Semaphorin7A: its role in the control of serotonergic circuits and functional recovery following spinal cord injury
Julie Fourneau, Florence M. Bareyre,
Abstract Serotonin is a monoamine neurotransmitter synthetized in various populations of brainstem neurons. In the spinal cord, descending serotonergic projections regulate postural muscle tone, locomotion and rhythm and coordination of movements via the Central Pattern Generator. Following a spinal cord injury, serotonergic projections to the lumbar spinal cord, where the Central Pattern Generators are located, are interrupted resulting in devastating locomotor impairments and changes in the expression and activation of serotonin and its spinal receptors. The molecular cues that control the precise patterning and targeting of serotonergic inputs onto Central Pattern Generator networks in healthy animals or after injury are still unknown. In our recent research work,we have been particularly interested in Semaphorin7A, which belongs to the Semaphorins family involved in guiding growing axons and controlling plasticity of synaptic connections.In this review, we discuss the role of Semaphorin7A signaling as an important molecular actor that instructs the patterning of serotonin inputs to spinal Central Pattern Generator networks. We show that Semaphorin7A controls the wiring of descending serotonin axons in the spinal cord. Our results reveal that mistargetting of serotonin fibers in the spinal cord is compensated in healthy uninjured Semaphorin7A deficient mice so that their gross locomotion proceeds accurately. We also demonstrate that when the system is challenged with a spinal lesion, the pattern of post-injury serotonin expression is significantly altered in Semaphorin7A deficient mice with specific ectopic targeting of serotonin fibers in the lumbar spinal cord. Compensatory mechanisms in place in uninjured Semaphorin7A deficient mice are lost and injured Semaphorin7A deficient mice exhibit a worsening of their post-injury locomotor abilities. Our findings identify Semaphorin7A as a critical determinant of serotonergic circuit formation in healthy or spinal cord injured mice.
Key Words: central pattern generator; guidance molecule; locomotion; recovery; rewiring;semaphorin7A; serotonergic patterning; serotonin; spinal cord injury
Introduction
Locomotion is an essential motor behavior that involves sequences of limb and body muscle activity following a specific rhythm and pattern, making possible to move through the surrounding environment. The organization of the structures underlying the genesis of locomotion is particularly well preserved and can be described at three main levels:the supraspinal level, including the mesencephalic locomotor region which initiates and controls locomotion, the spinal level which generates rhythm and finally, the peripheral level which allows rapid adaptation of movements to environmental constraints (S?awińska and Jordan, 2019). The spinal level is particularly interesting as it includes neural networks, which have the ability to transform a descending tonic command into simple rhythmic activity and produce a locomotor pattern that is transmitted to the motoneurons. These spinal neural networks, called “Central Pattern Generators” (CPG), have been shown to be distributed in the thoraco-lumbar spinal cord (Kiehn and Kullander, 2004). In this perspective article we aim to review the role of Semaphorin7A in the control of serotonergic circuits and locomotor recovery following spinal cord injury. We will do so by reviewing (i) the role of the serotonergic system in the control of locomotion, (ii)the remodeling of serotonergic circuits following spinal cord injury and (iii) the role of Semaphorin7A in those remodelings following injury.
Search Strategy and Selection Criteria
Studies cited in this review published between 2000 and 2021 were searched on the PubMed database using the following keywords: serotonergic system, 5-HT, locomotion, central pattern generator, spinal cord injury, semaphorin 7A and central nervous system.
Control of Locomotion by the Serotonergic System
The rhythmic discharge of the motorneurons that controls locomotor movements results from the activity and connections between the different spinal premotor interneurons. In this regard, neuromodulation plays a central role by modifying locomotor activity through several mechanisms: either by modulating the properties of motorneurons and CPG interneurons, or by modulating the synaptic connections between interneurons and motorneurons. It is now well established that locomotion is modulated by three monoaminergic systems that project to the spinal cord: serotonergic (5-HT), noradrenergic and dopaminergic (Pearlstein, 2013). Among those, the 5-HT system has been recognized as a powerful modulator of CPGdependent locomotion (Ballion et al., 2002; Takakusaki et al., 2004; Ghosh and Pearse, 2014; S?awińska and Jordan,2019). 5-HT axons observed in the spinal cord come from serotonergic neurons mostly localized in the raphe nuclei of the brainstem, including the raphe obscurus, raphe pallidus and raphe magnus. Descending brainstem 5-HT axons project then to the dorsal and ventral horn and on the intermediate area of the spinal cord (Figure 1A). Several studies in rodents have shown that locomotion is highly associated to 5-HT release in the lumbar spinal cord of rodents and the modulation of 5-HT and its receptors using agonists can produce a fictive locomotion (for review, see Ghosh and Pearse, 2014; S?awińska and Jordan, 2019). Indeed, 5-HT can regulate locomotion, rhythm and pattern of movements by modulating directly the intrinsic properties and excitability of motorneurons or indirectly through its interaction with spinal CPG interneurons to influence the amplitude and frequency of the final motor output. All these findings demonstrate that precise targeting and patterning of serotonergic projections onto thoraco-lumbar CPG networks is therefore crucial for efficient locomotion (Ghosh and Pearse, 2014; S?awińska and Jordan, 2019).
Remodeling of the Serotonergic Circuit after Spinal Cord Injury
Spinal cord injury (SCI) is a devastating condition with an overall global incidence of 10.5 cases per 100,000 people resulting in about 770,000 new cases annually worldwide(Kumar et al., 2018). The main consequence of SCI is the disruption of the dynamic connections between supraspinal structures and spinal cord areas below the site of injury,resulting in impaired sensorimotor function and locomotion(Bradley et al., 2019). In a situation of incomplete SCI, a spontaneous recovery of motor functions can be observed over time through several plastic mechanisms (Bareyre et al.,2004; van den Brand et al., 2012), in particular, rewiring of descending motor pathways including serotonergic system(Ghosh and Pearse, 2014; S?awińska and Jordan, 2019).
While in the healthy rat spinal cord, 5-HT levels in the different layers are constant, following SCI, studies point to a variation of 5-HT levels in the different layers of the spinal cord at different post-injury timepoints. Levels of 5-HT appear to increase directly after injury in the dorsal horn and then decrease over time (Noga et al., 2004), while serotonergic denervation causes a significant decrease in 5-HT levels in the ventral horn. Levels then rise significantly over the course of the subsequent weeks (Gerin et al., 2010).
The serotonergic system regulates motor function and hindlimb coordination through the activation of 5-HT receptors (e.g., 5-HT1A, 5-HT2A, 5-HT2C) to increase motoneuron and interneuron excitability and generates locomotion(Ghosh and Pearse, 2014). To compensate decreases in 5-HT levels, 5-HT receptors expression are up-regulated following SCI. 5-HT1Areceptors activation in spinal cord promotes locomotion in rodents. Its overexpression is known to be a major modification following SCI and would be dependent on afferent inputs (Ganzer et al., 2018). The expression of 5-HT2Areceptor is also drastically increased in the spinal cord early after SCI in rats. This receptor has an inhibitory effect by controlling the activity of the KCC2 potassium - chloride channels particularly present in the motorneurons membrane(Boulenguez et al., 2010). Finally, V2a spinal interneurons become hypersensitive to 5-HT after SCI in mouse due to overexpression of 5-HT2Creceptors, but without impacting their excitability (Husch et al., 2012).
Incomplete lesions of the spinal cord can preserve part of descending axonal pathways such as 5-HT axons. The conservation of these few serotonergic fibers is of genuine importance since they have the capacity to undergo sprouting in the spinal cord with time following SCI (Saruhashi et al.,2008; Leszczyńska et al., 2015). Endogenous restoration of locomotor function observed after SCI has been shown to be partially linked to the density of 5-HT fibers sprouting of those residual serotonergic fibers (Figure 1B).
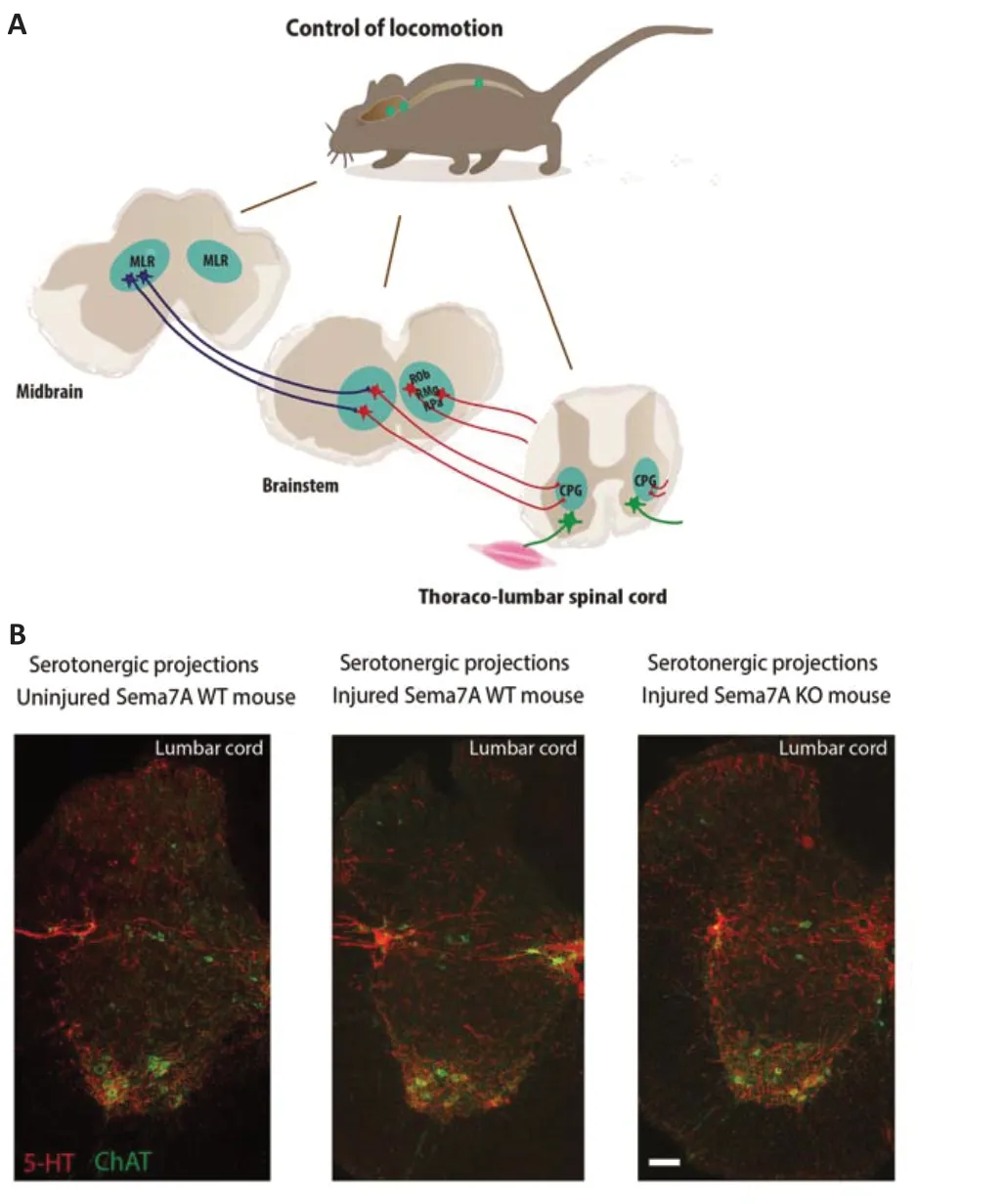
Figure 1|Serotonergic control of locomotion and pattern of serotonin fiber expression in the uninjured and injured mouse spinal cord.
Taken together, this indicates that serotonergic innervation,5-HT fibers sprouting and 5-HT availability in the lumbar spinal cord are crucial and indispensable for the restoration of CPGmediated locomotion and hindlimbs motor activity following SCI.
Molecular Mechanisms Involved in Serotonergic Circuit Remodeling: Role of Sema7A
Various strategies have been employed to repair the injured spinal cord and restore motor functions. One of those has been to elicit the sprouting or re-growth of severed serotonergic fibers below the lesion site in the thoracolumbar spinal cord, where is localized the CPG, to ensure an improvement of locomotor function after SCI (Ghosh and Pearse, 2014). Whether during development or following a CNS injury in adulthood, the proper wiring of neural networks in the brain and spinal cord is dependent on axonal guidance molecules (Pasterkamp, 2012; Jacobi et al., 2014).
Semaphorins are a family of identified repulsive axon guidance molecules that are required to ensure accurate neuronal axons connections to their appropriate targets during development and following injuries (Pasterkamp, 2012). A member of this family, Semaphorin 7A (Sema7A), or John Milton Hagen Blood Group antigen, is a membrane-anchored protein which has been widely studied for its role in the immune system via its interaction with its receptors PlexinC1 or integrin (Jongbloets et al., 2013). However, the role of Sema7A in neuroplasticity(Gatto, 2020) as an axonal guidance molecule is not to be underestimated (Pasterkamp et al., 2003).
In the developing nervous system, several studies have demonstrated that Sema7A controls axon outgrowth and patterning in different system such as the lateral olfactory tract (Pasterkamp et al., 2003), the barrel cortex (Carcea et al., 2014) or the cerebellum (Uesaka et al., 2014). In addition to these well-established roles in promoting outgrowth and patterning, Sema7A can also induce activity-dependent olfactory synapse formation in newborn mice (Inoue et al.,2018) and can affect the density and maturation of dendritic spines (Pasterkamp and Giger, 2009). Recently, it has been shown that Sema7A is able to control the correct targeting of monoaminergic axons of nigrostriatal and mesolimbic dopaminergic pathway in the striatum via its interaction with its receptor plexinC1 (Chabrat et al., 2017). In a previous study, we have shown that Sema7A is not only expressed during the CNS development but also consistently throughout the different area of the adult spinal cord (Jacobi et al., 2014)with no major changes in expression following injury of the spinal cord. In our recent research work (Loy et al., 2020), we therefore investigated the role of Sema7A following SCI to determine whether it can shape the targeting and patterning of 5-HT fibers and support functional recovery.
Using healthy young adult Sema7A deficient mice (2 to 3 months old), we observed that Sema7A homozygous deletion results in a significant increase of the serotonergic innervation affecting not only the dorsal and ventral horns but also the intermediate layers of the lumbar spinal cord (Loy et al., 2020;Figure2A). Despite this excess of serotonergic innervation, we did not observe an increase of contacts onto motoneurons or an increase in 5-HT2Areceptors expression.In addition, we did not detect any abnormalities of locomotor and gait functions evaluated by three different behavior tests such as (i) the ladder rung test which assess skilled walking and limb placement, (ii) the Catwalk system, in which several aspects of gait and locomotion are analyzed, (iii) the rotarod test, that evaluates the balance and motor coordination.These findings support the fact that when Sema7A is constitutively deleted, there are compensatory strategies put in place to preserve locomotor function. This is not surprising as the control of locomotion also involves monoaminergic neuromodulators other than serotonin (e.g., noradrenalin and dopamine) (Pearlstein, 2013) as well as several motor tract systems, whose sprouting could to be Sema7A-independant as demonstrated in Loy et al. (2020) in the corticospinal tract.Overall, these data allowed the identification of Sema7A as a key restrictive cue for serotonergic innervation in the adult spinal cord. Then, when we challenged the spinal cord with a spinal thoracic lesion, we found that the absence of Sema7A results in an exuberant serotonergic projection to the dorsal horn of the lumbar spinal cord overtime (Figures1Band2A). This indicates that Sema7A also restricts the post-injury remodeling of serotonergic connectivity in the adult spinal cord. This maladaptive serotonergic patterning was paralleled by an increase of contacts onto motoneurons,impaired recovery of gait and locomotor functions as well as the appearance of signs of hindlimbs spasticity in Sema7A deficient mice (S?awińska and Jordan, 2019; Loy et al., 2020)(Figure 2B).
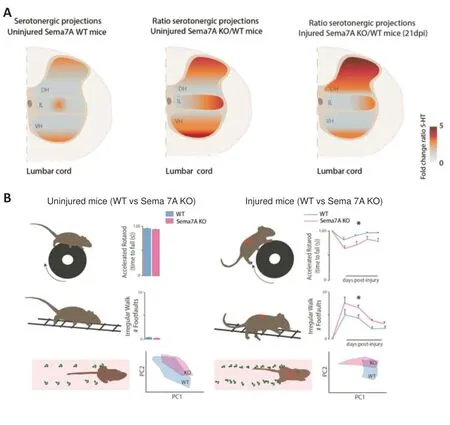
Figure 2|Sema7A is required for the proper serotonergic projections patterning in the lumbar spinal cord and for gait and locomotion functions in healthy uninjured and injured mice.
The change in serotonergic innervation pattern, with an abundant expression in the dorsal horn, is likely to be directly linked to the development of spasticity signs and lack of functional recovery in Sema7A mice. Whether this is a direct effect of Sema7A onto serotonergic patterning or whether there are additional perturbations in Sema7A deficient mice is still unclear. On the one hand, serotonergic projections originating from the brainstem initiate rhythmic locomotion activity by contacting directly the motoneurons or indirectly by modulating spinal interneurons that could be perturbated in absence of Sema7A. On the other hand, the lack of Sema7A could change the afferent inputs, leading to over-regulation of 5-HT sprouting in the dorsal horn. Afferent inputs during development are progressively retracted due to competition with descending systems. Following injury, where the descending systems are interrupted, and in the absence of Sema7A control, the 5-HT fibers sprouting are no longer counter-balanced and strengthens at the dorsal horn.
This study reinforces the idea that axonal guidance molecules play a central role in the patterning and adaptation of neuronal circuits both during development and following CNS lesions in adulthood. Therefore, the identification of important regulator and potential post-SCI druggable target such as Sema7A and their role in circuit remodeling following injury such as that of the serotonergic pathway following SCI to control the locomotor function, opens new opportunities to develop more refined therapies.
Author contributions:Data search and collection: JF. Manuscript writing:JF and FMB. Both authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:Work in FMB’s lab is supported by grants from the Deutsche Forschungsgemeinschaft (DFG? SFB 870 and CRC 274)? by the Munich Center for Neurosciences (MCN) and the International Foundation for Research in Paraplegia (IRP) (to FMB). FMB is also supported by the Munich Center for Systems Neurology (DFG? SyNergy; EXC 2145/ID 390857198).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal? and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License? which allows others to remix?tweak? and build upon the work non-commercially? as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Rodolfo Gabriel Gatto? University of Illinois at Chicago? USA; John C. Gensel? University of Kentucky? USA; Lukas Grassner?Trauma Center Murnau? Germany.
Additional file:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- From regenerative strategies to pharmacological approaches: can we fine-tune treatment for Parkinson’s disease?
- Glymphatic imaging and modulation of the optic nerve
- Time-to-enrollment in clinical trials investigating neurological recovery in chronic spinal cord injury:observations from a systematic review and ClinicalTrials.gov database
- Application value of biofluid-based biomarkers for the diagnosis and treatment of spinal cord injury
- Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer’s disease models
- Diffusion tensor tractography characteristics of axonal injury in concussion/mild traumatic brain injury

