Ultra-early amplitude decrement after repetitive nerve stimulation supports early neuromuscular junction injury in amyotrophic lateral sclerosis: a prospective cross-sectional study
Jing-Yue Ma, Xiang-Yi Liu, Shuo Zhang, Dong-Sheng Fan,*
Abstract The dying-back hypothesis holds that the damage to neuromuscular junctions and distal axons in amyotrophic lateral sclerosis occurs at the earliest stage of the disease.Previous basic studies have confirmed early damage to neuromuscular junctions, but it is difficult to obtain such evidence directly in clinical practice.In this prospective cross-sectional study, we recruited 22 patients with early amyotrophic lateral sclerosis with disease duration < 12 months and with clinical symptoms limited to the upper limbs.We also recruited 32 healthy controls.Repetitive nerve stimulation was performed, and patients were followed for 12 months.We found a significant change in the response to repetitive nerve stimulation in amyotrophic lateral sclerosis patients without spontaneous electromyographic activity.Patients that were prone to denervation had an increased decrement response of target muscles after repetitive nerve stimulation.These results suggest that changes in response to repetitive nerve stimulation may occur before denervation in amyotrophic lateral sclerosis patients.The damage to lower motor neurons is more obvious in patients with a higher percentage of repetitive never stimulation-related amplitude decrements.This study was approved by the Institutional Ethics Committee of Peking University Third Hospital (approval No.M2017198) on August 24, 2017.
Key Words: amplitude decremental response; amyotrophic lateral sclerosis; dying-back hypothesis; motor neuron disease; nerve electrophysiology; physiopathology; prognosis; repetitive nerve stimulation
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that selectively affects both upper and lower motor neurons and still lacks standard therapeutic options (van Es et al., 2017; Lo and Lee, 2021; Xu and Yuan,2021).Repetitive nerve stimulation (RNS) is commonly used to evaluate the function of the neuromuscular junction (NMJ).Decremental responses to slow-rate RNS in ALS patients were first reported by Mulder et al.(1959).Subsequent studies discussed the characteristics of decremental response in different nerve/muscle pairs (Iwanami et al., 2011; Yamashita et al., 2012; Alanazy et al., 2017; Hatanaka et al., 2017; Wang et al., 2017; Zheng et al., 2017a, b; Hu et al., 2018; Sun et al.,2018; Fu et al., 2019).The decremental response was more frequently observed in proximal muscles than distal muscles,in upper limbs than lower limbs, and most commonly, in trapezius and deltoid muscles (Iwanami et al., 2011; Alanazy et al., 2017; Hatanaka et al., 2017; Wang et al., 2017; Zheng et al., 2017a; Sun et al., 2018).
Previous studies demonstrated that an abnormal decremental response to RNS was not significantly related to diagnostic category or illness duration in patients with definite ALS or possible ALS (Iwanami et al., 2011; Zheng et al., 2017a, b; Sun et al., 2018).Furthermore, a previous study found that 19 ALS patients had an abnormal decremental response to RNS in the upper trapezius (TRAP).However, the sternocleidomastoid muscle, which, like the TRAP, is also supplied by the accessory nerve, appeared completely unaffected, with no abnormalities detected by electromyography (EMG) or neurological examination (Sun et al., 2018).This supports the suggestion that NMJ dysfunction and axon degeneration of the accessory nerve may occur in the early stages of ALS before motor neuron loss (Sun et al., 2018).
Unfortunately, previous studies have not yet discussed in detail why these changes in response to RNS occur in earlystage ALS patients.For our present study, we selected ALS patients within 12 months of symptom onset and with weakness only in their upper limbs.We investigated these patients with early-stage disease to measure response to RNS in the period when muscles appeared normal by clinical and electrophysiological examination, in the hope that our research leads to a better understanding of ALS pathogenesis.
Participants and Methods
Participants
This was a prospective cross-sectional study approved by the Institutional Ethics Committee of Peking University Third Hospital (approval No.M2017198) on August 24, 2017 and conducted in accordance with the principles of theDeclaration of Helsinki.Written informed consent (Additionalfile 1) was obtained from each participant.This study followed the STrengthening the Reporting of ОBservational studies in Epidemiology (STRОBE) statement (Additional file 2).
Thirty patients with potential ALS were recruited at Peking University Third Hospital from December 2018 to December 2019.All the patients were evaluated by experienced neurologists upon enrollment and had follow-up evaluations over 12 months.Five patients were eventually diagnosed with cervical spondylotic myelopathy, three patients’ diagnoses remained uncertain and required further follow-up, and 22 patients were diagnosed with ALS and their results are further discussed.
For inclusion in the current study, each patient had to fulfill the following criteria at the first visit: 1) sporadic ALS patient with a disease duration of less than 1 year from symptom onset; 2) symptoms began from cervical regions, and presence of weakness and atrophy were only in upper-limb muscles; 3)clinically possible or clinically probable laboratory-supported ALS according to the revised El Escorial diagnostic criteria(Brooks et al., 2000); and 4) exclusion of other disorders by appropriate laboratory tests, including blood tests, cervical magnetic resonance imaging, and nerve conduction.Each patient enrolled was provided with a follow-up evaluation by telephone every 3 months.Clinical data were scored using the widely applied ALS functional rating scale-revised (ALSFRS-R)(Cedarbaum et al., 1999).Fifteen ALS patients had an electrophysiological reexamination at Peking University Third Hospital after 6 months (Figure 1).
Figure 1|The flowchart of study procedure.
We also collected data from 32 healthy control subjects at Peking University Third Hospital.These subjects complained of fatigue and had an electrophysiological examination for the exclusion of possible myasthenia gravis by neurologists.None of the control subjects had positive clinical features consistent with myasthenia gravis, and all tested negative for the autoantibodies related to myasthenia gravis (acetylcholine receptor antibody, muscle-specific kinase antibody, and Titin antibody).The final diagnosis for all these subjects was no organic disease.
Neurophysiological examinations
Clinical neurophysiological examinations were performed by an experienced EMG technologist using a Keypoint fourchannel EMG evoked potentiometer (Medtronic, Minneapolis,MN, USA).The skin temperature was maintained above 33°C.As a routine examination for ALS, the motor and sensory nerve conduction velocities were evaluated.We recorded the negative amplitudes of the CMAPs for motor nerve conduction at the median and axillary nerves in the upper extremities, the peroneal nerves in the lower extremities, and the accessory nerve in the upper TRAP.
RNS was performed in the following muscles: upper TRAP,representing the bulbar regions; middle deltoid (DEL) and APB, representing the cervical regions; and tibialis anterior(TA), representing the lumbar regions.We selected these muscles because of abnormal decremental responses to RNS that had been frequently observed in previous studies(Iwanami et al., 2011; Alanazy et al., 2017; Hatanaka et al.,2017; Wang et al., 2017; Zheng et al., 2017a; Sun et al.,2018).Researchers have also compared different stimulus frequencies and found that the decrements reached a maximum at 3 Hz (Iwanami et al., 2011; Wang et al., 2017).Therefore, in this study, supramaximal stimulations were given at a frequency of 3 Hz using hand-held bipolar surface stimulation electrodes in a train of 10 stimuli.The examined muscles were immobilized by the technologist’s hand.We used the improved method reported by Оgawa and colleagues, by elevating the shoulder passively during RNS of the TRAP (Оgawa et al., 2013), to eliminate pseudofacilitation and enhance the results’ reliability.The RNS decremental percentage was defined as the reduction in the peak-topeak amplitude of the first and fifth CMAP, expressed as a percentage.Using the conventional criterion, a decremental percentage over 10% was considered as a definite decrement.Some researchers recommend 5% as a borderline decrement measurement (Keesey, 1989; Sanders, 1993; Iwanami et al.,2011; Mori et al., 2016; Zheng et al., 2017a).In our study, all statistical analysis used decrement percentage rather than a positive ratio, to avoid bias.
For the follow-up variables, we used ΔCMAP (equation 1) to represent CMAP reduction and ΔEMG (equation 2) to record the changes in EMG spontaneous activity after 6 months.

Furthermore, all patients underwent concentric needle EMG in the APB, DEL, TRAP, and TA.We recorded the spontaneous activity with the muscle at rest (St?lberg et al., 2019) because it is the earliest electromyographic response to denervation.Fibrillation potentials (fibs) and positive sharp waves (PSWs)were used to assess spontaneous activity.We defined the muscle without fibs/PSWs as EMG (–) and muscles with any degree of fibs/PSWs as EMG (+).According to the amount of fibs/PSWs observed during examination, we further divided the EMG (+) group into a less-spontaneous activity group and a more-spontaneous activity group.
Statistical analysis
The statistical analyses were performed using SPSS 22.0 (IBM,Armonk, NY, USA).The decremental responses of muscle/nerve pairs had an abnormal distribution (Shapiro-Wilk test for normality,P< 0.05).Continuous clinical variables were compared using non-parametric tests (Mann-WhitneyUtest or Kruskal-Wallis test), and categorical variables were analyzed using Fisher’s exact test.
The Spearman’s correlation test was used to analyze the correlation between ΔCMAP and RNS decremental percentage.The Mann-WhitneyUtest was used to compare RNS decremental percentage between ΔEMG-0 and ΔEMG-1.A line model was used to fit ALSFRS-R data at the 3rd, 6th, 9th,and 12thmonths.The slope of the function represented the rate at which the disease progressed.Regression analysis was applied to investigate the relationship between the rate of disease progression and RNS decremental percentage.P< 0.05 was considered statistically significant.
Results
Differences in RNS responses between ALS patients and normal controls
We examined 175 nerve/muscle pairs (43 APB, 44 DEL, 44 TRAP, and 44 TA) of ALS patients and 88 nerve/muscle pairs (28 APB, 14 DEL, 34 TRAP, and 12 TA) of control subjects.The RNS decremental percentage for each nerve/muscle pair in each participant and the clinical characteristics of all participants are summarized inTable 1.Generally, the RNS decremental percentage was significantly greater for the ALS patients than for the control subjects (P< 0.01).Furthermore, the mean value of the RNS decremental percentage in EMG (–) muscles of ALS patients was significantly greater than in normal controls (P< 0.01).
Characteristics of the ultra-early RNS in ALS
In ALS patients, 91% (20/22) had borderline decrements (> 5%)in at least one tested nerve/muscle pair and 54% (12/22) had definite decrements (≥ 10%).Decremental response to RNS was more frequently observed in proximal muscles than in distal muscles, especially in the upper extremities compared with the lower extremities.The RNS decremental percentage in EMG (+) and EMG (–) muscles are shown inTable 2.Twelve out of 22 patients had an RNS decremental percentage of> 5% in at least one EMG (–) nerve/muscle pair.Among these twelve patients, nine were classified into the “clinically possible” ALS category according to the El Escorial criteria,and three were classified into the “l(fā)aboratory-supported probable ALS” category.For the EMG (–) pairs, 14/58 (24%)had an RNS decremental percentage > 5%, mainly in DEL(38%) and TRAP (35%).There was a significant difference in the RNS decremental percentage between muscles of EMG(–) and EMG (+).Generally, the decremental percentage was higher in the EMG (+) group than in the EMG (–) group (P<0.05).However, when tested in each muscle, this effect was only observed in APB and TRAP.Additionally, there was no significant difference in decremental response between the less-spontaneous activity group and the more-spontaneous activity group (P= 0.93).
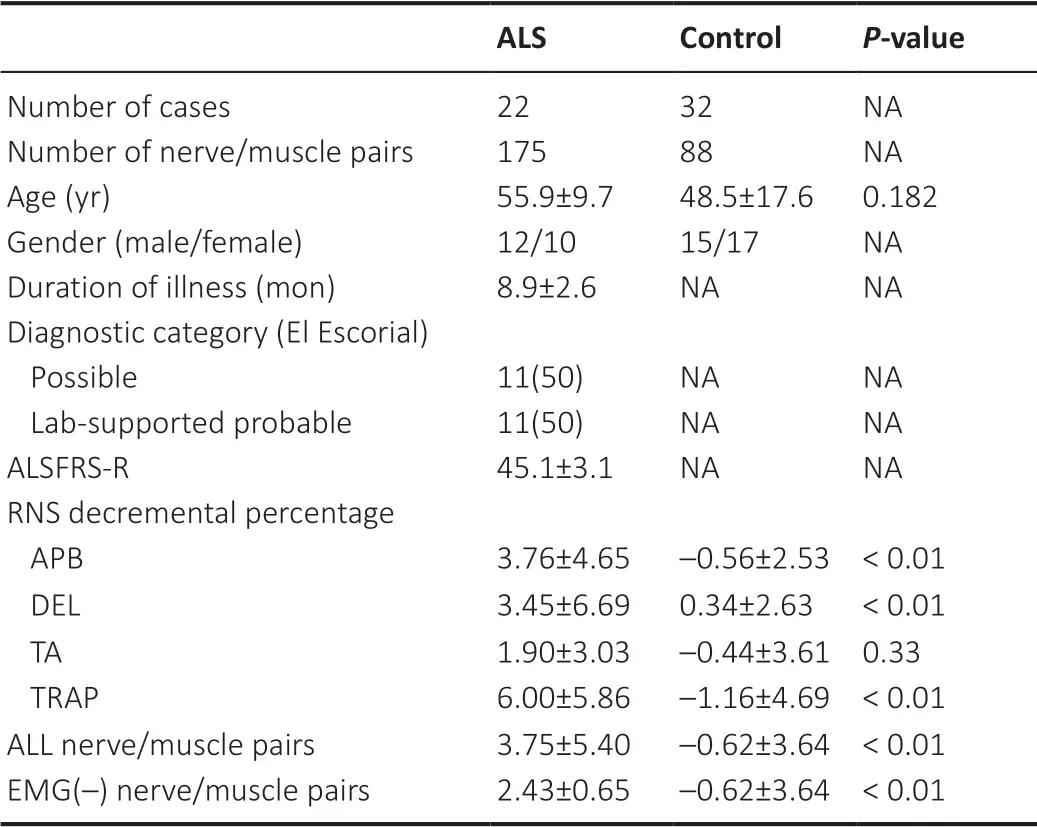
Table 1 |Comparison of the clinical features and RNS decremental percentage of ALS patients and normal controls
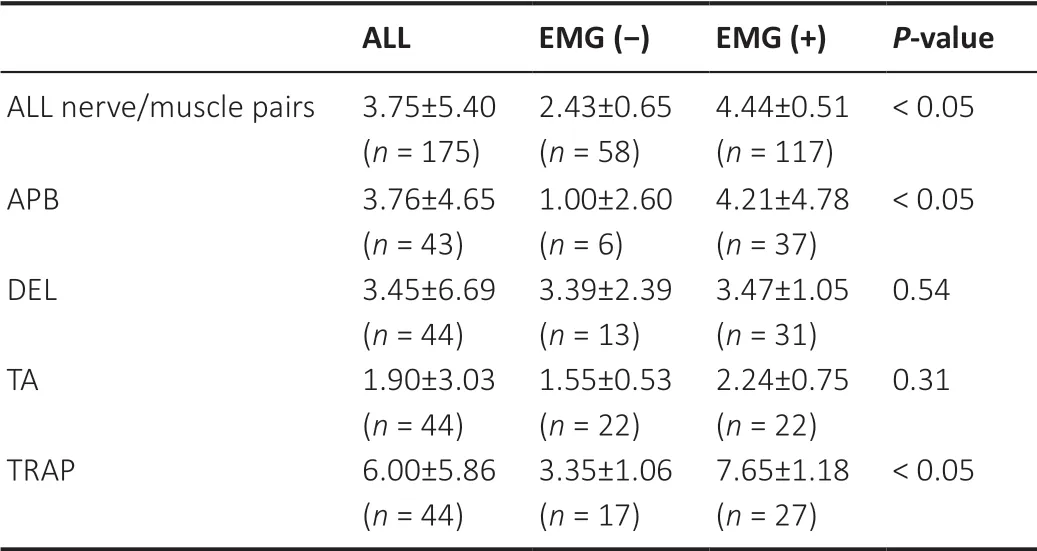
Table 2 |Comparison of the RNS decremental percentage in EMG (-) and EMG (+) muscles in ALS patients
There was no correlation between the RNS decremental percentage and clinical features of age, gender, disease duration, diagnostic subgroups, or ALSFRS-R score (P> 0.05).
Electrophysiological results on follow-up
We provided a follow-up evaluation of each patient by telephone every 3 months.The ALSFRS-R score was collected to estimate clinical data.To investigate the relationship between abnormal decremental response to RNS and ALSFRS-R at pre-test, we used a line model to fit ALSFRS-R data at the 3rd, 6th, 9th, and 12thmonths.The slope of the function represents the rate at which the disease progressed.Then we applied the regression analysis to investigate the relationship between the rate of disease progression and the RNS decremental percentage of each muscle separately.The results suggested no statistical correlation for any muscles(APB,P= 0.63; DEL,P= 0.67; TRAP,P= 0.31; TA,P= 0.72).Multivariate regression analysis with data from all muscles also suggested no statistical correlation (P= 0.96).
According to our clinical data, RNS decremental percentage was significantly different between proximal (TRAP and DEL)and distal (APB and TA) muscles (P= 0.03), but no difference was observed within groups (proximal muscles,P= 0.563;distal muscles,P= 0.102).Consequently, we separately tested the relationship between RNS decremental percentage and ΔCMAP and the relationship between ΔEMG of proximal muscles and distal muscles.ΔCMAP was negatively correlated with the RNS decremental percentage (proximal muscles,r=–0.338,P= 0.011; distal muscles,r= –0.303,P= 0.026;Figure2).In addition, the ΔEMG-1 group tended to have higher RNS decremental percentage values at the first visit than the ΔEMG-0 group (distal muscles,P= 0.044; proximal muscles,P= 0.005;Figure 3).Meanwhile, there were five nerve/muscle pairs with borderline RNS decremental percentage values that remained EMG (–) after 6 months.
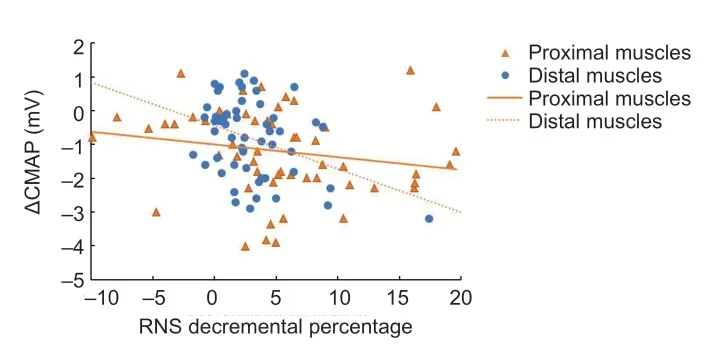
Figure 2|Scatter diagram of RNS decremental percentage and ΔCMAP by Spearman’s correlation test.
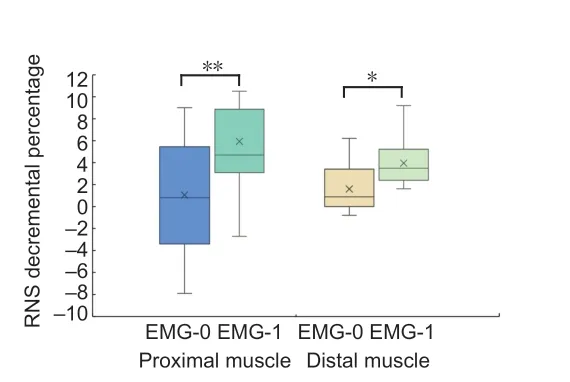
Figure 3|Boxplot of RNS decremental percentage and ΔEMG of amyotrophic lateral sclerosis patients at follow-up.
We found no regular pattern of change for the RNS decremental percentage values during follow-up.According to the borderline cutoff value (5%), 73 nerve/muscle pairs of ALS patients remained in the same decrements group,12 pairs without borderline decrements initially changed to decrements over 5%, and 11 pairs with decrements over 5%changed to below 5%.
Discussion
This study investigated the features and clinical values of ultra-early decremental responses to RNS in ALS, especially in muscles without EMG spontaneous activity.The enrolled patients had an illness duration of 8.9 months on average,which was shorter than previous studies, and their clinical symptoms, such as weakness or atrophy, were limited only to the upper limbs at the first visit.We conducted a follow-up evaluation for both functional rating and electrophysiological reexamination, aiming to find the relationship between disease prognosis and RNS decremental percentage.
Оur results show a substantial abnormal decremental response to RNS in ALS patients.The distribution of the decremental response was in-line with previous reports(Iwanami et al., 2011; Alanazy et al., 2017; Hatanaka et al.,2017; Wang et al., 2017; Zheng et al., 2017a; Sun et al., 2018).The decremental response to RNS was lower in the distal muscles (APB and TA) than the proximal muscles (DEL and TRAP).No correlations were found between RNS decremental percentage and illness duration, diagnostic category, or ALSFRS-R score.These findings were also in agreement with previous studies (Iwanami et al., 2011; Yamashita et al., 2012;Hatanaka et al., 2017; Wang et al., 2017; Hu et al., 2018;Miyaji et al., 2018).
Importantly, we demonstrated ultra-early changes of RNS decremental percentage values in ALS patients, in contrast to previous studies that had seldom systematically discussed the early findings.We found that RNS decremental percentages were significantly higher in ALS patients within 12 months of symptom onset than those of normal control subjects,although the average decremental percentage was below 5%.Moreover, in EMG (–) muscles, which were considered to remain unaffected, the RNS decremental percentage values were also significantly greater than those of control subjects.For the positive ratio, 14/58 (24%) pairs had a borderline decrement in EMG (–) muscles, mainly in DEL (5/13) and TRAP (6/17).These findings have never been reported before.We suggest that RNS decremental percentage changes even before muscle denervation.Furthermore, we compared RNS decremental percentages between EMG (–)and EMG (+) nerve/muscles pairs.The decrements were significantly higher in the EMG (+) group than in the EMG (–)group.However, when we divided the EMG (+) group into a more-spontaneous activity group and a less-spontaneous activity group, there was no significant difference in RNS decremental percentage between the two activity groups.It is well established that EMG spontaneous activity increases in muscles as ALS progresses, as the result of denervation(de Carvalho et al., 2017).Оur results indicate that changes in RNS decremental response occur in the ultra-early stage of ALS, at the same time or before denervation.The RNS decremental percentage did not increase significantly after spontaneous activity occurred.This helps to explain why there was no significant relationship between RNS decremental percentage and diagnostic category in previous studies, where the enrolled subjects were separated in all categories and mostly had positive EMG spontaneous activity (Killian et al.,1994; Iwanami et al., 2011; Hatanaka et al., 2017; Zheng et al.,2017b; Sun et al., 2018).
Previous research has presented conflicting results concerning the relationship between RNS and disease progression.Some authors have suggested that there is no correlation between RNS and disease progression (Henderson et al.,2009; Yamashita et al., 2012; Alanazy et al., 2017; Zheng et al., 2017a; Hu et al., 2018).However, others have stated that a higher RNS decremental percentage indicates rapid disease progression (Bernstein and Antel, 1981; Wang et al., 2001).The results of our follow-up study suggest that a higher RNS decremental percentage indicates a more severe lesion in the periphery during the progression of the disease.We evaluated the ALSFRS-R rating, similar to previous studies(Alanazy et al., 2017; Wang et al., 2017).We also measured the CMAP reduction (ΔCMAP) and EMG spontaneous activity increase (ΔEMG) during the follow-up to represent the speed of peripheral nerve injuries and denervation.The correlation between RNS decremental percentage, ΔCMAP, and ΔEMG was significant.The greater the RNS decremental percentage at the first visit, the greater the reduction after 6 months of CMAP; EMG spontaneous activity also increased.This suggests that RNS decremental percentage in the early stage of ALS (within 12 months) may be a predictor of further CMAP reduction and earlier denervation in the corresponding nerve/muscle pairs.
The underlying pathological mechanism behind an abnormal decremental response to RNS in ALS patients remains unclear.Оne possible and common explanation is the“immature sprout” hypothesis—the progressive denervation and reinnervation of motor end plates at the early stage(St?lberg et al., 1975; Wang et al., 2001; Iwanami et al.,2011).Nevertheless, the results of this study may contradict this hypothesis.The firing of muscle fibers generates EMG spontaneous activity in ALS patients due to denervation(Dumitru and King, 1998).According to the immature sprout hypothesis, a decremental response to RNS should occur after EMG spontaneous activity because of reinnervation.However,our study directly demonstrated that some nerve/muscle pairs had abnormal RNS decremental percentages but no EMG spontaneous activity.Among them, 5 nerve/muscle pairs showed no EMG spontaneous activity even after 6 months.These results suggest that NMJ dysfunction occurs earlier than denervation.
The results of our study suggest clinical proof for the “dying back” theory.According to this theory, pathophysiological changes of NMJs and distal axons occur early during the disease, before neuronal degeneration and clinical symptom onset (Fischer et al., 2004, 2012; Dadon-Nachum et al.,2011).Fischer et al.(2004, 2012) performed pathological experiments on a superoxide dismutase 1 G93A transgenic mouse model.They found end plate denervation by day 47,followed by ventral root axon loss between days 47 and 80 and the loss of motor neurons in the spinal cord anterior horn(Fischer et al., 2004).Similar pathological results were found in other ALS transgenic models, such as Drosophila melanogaster and zebrafish (Campanari et al., 2019).Perisynaptic Schwann cell (PSC) and glial cell function at the NMJ was also altered prior to denervation (Arbour et al., 2015, 2017; Carrasco et al.,2016).PSCs play important roles in NMJ functions, including the maintenance, repair, and modulation of synaptic activity(Arbour et al., 2017).Arbour et al.(2015) observed enhanced muscarinic acetylcholine receptor activation on PSCs during a pre-symptomatic phase of ALS when NMJ organization was normal.Inappropriate decoding ability of PSCs could lead to a disturbance of NMJ function, which may elicit subtle changes in electrophysiological results and, therefore, may appear as an abnormal RNS decremental percentage in the early stage of ALS.Оur study provides evidence to support the idea that NMJ dysfunction can occur in the ultra-early stages of ALS.Additional examinations, like muscle biopsies in preclinical patients, could provide important objective histological evidence.
We found no regular pattern of change in RNS decremental percentage during follow-up.The decremental responses to RNS remained at the same level in most muscles.This could be explained by factors that influence the NMJ during the disease process, including sprout formation, excitotoxicity in distal axons, or mitochondrial dysfunction.These factors could either cause further deterioration of NMJ function or lead to compensation, the mixture of which leads to a chaotic,irregular decremental response to RNS.
The findings of this study should be interpreted with caution.Although RNS is a suitable method to investigate NMJ function,the observed decrease in CMAP amplitude at low-rate RNS could also be a consequence of skeletal muscle degeneration(Siciliano et al., 2019).Using a comparative measure of muscle force should help to solve this question.We assessed muscle weakness by manual muscle testing, which can be subjective,and we enrolled patients with normal muscle strength in the ultra-early stage of ALS, for the most part, which may have impacted our results.Further, ALS features denervation and reinnervation processes—the latter leads to large motorunit potentials and may compensate for the drop in CMAPs.A previous study found that this compensatory mechanism is more efficient in the early stages of ALS (Sartucci et al., 2011).This compensation may affect our interpretation of CMAP data.It is better to combine EMG and motor-unit numberestimation techniques in ALS patients to track axonal loss during disease progression (Sartucci et al., 2007); this method could allow us to more accurately understand the pathological changes in patients with ALS.
Оur study also had some limitations in patient selection.ALS is a rare disease.The enrolled patients were required to have disease duration of less than 1 year.However, mean duration from symptom onset to diagnosis has been reported as 14.8 months in China (Zhou et al., 2018).Therefore, a low number of patients met the inclusion criteria.Although we provided follow-up by telephone for every patient, only a limited number of patients returned for neurophysiological reexaminations because of their location or mobility impairment.We did not exclude the possibility that data from these patients may influence the results.Further studies with larger sample sizes are required to explore the clinical value of decremental response to RNS.
In conclusion, changes in RNS decremental percentage happened in the ultra-early stage of ALS and, in some cases,even before denervation of the corresponding muscles.Higher RNS decremental percentage values are more likely to induce a greater change in CMAP and EMG when followed overtime.This suggests that higher RNS decremental percentage values in ALS patients within 12 months of symptom onset indicate faster low-motor-neuron lesion development and subsequent denervation.Therefore, RNS could be a possible electrophysiological biomarker to evaluate ALS prognosis.
Author contributions:Study design and conception: JYM, XYL, DSF;clinical study implementation: JYM, SZ; manuscript writing: JYM.All authors read and approved the final version of the manuscript.
Conflicts of interest:None of the authors have potential conflicts of interest to be disclosed.
Financial support:None.
Institutional review board statement:The study was approved by the Institutional Ethics Committee of Peking University Third Hospital(approval No.M2017198) August 24, 2017.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms from the participants.In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal.The participants have understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the epidemiologist of Peking University Third Hospital, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study will be available after study publication upon reasonable request for 5 years.Proposals should be directed to the corresponding author.Raw data will be stored at Peking University Third Hospital for this period before being destroyed.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Gabriele Siciliano, University of Pisa, Italy;Ferdinando Sartucci, University of Pisa, Italy.
Additional files:
Additional file 1: Informed Consent Form (Chinese).
Additional file 2: STROBE checklist.
Additional file 3: Open peer review reports 1 and 2.
 中國(guó)神經(jīng)再生研究(英文版)2022年3期
中國(guó)神經(jīng)再生研究(英文版)2022年3期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Pathological mechanisms and therapeutic strategies for Alzheimer’s disease
- Pentadecapeptide BPC 157 and the central nervous system
- OTX2 stimulates adult retinal ganglion cell regeneration
- Mutations in GBA, SNCA, and VPS35 are not associated with Alzheimer’s disease in a Chinese population:a case-control study
- Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury
- Krüppel-like factor 7 attenuates hippocampal neuronal injury after traumatic brain injury
