Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury
Jingmin Liu, Lixia Li, Ying Zou, Lanya Fu, Xinrui Ma, Haowen Zhang,Yizhou Xu,, Jiawei Xu, Jiaqi Zhang, Mi Li, Xiaofang Hu, Zhenlin Li,Xianghai Wang,, Hao Sun, Hui Zheng, Lixin Zhu, Jiasong Guo,,,5,*
Abstract Wallerian degeneration, the progressive disintegration of distal axons and myelin that occurs after peripheral nerve injury, is essential for creating a permissive microenvironment for nerve regeneration, and involves cytoskeletal reconstruction.However, it is unclear whether microtubule dynamics play a role in this process.To address this, we treated cultured sciatic nerve explants, an in vitro model of Wallerian degeneration, with the microtubule-targeting agents paclitaxel and nocodazole.We found that paclitaxel-induced microtubule stabilization promoted axon and myelin degeneration and Schwann cell dedifferentiation, whereas nocodazole-induced microtubule destabilization inhibited these processes.Evaluation of an in vivo model of peripheral nerve injury showed that treatment with paclitaxel or nocodazole accelerated or attenuated axonal regeneration, as well as functional recovery of nerve conduction and target muscle and motor behavior,respectively.These results suggest that microtubule dynamics participate in peripheral nerve regeneration after injury by affecting Wallerian degeneration.This study was approved by the Animal Care and Use Committee of Southern Medical University, China (approval No.SMUL2015081) on Оctober 15, 2015.
Key Words: axon; demyelination; microtubule dynamics; nerve regeneration; nocodazole; paclitaxel; peripheral nerve injury; Schwann cell;Wallerian degeneration
Introduction
Wallerian degeneration (WD) is a progressive process of demyelination and anterograde disintegration of the distal axonal segment following transection of the axon, which may occur in peripheral nerve injury (PNI) or traumatic brain injury(Conforti et al., 2014).During WD, debris from degraded axons and myelin is removed by macrophages and Schwann cells to ensure a permissive microenvironment for axonal regeneration (Nocera and Jacob, 2020; Qu et al., 2021).Therefore, gaining a better understanding of WD could be helpful in developing novel therapeutic strategies for PNI.The morphology of axons and the myelin sheath changes dramatically during WD, suggesting that the cytoskeleton,which includes microfilaments, intermediate filaments, and microtubules, is involved in this process.Indeed, the roles that microfilaments and intermediate filaments play in WD are well-established (Triolo et al., 2012; Wang et al., 2018).However, the relationship between microtubules and WD remains unclear.
Microtubules are intrinsically dynamic polymers.They have two states: stable and labile.The continuous transition between stable and labile states is called microtubule dynamics(Fees and Moore, 2018; Manka and Moores, 2018; Honoré et al., 2019).Microtubule dynamics are a key regulator of various biological activities (Maciel et al., 2018).For example,microtubule-targeting agents are widely used to treat cancer (Senese et al., 2017; Roviello et al., 2019).As axon regeneration may involve internal protrusive forces generated by microtubules, these agents are also considered to be potential therapies for central nervous system injury.Many studies have demonstrated that treatment with paclitaxel, a microtubule stabilizer, can promote central nervous system axonal regeneration (Hellal et al., 2011; Sengottuvel et al.,2011; Austin et al., 2017).In contrast, treatment with the microtubule-destabilizing agent nocodazole inhibits axonal regeneration in the central nervous system (Hu et al., 2018).However, little is known about the relationship between microtubule dynamics and peripheral nerve regeneration, and it remains unclear whether microtubule-destabilizing agents affect WD and nerve regeneration after PNI.While a few studies have investigated the effects of microtubule-stabilizing agents on PNI, the results are contradictory.For example, Hsu et al.(2017) reported that paclitaxel impairs nerve regeneration in a rat model of sciatic nerve transection, while Zhou et al.(2020) found that epothilone B, another U.S.Food and Drug Administration–approved microtubule-stabilizing agent,facilitates peripheral nerve regeneration.To our knowledge,the current study is the first to describe the role of microtubule dynamics in WD, and the first to explore the effects of both a microtubule-stabilizing agent (paclitaxel) and a microtubuledestabilizing agent (nocodazole) on PNI repair.
Materials and Methods
Experimental animals and ethics statement
Twenty-seven specific pathogen-free Sprague-Dawley female adult rats (aged 8 weeks) weighing 200–250 g and five neonatal rats (postnatal day 3) were provided by the Animal Center of Southern Medical University, China (license No.SCXK (Yue) 2016-0041).The rats were housed in an animal room maintained at 21°C and 55% relative humidity with a 12-hour light/dark cycle and given free access to water and food.All procedures, including surgery and tissue collection,were carried out with the approval of the Southern Medical University Animal Care and Use Committee (approval No.SMU-L2015081, approval date Оctober 15, 2015) in accordance with the guidelines for the ethical treatments of animals.All efforts were made to minimize the number of animals used and their suffering.
Sciatic nerve explant culturing and treatment
Sciatic nerve explant culturing, which is anin vitromodel of WD, was performed as described previously (Shin et al.,2013; Park et al., 2015; Li et al., 2021).Briefly, nine adult rats were anesthetized by intraperitoneal injection of 12 mg/mL tribromoethanol (180 mg/kg; Sigma-Aldrich, St.Louis, MО,USA) and decapitated by guillotine.Sciatic nerve segments (0.5 cm in length) were isolated and incubated in the Dulbecco’s modified Eagle’s medium/F12 (Corning, New York, NY, USA)containing 3% fetal bovine serum (Corning), 3 mM forskolin(Sigma-Aldrich), 10 ng/mL heregulin (PeproTech, Rocky Hill, NJ,USA), and 100 mg/mL penicillin-streptomycin (Gibco, Grand Island, NY, USA).Paclitaxel (Sigma-Aldrich) or nocodazole(Sigma-Aldrich) was dissolved in anhydrous dimethyl sulfoxide(Sigma-Aldrich) to produce a 2 or 10 mg/mL stock solution,respectively, which was stored at –20°C until administration.Paclitaxel or nocodazole was added to the culture medium,and the nerve explants were collected after culturing for 5 or 8 daysin vitro(div) for subsequent analysis.A vehicle-only control group was included.
Schwann cell culturing and treatment
The concentrations of paclitaxel and nocodazole to be used for subsequent experiments were determined based on a previous report (Imai et al., 2017) and preliminary experiments performed using cultured Schwann cells.First, Schwann cells were isolated from neonatal rats as we described earlier (Wen et al., 2017, 2018; Tan et al., 2018).Briefly, five neonatal rats were subjected to hypothermic anesthesia by placing them on ice, and then disinfected with 75% alcohol.Next, all the spinal nerves were aseptically isolated and digested with 0.25% Trypsin-ethylene diamine tetraacetic acid (Gibco) to obtain a single-cell suspension.After incubation in Dulbecco’s modified Eagle’s medium/F12 containing 10% fetal bovine serum in poly-L-lysine (Sigma-Aldrich)-coated petri dish (Jet Biofil, Guangzhou, China) for 24 hours, the cells were treated with 10 μM of cytosine arabinoside (Sigma-Aldrich) for 48 hours to eliminate the fibroblasts.Subsequently, the cells were cultured in the Dulbecco’s modified Eagle’s medium/F12 containing 3% fetal bovine serum, 3 μM forskolin (Sigma-Aldrich), and 10 ng/mL heregulin (PeproTech).The passage 3 cells were then treated with paclitaxel (1, 10, 50, 100, or 200 nM) or nocodazole (1, 10, 100, 500, or 1000 nM) for 24 hours.Morphologic changes were observed with a phase contrast microscope (Leica, Wetzlar, Germany) immediately after treatment, or after an additional 24 hours of culturing in fresh medium to assess washout effects (Felitsyn et al., 2007).
Sciatic nerve crush injury model and treatment
A rat model of sciatic nerve crush injury was established as described previously (Qian et al., 2018).Briefly, adult rats were randomly divided into vehicle, paclitaxel, and nocodazole groups (n= 6 for each group) and anesthetized by intraperitoneal injection of 180 mg/kg tribromoethanol.Then, the bilateral sciatic nerves were bluntly exposed, and a crush injury 0.5 cm distal to the sciatic notch was performed by clamping the nerve with a smooth, straight hemostat (tip width 2 mm) for 2 minutes.Before the incision was closed, the crush site was marked with a 9-0 nylon suture.Immediately after the surgery, rats were injected intraperitoneally with 100 μL paclitaxel (200 μM, stock solution diluted with saline),nocodazole (1000 μM, stock solution diluted with saline), or vehicle (dimethyl sulfoxide diluted with saline), as described previously (Cook et al., 2018).At 3 days post injury (dpi), three rats from each group were anesthetized by intraperitoneal injection of 180 mg/kg tribromoethanol, after which the left sciatic nerves were collected for western blot assay.Next, transcardial perfusion with 4% paraformaldehyde was performed, and the right sciatic nerves were collected for immunohistochemistry.The remaining three rats in each group were treated with the appropriate drug (or control injection) every 2 days for 2 weeks following the surgery.At 28 dpi, behavioral assessments and electrophysiological tests were performed, after which the rats were sacrificed andtissues were collected for further assays (Additional Figure 1).
Behavioral assessment and electrophysiological testing
Recovery of motor function controlled by the injured sciatic nerve was detected using a catwalk analysis system (DigiGait,Framingham, MA, USA) (Glajch et al., 2012).From 25 to 27 dpi,the rats were trained to walk on the motorized transparent treadmill belt three times per day.At 28 dpi, their footprints were captured using a video camera set beneath the belt to calculate the sciatic functional index (SFI) using the associated software (Wu et al., 2011).The SFI was calculated according to the following formula: SFI = –38.3(EPL– NPL)/NPL + 109.5(ETS– NTS)/NTS + 13.3(EIT– NIT)/NIT – 8.8.Three different parameters were measured: (1) distance from the heel to the third toe (print length; PL); (2) distance from the first toe to the fifth toe (toe spread; TS); and (3) distance from the second toe to the fourth toe (intermediate toe spread; ITS); E indicates experimental animals, and N indicates normal animals.
Six hours after the final behavioral test, the rats were anesthetized with 180 mg/kg tribromoethanol, and electrophysiological testing was performed (Pan et al., 2017;Li et al., 2019; Hu et al., 2020; Lai et al., 2020).Briefly, a pair of needle electrodes was inserted to stimulate the sciatic nerve 3 mm proximal to the crush site, and a pair of recording electrodes was inserted subcutaneously into the middle of the intrinsic foot muscle.After a single stimulation with a strength of 10 mA and a duration of 0.1 ms, the amplitude and latency of the compound muscle action potential (CMAP)were recorded with a set of electrophysiological recorders(frequency of 20 Hz and pulse width of 0.1 ms) (Axon Digidata 1550 Digitizer, Molecular Devices, Sunnyvale, CA,USA).Immediately after the CMAP recording, the rats were transcardially perfused, and the gastrocnemius muscles and sciatic nerves were collected for the following assessments.
Histomorphometry of the gastrocnemius muscle
To assay the level of myoatrophy of the target muscle of the injured nerve, the wet weight of the bilateral gastrocnemius muscles collected at 28 dpi was measured on a laboratory scale(METTLER TОLEDО, Zurich, Switzerland).Then the mid-belly of the muscles was trimmed for routine paraffin embedding,transversal sectioning, and hematoxylin-eosin staining to visualize the myofibers.As described previously (Wang et al.,2014; Pan et al., 2017), six non-overlapping images of every eighth section from each animal were captured, and the total myofiber area was quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemistry of cryosections and teased nerve fibers
The distal trunk of the injured sciatic nerve was harvested from the perfused rats at 3 or 28 dpi, and the nerve explants were collected at 5 or 8 div.Both types of nerve segments were fixed in 4% paraformaldehyde for 24 hours and dehydrated in 30% sucrose overnight.Then they were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance CA,USA) for cryosectioning and subsequent immunofluorescence staining.To assess the myelin ovoid formation that occurs during WD, five nerve explants from each group were also teased into individual fibers and mounted on adhesion slides(Guo et al., 2014; Wen et al., 2017).
The 10 μm-thick cryosection slices and teased fibers were stained for immunohistochemistry as follows.The samples were first permeabilized with 0.5% Triton X-100 (Sigma) for 30 minutes, then blocked with 5% fish gelatin (Sigma) containing 0.3% Triton X-100 at room temperature for 1 hour, followed by incubation with the primary antibodies diluted in blocking buffer overnight at 4°C.Goat anti-rabbit Alexa Fluor 488(1:400, Cat# A-11008), goat anti-rabbit Alexa Fluor 568 (1:400,Cat# A-11011), and goat anti-mouse Alexa Fluor 568 (1:400,Cat# A-11031) fluorescent-conjugated secondary antibodies(all from Molecular Probes, Eugene, ОR, USA) were applied for 2 hours at room temperature.The samples were then incubated with 4′,6-diamidino-2-phenylindole (1:5000; Sigma)for 2 minutes to counterstain the cell nuclei.Finally, images were captured using a fluorescence microscope (Leica).The primary antibodies used for immunocytochemistry were as follows: mouse anti-myelin basic protein (MBP; 1:200, Cat#NE1018, Calbiochem, Darmstadt, Germany), rabbit antineurofilament 200 (NF200; 1:400, Cat# N0142, Sigma), mouse anti-α-tubulin (1:1000; Cat# AB7291, Abcam, Cambridge, UK),rabbit anti-myelin-associated glycoprotein (MAG; 1:400; Cat#AB89780, Abcam), mouse anti-c-Jun (1:400; Cat# 610326, BD Biosciences, New York, NY, USA), rabbit anti-growth-associated protein 43 (GAP43; 1:400, Cat# ab16053, Abcam), and rabbit anti-superior cervical ganglia protein 10 (SCG10; 1:500; Cat#NBP1–49461, Novusbio, Littleton, CО, USA).
Oil red O staining
To assess myelin degradation during WD, oil red О (ОRО)staining was performed on the nerve explant cryosection(Li et al., 2021).Briefly, the 0.3% ОRО staining solution was prepared by mixing ОRО (dissolved in 60% 2-propanol) with deionized water (ratio = 3:2).The sections were rinsed in 0.01 M phosphate buffered saline and 60% isopropanol, then incubated in the ОRО solution for 15 minutes at 37°C and rinsed in 60% isopropanol and 0.01 M phosphate buffered saline and mounted.Images of each section were captured using a fluorescence microscope.
Western blot assay
Protein extracts from the collected nerve segments (1 cm of sciatic nerve spanning the injury site and the distal trunk), as well as thein vitrocultured nerve explants, were prepared by routine procedures, then separated on 10% dodecyl sulfate sodium salt-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA).After blocking with 5% bovine serum albumin (GBCBIО, Guangzhou, China) in Tris-buffered solution containing 0.5% Tween-20 for 2 hours, the blots were probed overnight at 4°C with the following primary antibodies: rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (1:3000, Cat#CW0101, Cwbiotech, Beijing, China), rabbit anti-tyrosinated(tyr)-tubulin (1:2000; Cat# MAB1864-I, Sigma), mouse antiacetylated (ace)-tubulin (1:3000; Cat# T7451, Sigma), mouse anti-MBP (1:200; Cat# NE1018, Calbiochem, Darmstadt,Germany), rabbit anti-NF200 (1:1000, Cat# N0142, Sigma),rabbit anti-MAG (1:400; Cat# AB89780, Abcam), mouse antic-Jun (1:400; Cat# 610326, BD Biosciences), rabbit anti-GAP43 (1:1000; Cat# ab16053, Abcam), and rabbit anti-SCG10(1:1000; Cat# NBP1–49461, Novusbio).Next, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies (1:2000, Cat# 65-6120, Molecular Probes)for 2 hours at room temperature, and were visualized using enhanced chemiluminescence (EpiZyme, Shanghai, China).Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal reference.Finally, the density was calculated using Image-Pro Plus 6.0 software (Media Cybernetics).The relative protein expression levels were normalized to GAPDH.
Statistical analysis
All data are presented as mean ± standard error of the mean(SEM).All statistical analyses were performed with GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA)using one-way analysis of variance followed by Bonferroni’s multiple test.APvalue of < 0.05 was considered statistically significant.
Results
Effects of paclitaxel and nocodazole on microtubule dynamics
First we tested the effect of microtubule-targeting agents on microtubule stabilization and destabilization.Based on concentrations used in a previous report (Imai et al., 2017),we treated nerve explants with a series of concentrations of paclitaxel (0, 1, 10, 50, 100, or 200 nM) or nocodazole(0, 1, 10, 100, 500, or 1000 nM).As expected, expression of acetylated tubulin (ace-tub), a marker of stable microtubules(Xiong et al., 2019), was up-regulated with increasing paclitaxel concentrations, while expression of tyrosinated tubulin (tyr-tub), a marker of unstable microtubules (Hu et al., 2017), was down-regulated with increasing paclitaxel concentrations.In contrast, treatment with nocodazole led to a decrease in ace-tub levels and an increase in tyr-tub levels (P< 0.05;Figure 1A–F).
Next, primary Schwann cells were isolated from neonatal rats,and passage 3 cells were treated with paclitaxel (1,10, 50, 100,or 200 nM) or nocodazole (1, 10, 100, 500, or 1000 nM) for 24 hours.As the drug concentration increased, the changes in cell morphology became more and more pronounced, and cell growth was increasingly hampered (Figure 1G&H).However,when the drugs were washed out, even the cells that had been treated with the highest concentrations recovered their normal morphology and regrew quickly (Figure 1I&J).These results indicate that the highest drug concentrations tested,200 nM paclitaxel and 1000 nM nocodazole, safely regulate the microtubule dynamics of Schwann cells without inducing cell death; therefore, we chose these concentrations for use in subsequent experiments.
Effects of paclitaxel and nocodazole on axon and myelin degeneration
Nerve explant cultures have been demonstrated to be a reliablein vitromodel for studying WD (Shin et al., 2014;Park et al., 2015; Li et al., 2021).This simplified model eliminates unpredictable factors that can interfere within vivoexperiments.The nerve explants were cultured for 5 or 8 days,after which longitudinal sections were stained with NF200 (an axonal marker (Hu et al., 2019)) and MBP (a myelin marker (Li et al., 2021)).The immunostaining results showed that both the axons and myelin were undergoing degeneration, which is characteristic of WD.We found that treatment with paclitaxel significantly accelerated axon and myelin degeneration, while treatment with nocodazole significantly inhibited this process(bothP< 0.05;Figure 2A–J).Similar trends were observed when NF200 and MBP levels were assessed by western blot (P< 0.05;Figure 2K–N).
Effects of paclitaxel and nocodazole on myelin degeneration and fragmentation
ОRО staining is used to label lipid droplets in adipocytes(Schachner-Nedherer et al., 2019; Velickovic et al., 2020).Recent studies have indicated that degenerated myelin fibers,which have a shrunken shape, also stain heavily with ОRО,while normal myelin fibers, which have a circular shape, only stain faintly (Wang et al., 2020; Zou et al., 2020).Therefore,we stained cross sections of nerve explants cultured for 5 or 8 days with ОRО and calculated the proportion of degenerated nerve fibers (shrunken myelin fibers with high fluorescence intensity) in each sample, as well as the mean intensity of ОRО fluorescence.The proportion of degenerated myelin in the paclitaxel group was significantly higher than that in the vehicle group, and was the lowest in the nocodazole group (P< 0.05;Figure 3).Next, the nerves were teased into single nerve fibers and immunostained for α-tubulin to facilitate visualization of the myelin sheath by phase contrast microscopy.Myelin ovoids, which are the typical structure of a fragmented myelin sheath, was easily identified and quantified using this method(Jung et al., 2011) (Figure 4AandB).Consistent with the immunohistochemistry results, the number of myelin ovoids present in every 200-μm length of teased fiber (the ovoid index) (Jung et al., 2011) was highest in the paclitaxel group,followed by the vehicle group and then the nocodazole group (P< 0.05 among the three groups;Figure 4C).
Effects of paclitaxel and nocodazole on Schwann cell dedifferentiation
A WD progresses after PNI, mature Schwann cells undergo dedifferentiation.The dedifferentiated Schwann cells play a crucial role in WD; for example, they release chemoattractants to recruit macrophages that clear the debris of degenerated axons and myelin, and can phagocytose and degrade this debris themselves (Jang et al., 2017).Thus, we next performed immunostaining and western blotting with c-Jun (a marker of immature Schwann cells (Scapin et al., 2020)) and MAG (a marker of mature Schwann cells (Bolívar et al., 2020))antibodies to evaluate Schwann cell dedifferentiation in nerve explants cultured for 5 or 8 days.The ratio of c-Jun/DAPI double-positive cells to all DAPI-positive cells and the level of c-Jun expression were dramatically higher in the cells induced with paclitaxel than in the vehicle and nocodazole groups,while both values were lowest in the nocodazole group.In contrast, the ratio of MAG/DAPI double-positive Schwann cells to all DAPI-positive Schwann cells and the level of MAG expression were significantly higher in the paclitaxel group than in the vehicle group, and both values were lowest in the nocodazole group (P< 0.05;Figure 5).
Effects of paclitaxel and nocodazole on nerve regeneration after sciatic nerve crush injury
To assess the role of microtubule dynamics on nerve regeneration, rats were subjected to sciatic nerve crush injury and immediately treated with paclitaxel, nocodazole, or saline(vehicle).Three days later, immunohistochemistry for GAP43(a marker of axonal regeneration (Romeo-Guitart et al., 2020))or SCG10 (a marker for regenerating sensory axons (Lai et al., 2020)) was performed on the injured nerves to detect regenerating axons.Оn cross sections taken 5 mm distal to the injured site, we found the highest density of both GAP43- and SCG10-positive axons in the paclitaxel group and the lowest density in the nocodazole group (P< 0.05;Figure 6A–D).To verify these results, total protein isolated from a 1-cm segment of the nerve spanning the injured site and the distal trunk was subjected to western blot.GAP43 and SCG10 expression in the injured nerve were significantly higher in the paclitaxel group compared with the vehicle group, and significantly lower in the nocodazole group (bothP< 0.05;Figure 6E–G).
Based on our and others’ previous studies, we selected 28 dpi as the time point for evaluating nerve recovery outcomes.At this time point, the numbers of NF200-positive axons and MBP/NF200 double positive myelinated axons in a cross section taken 5 mm distal to the injured site were highest in the paclitaxel group and lowest in the nocodazole group(P< 0.05;Figure 7).Next, we assessed the wet weight and total myofiber area of the gastrocnemius muscle, a key target muscle of the sciatic nerve, as these measures are widely used to assess nerve regeneration (Hu et al., 2020).Gross observation of the gastrocnemius muscles and closet observation of the hematoxylin-eosin-stained cross sections showed that myoatrophy was attenuated in the paclitaxel group compared with the vehicle group; however, myoatrophy was increased in the nocodazole group compared with the vehicle group (P< 0.05;Figure 8).
The ultimate aim of nerve repair is to restore nerve conduction and reverse functional losses (Wang et al., 2014; Li et al., 2019).SFI assessment is widely used to test the functional recovery of the injured hindlimb after sciatic nerve injury in animal models(Hu et al., 2020; de Оl(fā)iveira Marques et al., 2021).Therefore,we performed this assessment using a footprint test system,and found that there was significantly greater motor function recovery in the paclitaxel group compared with the vehicle group, and significantly lower recovery in the nocodazole group(P< 0.05;Figure 9A–C).CMAP amplitude and latency can be evaluated to assess nerve conduction (Pan et al., 2017; Park et al., 2018) (Figure 9D).Statistical analysis of the CMAP images generated in our study showed that, compared with the vehicle group, treatment with paclitaxel resulted in a higher amplitude(which indicates that more axons regenerated and arrived at the muscles measured in the paws (Zhan et al., 2013)), as well as a shorter latency (which corresponds to quicker nerve conduction (Pan et al., 2017)).The nocodazole group exhibited the lowest amplitude and longest latency among all three groups (P< 0.05;Figure 9E&F).
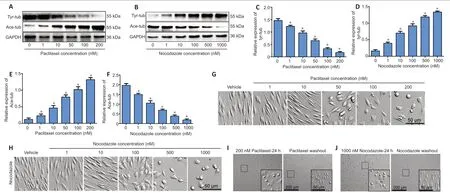
Figure 1|The effects of paclitaxel and nocodazole on microtubule dynamics in nerve explant Schwann cells.
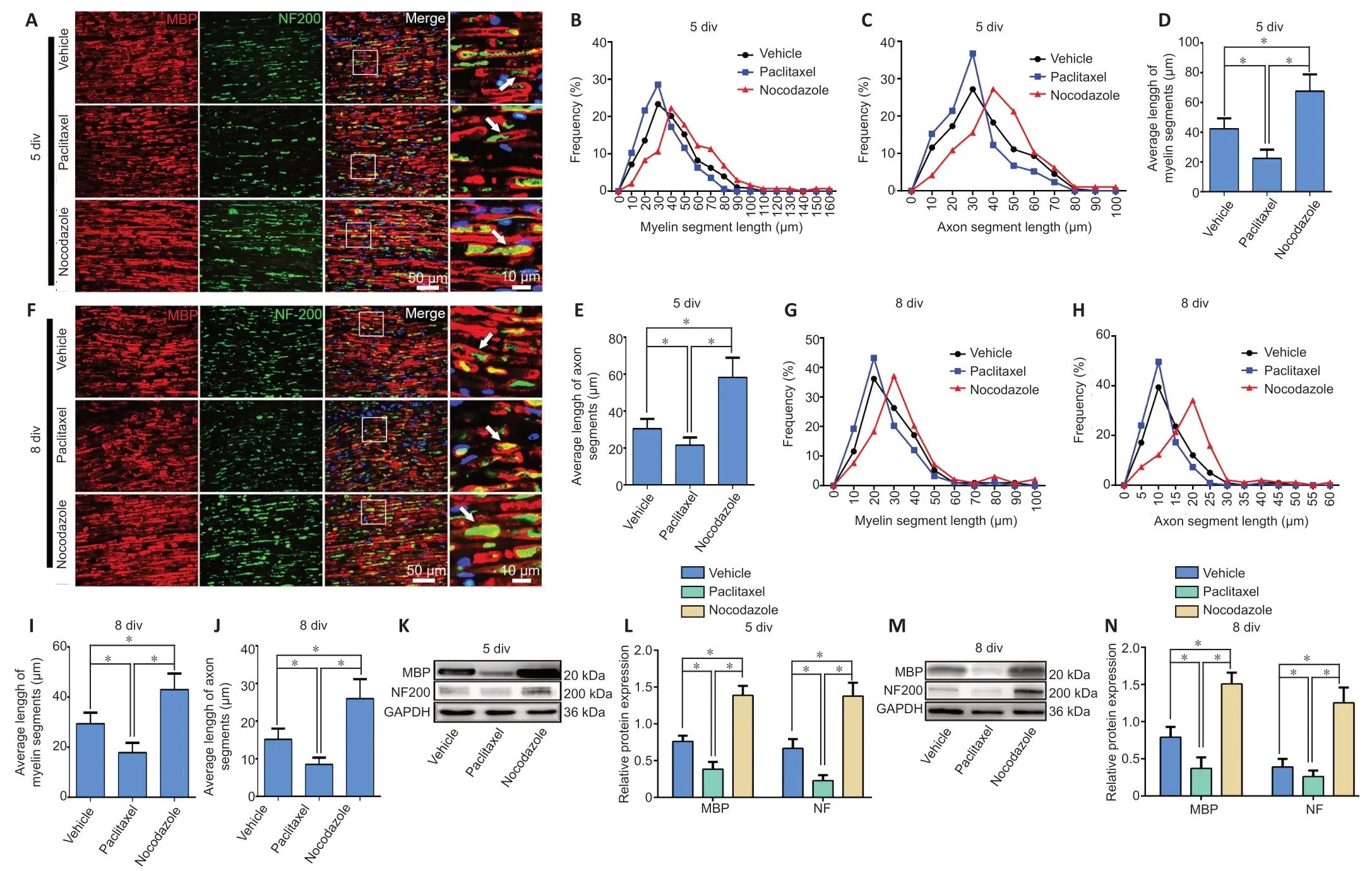
Figure 2|Paclitaxel and nocodazole affect axon and myelin degeneration in nerve explants.
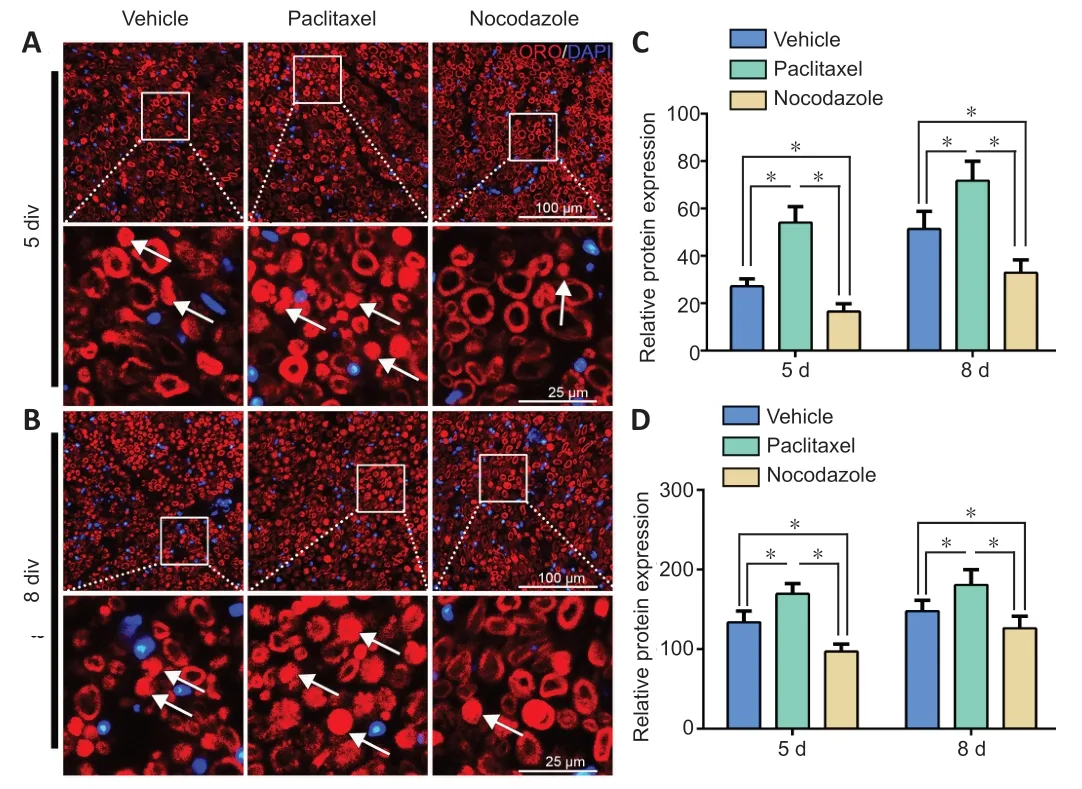
Figure 3|The effects of paclitaxel and nocodazole on myelin degeneration in nerve explants.
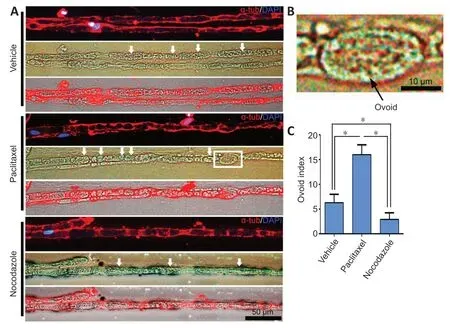
Figure 4|Teased nerve explant fibers exhibit myelin ovoid formation.
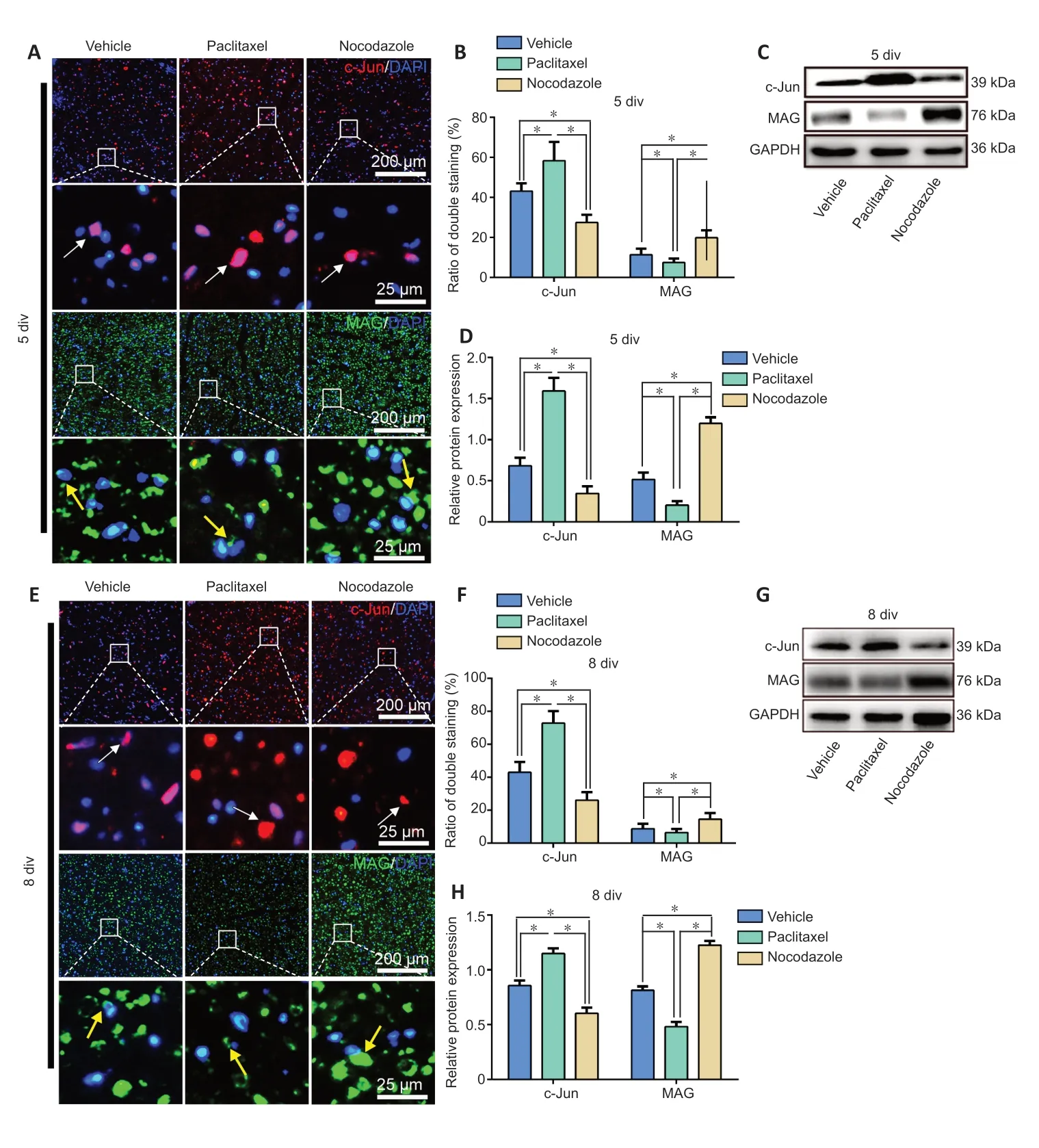
Figure 5|Effects of paclitaxel and nocodazole on Schwann cell dedifferentiation in sciatic nerve explants.
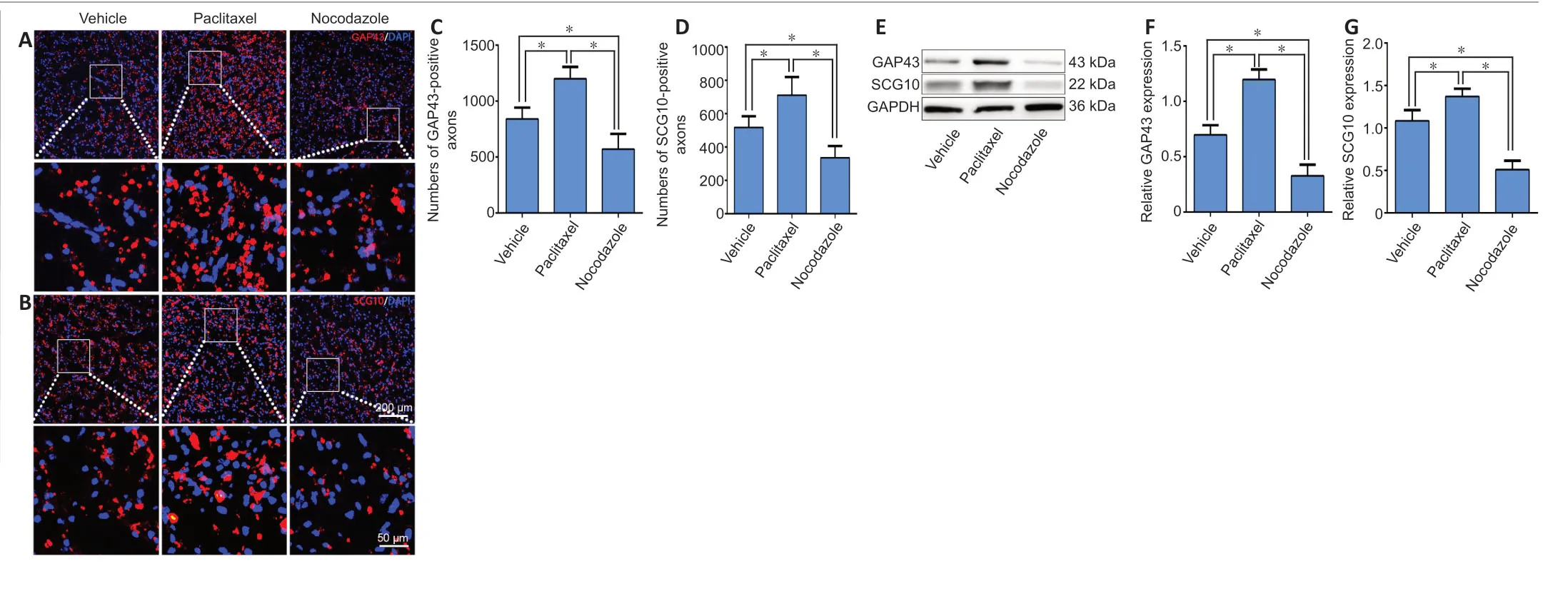
Figure 6|Paclitaxel and nocodazole affect axonal regeneration in the distal trunk of the injured sciatic nerve at 3 dpi.
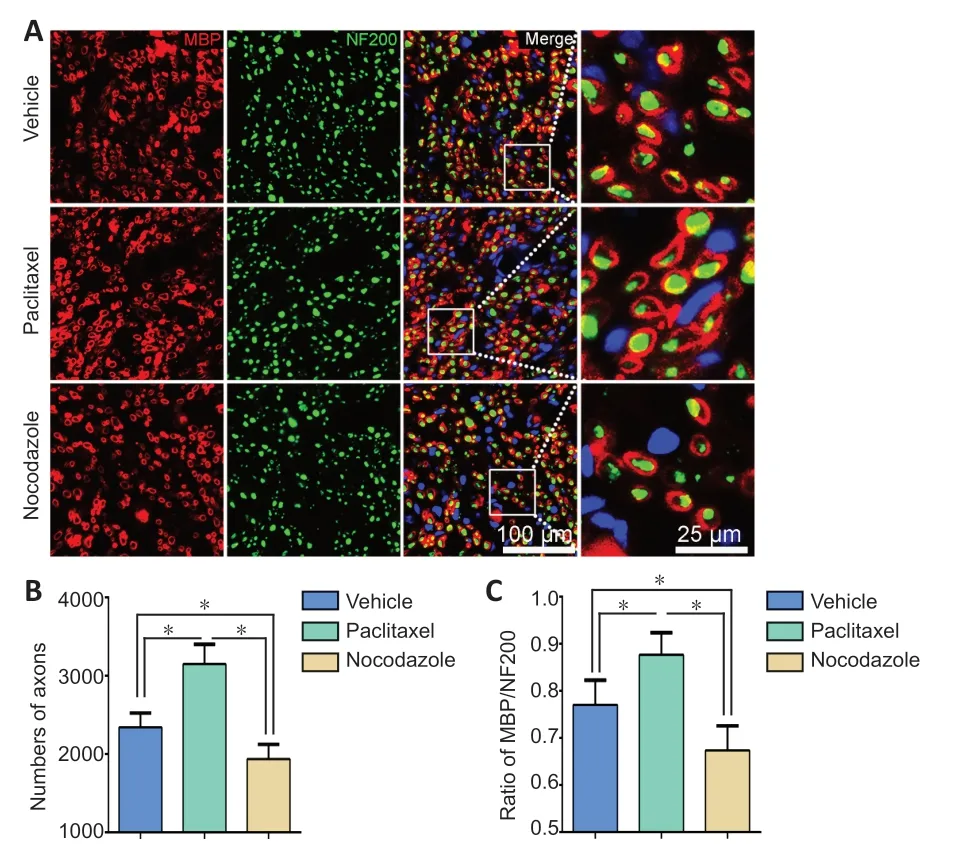
Figure 7|Effects of paclitaxel and nocodazole on nerve regeneration of the injured sciatic nerve at 28 dpi.
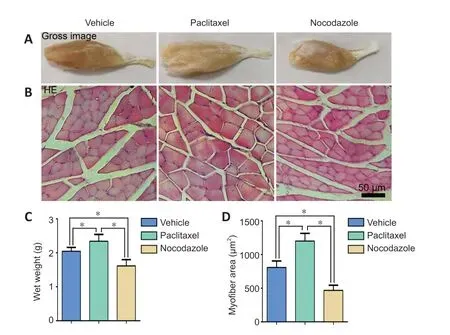
Figure 8|Effects of paclitaxel and nocodazole on gastrocnemius muscle myoatrophy in rats with sciatic nerve injury.
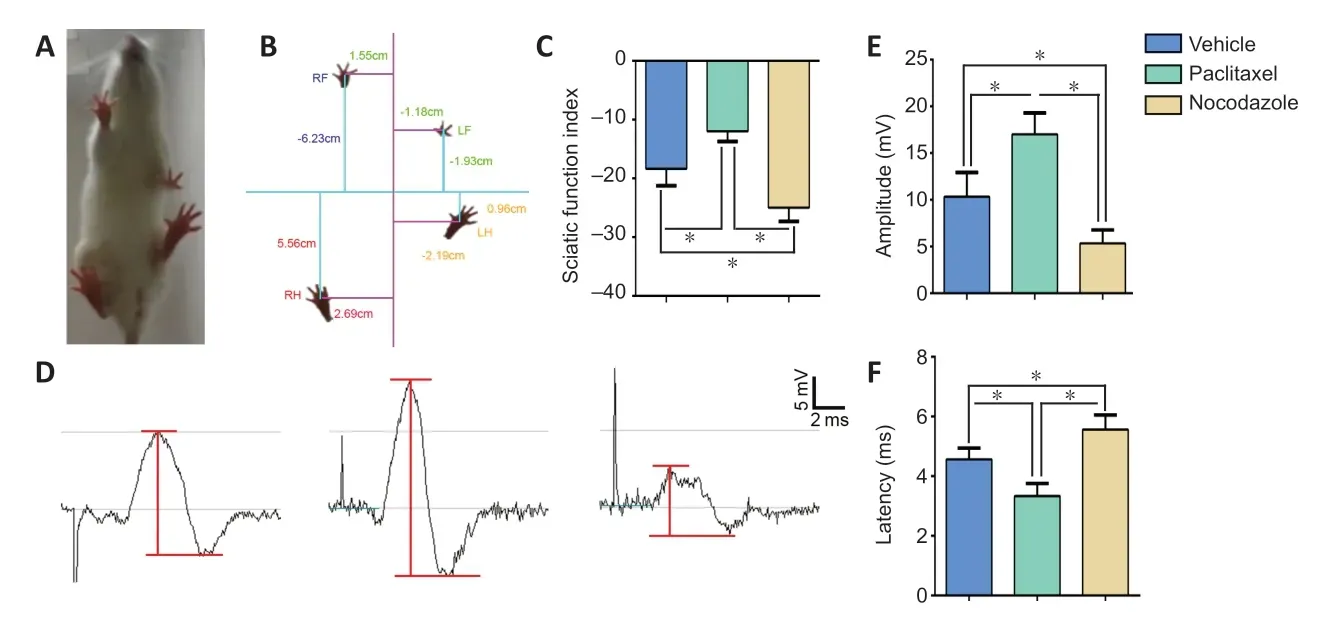
Figure 9|Effects of paclitaxel and nocodazole on functional recovery after sciatic nerve injury.
Discussion
It is well-known that microtubules play pivotal roles in most cellular activities, and that microtubule dynamics are crucial to their function (Zhang et al., 2015; Fedorov et al., 2019).Microtubule-targeting agents are used in the treatment of various diseases to stabilize or destabilize microtubules for therapeutic effect (Kaul et al., 2019; Peronne et al.,2020).To date, however, there have been no reports of the role of microtubule dynamics in WD after PNI.This study demonstrates, for the first time, that paclitaxel and nocodazole treatment can accelerate and attenuate demyelination and Schwann cell dedifferentiation during WD,respectively, and suggests that altering microtubule dynamics has a profound impact on WD.Indeed, earlier studies have shown that paclitaxel treatment results in a higher frequency of dysmyelination or demyelination in untreated sciatic nerves(Benbow et al., 2016; Cook et al., 2018), disrupts myelin formation in Schwann cell/dorsal root ganglion neuron cocultures, and leads to dedifferentiation of Schwann cells (Imai et al., 2017).Consistent with these studies, we also found that paclitaxel promotes demyelination and dedifferentiation.Taken together with the existing literature, our findings indicate that paclitaxel-induced microtubule stability promotes WD, while nocodazole-induced microtubule instability inhibits WD.
Many studies have shown that paclitaxel promotes axonal regeneration after central nervous system injury (Hellal et al., 2011; Sengottuvel et al., 2011; Austin et al., 2017).In addition, epothilone B, another microtubule-stabilizing agent, facilitates peripheral nerve regeneration (Zhou et al.,2020).Similar to these earlier studies, the present study illustrates that paclitaxel treatment significantly enhances morphologic and functional recovery in the injured sciatic nerve and its target muscles.However, the results reported by Hsu et al.(2017) suggest that paclitaxel inhibits peripheral nerve regeneration.To address this apparent controversy,in the present study we assessed the effects not only of paclitaxel, but also of nocodazole, which has the opposite effect in that it promotes microtubule destabilization (Endo et al., 2020; Marquis et al., 2021).As expected, nocodazole treatment not only inhibited WD but also attenuated post-PNI morphologic and functional regeneration.WD is essential for nerve regeneration, as the debris of degenerated axons and myelin needs to be cleaned up as quickly as possible to create a favorable microenvironment for promoting subsequent nerve regeneration (Bombeiro et al., 2020).Although the deeper underlying mechanisms require further investigation,the results from the present study suggest that microtubule stabilization may be conducive to peripheral nerve regeneration, due at least in part to the role of this process in WD.
In summary, the current study used anin vitronerve explant model and anin vivorat model of sciatic nerve crush injury to show that paclitaxel-induced microtubule stabilization promotes WD and facilitates structural and functional recovery after PNI.In contrast, nocodazole-induced microtubule destabilization has the opposite effect.Regarding the limitations of this study, there was no inherent bias, but the underlying mechanisms of how microtubule dynamics regulate WD have yet to be elucidated.
Author contributions:Study conception and design: JG; animal surgery:JL, LL, YX; immunohistochemistry: JL, LF, JZ, ML; western blot assay: JL,YZ, HZ, XH; Schwann cells culture: JL, XM, JX; behavioral assessment and electrophysiological test: JL, XW, ZL; histomorphometry of the gastrocnemius muscle: JL, data analysis: JL, HS, HZ, LZ; manuscript writing: JL, JG.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, Nos.82071386 (to JS), 81870982 (to JS)& 81571182 (to JS); National Key Basic Research Program of China,No.2014CB542202 (to JS); the Program for Changjiang Scholars and Innovative Research Team in University of China, No.IRT-16R37 (to JS); Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory of China, No.2018GZR110104008 (to HZ); Research Grant of Guangdong Province Key Laboratory of Psychiatric Disorders of China, No.N201904 (to JS); and Natural Science Foundation of Guangdong Province of China, No.2017A030312009 (to JS).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Southern Medical University Animal Care and Use Committee (approval No.SMU-L2015081) on October 15, 2015.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Kathryn R Moss, Johns Hopkins Medicine, USA.
Additional files:
Additional Figure 1: The experiment process.
Additional file 1: Open peer review report 1.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Pathological mechanisms and therapeutic strategies for Alzheimer’s disease
- Pentadecapeptide BPC 157 and the central nervous system
- OTX2 stimulates adult retinal ganglion cell regeneration
- Mutations in GBA, SNCA, and VPS35 are not associated with Alzheimer’s disease in a Chinese population:a case-control study
- Krüppel-like factor 7 attenuates hippocampal neuronal injury after traumatic brain injury
- Ultra-early amplitude decrement after repetitive nerve stimulation supports early neuromuscular junction injury in amyotrophic lateral sclerosis: a prospective cross-sectional study

