Severe aortic stenosis and acute coronary syndrome in an elderly patient with idiopathic thrombocytopenic purpura:a therapeutic challenge
Mehmet Rasih Sonsoz?, Selin Berk, Hamdi Pusuroglu Ahmet Guler Fatih Uzun
1. Department of Cardiology, Basaksehir Cam & Sakura City Hospital, Istanbul, Turkey; 2. Department of Hematology,Basaksehir Cam & Sakura City Hospital, Istanbul, Turkey
Severe valvular aortic stenosis is a commonly encountered disorder in the elderly, and the intervention is indicated when the patient is symptomatic. Transcatheter aortic valve implantation (TAVI) is a safer therapeutic option in symptomatic patients who cannot undergo surgery or who have high, intermediate, or even low surgical risk.[1]Idiopathic thrombocytopenic purpura (ITP)is acquired thrombocytopenia caused by autoantibodies against platelet antigens.[2]Herein, we report an 81-year-old patient, who was diagnosed with primary ITP two years ago, was admitted to the hospital for heart failure with acute coronary syndrome and severe aortic stenosis. We describe our experience in the peri-operative management of the case of coronary and structural intervention in a patient with ITP.
An 81-year-old lady presented with shortness of breath and chest pain to the emergency room. Her medical history included hypothyroidism and primary ITP, which was diagnosed two years ago,and she wasn’t taking any medication for it. She had a temperature of 36.5 ℃, a pulse rate of 105 beats/min, a respiratory rate of 30 breaths/min,and a blood pressure of 110/60 mmHg. Physical examination revealed pretibial edema with godet leaving on both legs, 3/6 systolic murmur on all cardiac auscultation sites, left-sided S4, tachypnea and bibasilar crackles. Electrocardiogram was consistent with sinus tachycardia and anterior negative T waves. Chest X-ray demonstrated increased cardio-thoracic index and interstitial pulmonary edema with bilateral pleural effusions. Complete blood count demonstrated mild anaemia with a haemoglobin level of 12.2 g/dL. The white blood cell count was normal. Platelet count was 122 ×103/μL. Liver function tests demonstrated elevated levels of aminotransferase (AST) 44 U/L, alanine transaminase (ALT) 62 U/L, and alkaline phosphatase 117 U/L, with slightly elevated levels of total bilirubin at 1.7 mg/dL and direct bilirubin at 0.7 mg/dL. Kidney function tests demonstrated elevated levels of creatinine 1.35 mg/dL. Additional test results included pro-BNP 25770 pg/mL and high sensitivity troponin T 105 pg/mL. Her transthoracic echocardiogram disclosed severely reduced left ventricular systolic function (ejection fraction (EF) measured as 30%), left ventricular hypertrophy, akinesia in anterior wall and severe aortic stenosis with a mean gradient of 41 mmHg,transaortic velocity of 3.95 m/s and valve area of 0.82 cm2(Figure 1). She was treated with intravenous furosemide. Within days, her signs and symptoms of congestion resolved. Acetylsalicylic acid 100 mg and clopidogrel 75 mg were initiated orally.A blood smear showed decreased platelets. She underwent invasive coronary angiography while having a platelet count of 40 × 103/ μL (Figure 2 and 3).There was stenosis of 90% in the proximal left anterior descending artery. We implanted a sirolimus eluting stent into the lesion (Biomime 30 × 16 mm)on 20thMay. The patient was continued on steroids for four days (dexamethasone 40 mg q.i.d.).
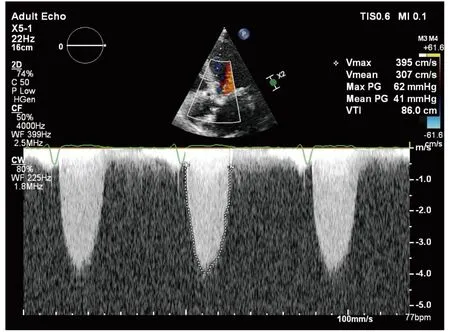
Figure 1 The Doppler echocardiography of the transvalvular flow consistent with severe aortic stenosis.
The patient received multiple platelet transfusions, and underwent transfemoral aortic valve implantation on 9thJune while having a platelet count of 50 × 103/ μL. The 6-Fr and 7-Fr sheaths were placed in the right femoral vein and right femoral artery, respectively. An 18-Fr Ultimum e-sheath was inserted into the left femoral artery under fluoroscopy following pre-placement of two Perclose devices (Abbott, Abbott Park, IL, USA). A 25-mm Portico Aortic Valve was then advanced across the aortic valve following an 18-mm Simeks-valvuloplasty balloon (Figure 4). After confirmation of position by aortography, the valve was successfully deployed during right ventricular pacing at 170 beats/min.The access was closed with the pre-positioned Perclose devices. The right femoral vein temporary pacer was removed. The patient was hemodynamically stable.
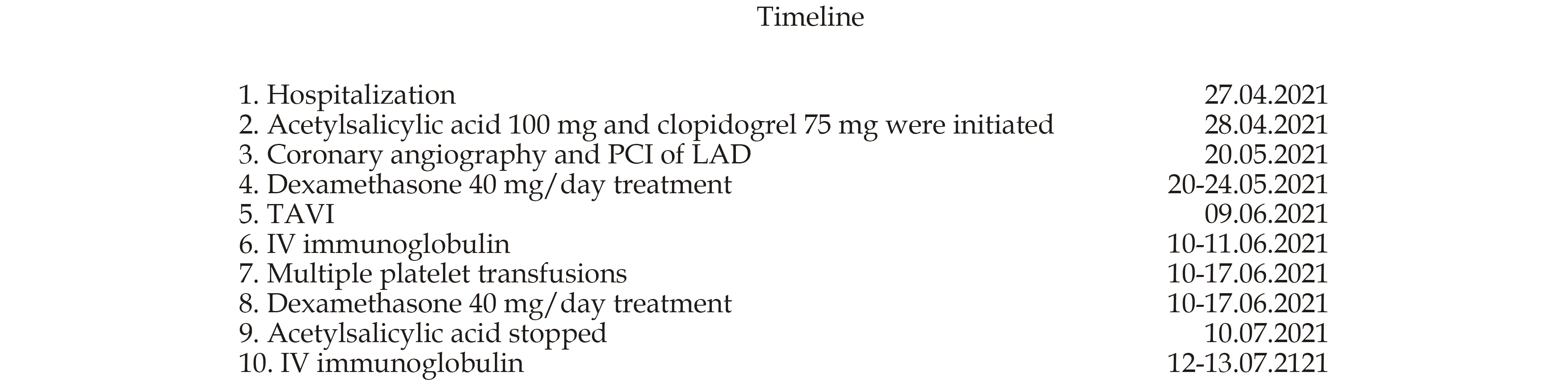
Figure 2 Timeline of the clinical course.
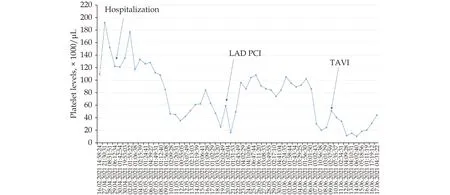
Figure 3 The course of the platelet count throughout the hospitalization. LAD: left anterior descending artery; PCI: percutaneous coronary intervention; TAVI: trans-catheter aortic valve implantation.
After the procedure, the patient was continued on acetylsalicylic acid 100 mg and clopidogrel 75 mg but platelet count decreased, therefore she was supported with platelet infusions. Intravenous immunoglobulin 1 g/kg was administered for two days.There was no evidence of access site or non-access site bleeding. The patient was subsequently discharged. At 1-month follow-up, the patient reported significant improvement in exercise tolerance and exertional symptoms. There were no bleeding issues, but the platelet count was 15 × 103/μL. Acetylsalicylic acid was stopped on 10thJuly. Intravenous immunoglobulin was repeated for two days in an outpatient setting, and the platelet count increased to 50 × 103/μL. Repeat transthoracic echocardiogram (TTE) showed improved left ventricular function (EF = 50%) and a functional bioprosthetic aortic valve with no significant stenosis or regurgitation (aortic valve area was 1.5 cm2, aortic valve area index was 0.9 cm2/m2, and mean gradient was 15 mmHg).
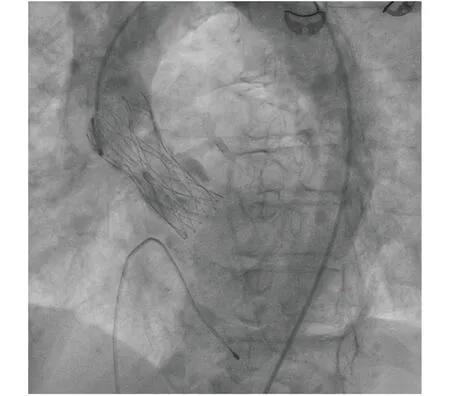
Figure 4 The angiographic image of the transcatheter implanted aortic bioprosthesis.
This case illustrates one of the challenging aspects of interventional cardiology in the setting of ITP. Severe aortic stenosis and critical proximal LAD lesion in our patient were the targets for intervention, but there was an increased risk for bleeding because of the low platelet count and the need for antiaggregants. Ito,et al.[3]recently reported that thrombocytopenia at baseline was associated with a higher incidence of composite late bleeding events(life-threatening, disabling bleeding or major bleeding) throughout the 3 years after the index TAVI procedure than no thrombocytopenia. Flaherty,et al.[4]reported that pre-existing pre-TAVI thrombocytopenia was associated with a statistically significant increased risk of vascular complications (53%),major bleeding (42%), and requirement for red blood cell transfusions (75%). It is also prudent to remember that the Academic Research Consortium for high bleeding risk included moderate or severe thrombocytopenia as a major criterion in patients who underwent percutaneous coronary intervention.[5]Our patient first underwent percutaneous coronary stent implantation, and then TAVI. She was in good health status after each intervention and at the 1-month visit. Control TTE disclosed improved left ventricular function and a functional bioprosthetic aortic valve, and she did not experience any bleeding event despite low platelet level.
Al’Aref,et al.[6]reported a case of successful TAVI in an elderly patient with Evans syndrome (autoimmune hemolytic anemia and immune thrombocytopenia). The authors stated that despite hydrocortisone 100 mg TID and 2 g/kg of intravenous immunoglobulins, the platelet count did not improve(count dropped from 64 × 103/ μL to 29 × 103/ μL).Post-procedure, the patient received romiplostim 1 μg/kg and was supported with platelet transfusions. After one month, the platelet count increased to 127 × 103/ μL. Treatment is generally recommended in ITP patients who are not bleeding if the platelet count is < 20 × 103/μL, as the bleeding risk substantially increases.[7]Any patient requiring invasive procedure and has a platelet count below the critical threshold, platelet infusions, glucocorticoids and/or intravenous are indicated according to the 2019 British Society for Hematology guideline. Because the intervention for the proximal LAD lesion and severe aortic stenosis was a necessity for the patient, we treated her with steroids, intravenous immunoglobulin and supported with multiple platelet infusions. Despite all attempts, the response of the platelet count was limited. Nevertheless, the patient successfully underwent both interventions and did not experience any bleeding events.
In conclusion, in elderly patients with ITP who are candidates for TAVI and PCI, close collaboration between hematology and cardiology is crucial for the management. With careful pre-procedural planning, vascular and bleeding complications can be avoided.
ACKNOWLEDGEMENTS
We would like to thank Emre Onar for proof editing of the text, who works as digital marketing manager in Kimberly Clark in London, the UK.
 Journal of Geriatric Cardiology2022年3期
Journal of Geriatric Cardiology2022年3期
- Journal of Geriatric Cardiology的其它文章
- COVID-19: cardiovascular manifestations—a review of the cardiac effects
- Associations of body mass index and hospital-acquired disability with post-discharge mortality in older patients with acute heart failure
- Mortality in patients with heart failure and suicidal ideation discharged to skilled nursing facilities
- Association of time-varying changes in physical activity with cardiac death and all-cause mortality after ICD or CRT-D implantation
- Caseous calcification of mitral annulus in the setting of multivessel disease
- Relationship of body fat and left ventricular hypertrophy with the risk of all-cause death in patients with coronary artery disease
