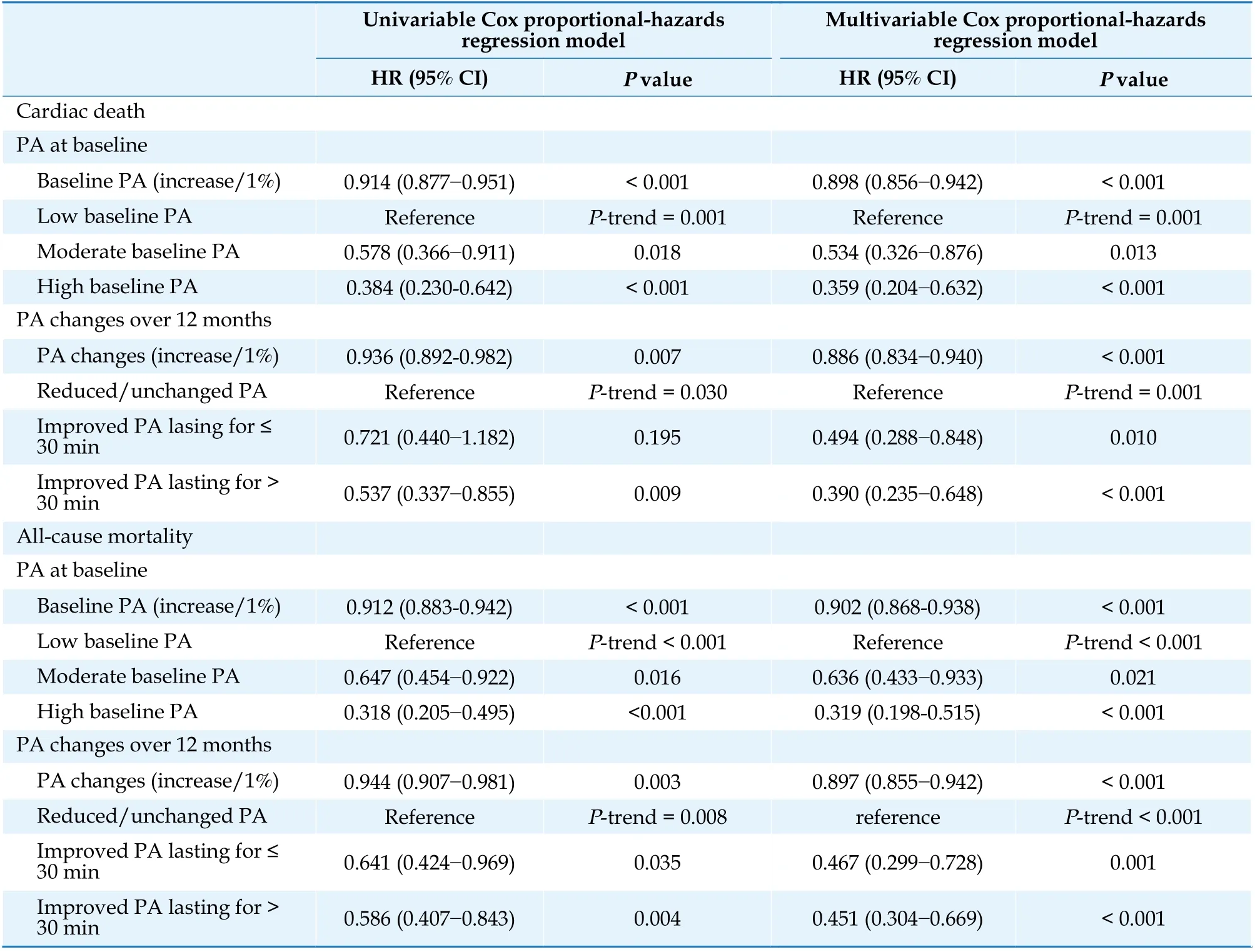
Table 2 Effects of PA changes on long-term outcomes.
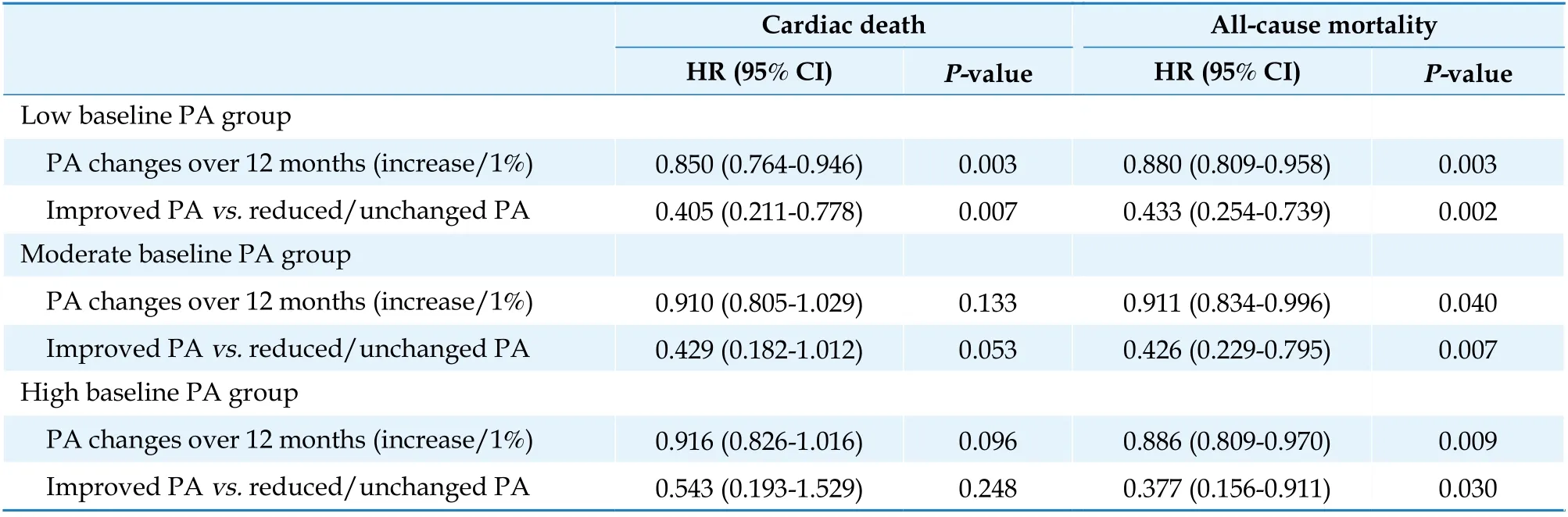
Table 3 Effects of PA changes at different baseline PA levels.
DISCUSSION
In this retrospective study, time-varying PA changes during the early 12 months after ICD/CRT-D implantation and their effects on the long-term clinical outcomes were demonstrated. The main findings were as below: (1) 63.2% of patients had improved PA over 12 months after implantation, and more patients with reduced/unchanged PA were in the higher levels of baseline PA groups; (2) improved PA lasting for ≤ 30 min and improved PA lasting for > 30 min can result in more than 50%lower risks of cardiac death and all-cause mortality,but they were not combined with more VA events or ICD shocks; and (3) the beneficial effects on cardiac death were only observed in patients with low baseline PAs, but the beneficial effects of improved PA on all-cause mortality were not dependent on the PA levels at baseline.
Previous studies have reported that high levels of baseline PA protected against cardiac death and allcause mortality.[1?4]The time-varying changes in PA was not discussed as much as the baseline PA.[6]Westerterp KR measured PA over a lifetime using total energy expenditure and reported that the changes in PA gradually increased from the early ages to adulthood but declined in old age, reflecting the changes in muscle mass and muscle strength.[20]Some studies accessed changes in PA by comparing the categories of self-reported PA intensity (light, moderate, vigorous, etc) during follow-ups.[6?8,21,22]In the Copenhagen City Heart Study, changes in PA in patients with chronic obstructive pulmonary disease(COPD) and those without COPD were compared.In contrast with patients without COPD, a longitudinal decline in PA during follow-up was more common in patients with COPD, irrespective of the PA levels at baseline.[6]Airflow obstruction and smoking status may affect PA levels in patients with COPD.[6,7]Among patients who received cardiovascular implantable electronic device implantation, changes in PA can be quantified using the accelerometer measurements.[18]The ALTITUDE Activity Study found the overall PA over 2.2 years after ICD implantation to be approximately 7.7%(111 min/day), which is comparable to 7.5% at baseline (107.5 minutes/day).[3]In the present study, the PA changes were also quantified, and the individual time-varying PA changes were evaluated. Overall, improvement in PA over 12 months after implantation occurred in 63.2% of the patients,and an increase from 11.5% (167 min/day) to 12.7%(183 min/day) was observed, demonstrating a gradually increasing curve. Moreover, patients with improved PA had lower levels of baseline PA (12.4%vs.10.2%vs.10.5%,P< 0.001) but experienced more pre-implant syncope episodes (14.3%vs.26.6%vs.23.1%,P= 0.004) than those with reduced/unchanged PA. Two possible explanations for improved PA after device implantation: first, patients were probably willing to perform appropriate behavioral modification or exercise training with the application of ICD or CRT-D devices; second, satisfactory clinical efficacy with ICD/CRT-D therapy might contribute to active physical exercise.
Regarding the effects of time-varyinig changes in PA, previous studies initially discussed those in the general population.[8,21,22]Another analysis from the Copenhagen City Heart Study evaluated the influence of leisure PA changes over 5 years on death in 7023 healthy adults across various age groups and both sexes, showing that increasing PA can facilitate longevity.[21]Petersen,et al.and Wannamethee,et al.also demonstrated that maintaining or adopting moderate to high PA could protect against heart attack,MI, and ischemic heart disease.[22,23]Thereafter, the important beneficial consequences of improved PA were verified among patients who developed cardiovascular diseases and COPD.[6,24]The mechanisms for the association between increased PA and decreased mortality appeared to be mediated by systemic changes in health status, such as improved cardiovascular function, increased maximum oxygen intake, lowered heart rate at rest and blood pressure, improved insulin sensitivity and lipid profile,and reduced platelet aggregation.[6,21]In the present study, changes in PA were obtained over 12 months after ICD or CRT-D implantation, and the results were consistent with those of previous studies.[8,21,22]Improved PA can reduce the risks of cardiac death and all-cause mortality at 5 years by 50% or more.The effects of PA changes in different baseline PA groups were also discussed. For cardiac death, only patients with low levels of baseline PA could benefit from improved PA; for all-cause mortality, the beneficial effects of improved PA were independent of PA at baseline. This finding emphasized that increasing PA may reduce the risks of cardiac death and all-cause mortality in patients with ICD or CRTD implantation, regardless of the PA levels before implantation. Thus, for patients with low baseline PA, increasing PA appropriately after implantation can help reverse the high risks of cardiac death and all-cause mortality. For patients with high baseline PA, there were about 45.5% patients who experienced reduced or unchanged PA at 12 months, and maintaining regular PA after ICD or CRT-D implantation should not be ignored in patients with high levels of PA at baseline, either.
For patients at high risk of SCD, safety should be guaranteed while increasing exercise training.[12,17]It was essential to ensure that the risks of VAs and ICD shock would not further rise while increasing physical training.[17]Based on the monitoring and reporting of ICD devices, no differences in the first VA episode and first appropriate ICD shock were observed across the reduced/unchanged PA, improved PA lasting for ≤ 30 min, and improved PA lasting for >30 min groups. In addition, we made the comparison between the improved PA lasting for ≤ 30 min and improved PA lasting for > 30 min groups. Different from baseline PA, the changes in PA seemed not to have a dose-response relationship with longterm mortality in ICD/CRT-D recipients. The average increase in the improved PA lasting for ≤ 30 min group was 1.15% (16.6 min per day) while that in the improved PA lasting for > 30 min group was 5.44% (78.3 min per day). However, the beneficial effects of improved PA lasting for ≤ 30 min and improved PA lasting for > 30 min on the risks of cardiac death (50.6% and 61.0% lowered risks of cardiac death, respectively) and all-cause mortality (53.3%and 54.9% lower risks of all-cause mortality, respectively) were comparable. This suggested that low-intensity exercise training or positive homebased behavioral modification during the early stages of 12 months after ICD or CRT-D implantation might be sufficient for satisfactory long-term clinical outcomes. The 2021 ISHNE/HRS/EHRA/APHRS expert collaborative statement on mHealth in arrhythmia management encouraged behavioral modification in patients.[25]In addition, this study also emphasized the importance of PA monitoring,which supported that self-monitoring digital medical tools could be used for promoting PA and achieving sustainable health behavioral changes.[25,26]
Strength and Limitation
In this study, ICD/CRT-D recipients were equipped with a remote home monitoring system, which can provide accurate continuous recordings about PA and ventricular tachycardia episodes. However,there were still several limitations. First, this was a retrospective cohort study. Basesd on the available evidence in this study, it was difficult to explore the underlying mechanisms of the beneficial effects of improved PA on long-term cardiac death and allcause mortality. Second, changes in PA can be affected by smoking, diet, anxiety, and cardiac autonomic activity, but these data were unattainable. In this analysis, a significant sex difference was indicated, and further discussion was not conducted because of the small number of female patients. These factors limited the interpretation of results. Third,the potential selection bias should be considered.Caution should be exercised in generalizing the results to other populations. Fourth, the accelerometerderived PA, defined from the aspect of exercise duration, cannot fully reflect the exercise intensity. The precision of the results might be affected to some extent.
CONCLUSIONS
Increasing PA during the early stages of 12 months after ICD/CRT-D implantation was beneficial in preventing cardiac death and all-cause mortality without observed rising incidences of VAs or ICD shocks. The importance of PA monitoring should be emphasized, and low-intensity exercise training might be encouraged among patients with different levels of baseline PA after ICD/CRT-D implantation.
CONTRIBUTIONS
SUN XR, CHENG CD, and ZHOU B contributed to the conception or design of the work. SUN XR,CHENG CD, ZHOU B, ZHAO S, CHEN KP, HUA W, SU YG, XU W, WANG F, FAN XH, DAI Y and LIU ZM contributed to the acquisition, analysis, or interpretation of data for the work. SUN XR and CHENG CD drafted the manuscript. ZHOU B and ZHANG S critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
FUNDINGS
This work was supported by Natural Science Foundation of China (81470466) and the National Science & Technology Pillar Program during the 12th Five-Year Plan Period (2011BAI11B02).
COMPETING INTERESTS
None.
ETHICS
The present study was approved by the ethics committee of Fuwai Hospital (the chief institute)and all other participating organisations. All patients provided written informed consent before entering this study.
DATA SHARING STATEMENT
The datasets generated and analysed during the current study are not publicly available due to the Fuwai Hospital regulations but are available from the corresponding author on reasonable request.
PATIENT AND PUBLIC INVOLVEMENT STATEMENT
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
 Journal of Geriatric Cardiology2022年3期
Journal of Geriatric Cardiology2022年3期
- Journal of Geriatric Cardiology的其它文章
- COVID-19: cardiovascular manifestations—a review of the cardiac effects
- Associations of body mass index and hospital-acquired disability with post-discharge mortality in older patients with acute heart failure
- Mortality in patients with heart failure and suicidal ideation discharged to skilled nursing facilities
- Severe aortic stenosis and acute coronary syndrome in an elderly patient with idiopathic thrombocytopenic purpura:a therapeutic challenge
- Caseous calcification of mitral annulus in the setting of multivessel disease
- Relationship of body fat and left ventricular hypertrophy with the risk of all-cause death in patients with coronary artery disease
