Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer’s disease models
Dai-Di Li , Chang-Qing Zheng Feng Zhang , Jing-Shan Shi
Abstract At present, treatments for Alzheimer’s disease can temporarily relieve symptoms but cannot prevent the decline of cognitive ability and other neurodegenerative changes.Dendrobium nobile Lindl alkaloid is the main active component of Dendrobium nobile Lindl. Dendrobium nobile Lindl alkaloid has been shown to resist aging, prolong life span, and exhibit immunomodulatory effects in animals. This review summarizes the mechanisms behind the neuroprotective effects reported in Alzheimer’s disease animal models. The neuroprotective effects of Dendrobium nobile Lindl alkaloid have not been studied in patients. The mechanisms by which Dendrobium nobile Lindl alkaloid has been reported to improve cognitive dysfunction in Alzheimer’s disease animal models may be associated with extracellular amyloid plaque production, regulation of tau protein hyperphosphorylation, inhibition of neuroinflammation and neuronal apoptosis, activation of autophagy, and enhanced synaptic connections.
Key Words: Alzheimer’s disease; amyloid β plaques; animal models; Dendrobium nobile Lindl alkaloid; neural regeneration; neurofibrillary tangle; neuroinflammation;neuroprotection; pharmacokinetics; Tau protein
Introduction
Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disease (Wang et al., 2020; Li et al., 2021;Zeng et al., 2021) and it is predicted to affect 131.5 million people by 2050 (Lane et al., 2018; Sengoku, 2020). AD is becoming a major public health issue with an increasing burden on caregivers and on the health care system (Dos Santos TC et al., 2018). The main clinical feature of AD is progressive cognitive and memory decline, and AD has been reported to be the most common form of dementia in older adults (Hebert et al., 2003; Povova et al., 2012).Despite large gains in understanding AD, there are gaps in our knowledge regarding the molecular mechanisms of AD pathogenesis. AD has a complex and multifactorial etiology,involving amyloid β (Aβ) plaques, neurofibrillary tangles (NFTs),neurodegeneration, synaptic loss, neurovascular dysfunction and neuroinflammation, which interact with each other to promote neurodegeneration (Behl and Ziegler, 2017).
Several therapeutic strategies are currently used in clinical practice to prevent or delay the process of AD. However, all the available AD drugs have limited efficacy and undesirable side effects (Vaz and Silvestre, 2020). Therefore, there is a great need to find new drug options for AD. Considering that AD is a multifactorial disease, in contrast with the single-target activity of the drugs used for AD treatment,nutraceutical compounds have the advantage of a multi-target approach, which can mark different molecular locations in the human brain (Calfio et al., 2020). Natural compounds have traditionally been a rich source for drug discovery (Ciccone et al., 2021). Active substances with anti-amyloid, anti-apoptosis,anti-inflammatory and neuronal regeneration-promoting activities have been widely studied (Ciccone et al., 2020).Several compounds isolated from plants, targeting different pathological mechanisms of AD, have been reported to exhibit beneficial effects in AD in preclinical and clinical trials (Andrade et al., 2019).
Dendrobiumis the second largest orchid genus. About 70Dendrobiumspecies are found in China.Dendrobiumherb is a well-known traditional Chinese herbal medicine that has immunomodulatory, anti-tumor, anti-diabetic and anti-oxidant properties (Teixeira and Ng, 2017). In China,Dendrobiumnobile Lindl has been used as an ingredient for nutraceutical beverages and food products for thousands of years (Sha and Luo, 1980).Dendrobiumnobile Lindl alkaloid (DNLA) is the main active component ofDendrobiumnobile Lindl. Several reports have demonstrated the neuroprotective effects of DNLA against memory deficits (Li et al., 2011), neuronal and synaptic loss (Li et al., 2016), inhibition of tau protein hyperphosphorylation (Liu et al., 2020a) and apoptosis (Chen et al., 2008) in the hippocampus and cortex.
Dendrobiumnobile Lindl has been traditionally used in traditional Chinese medicine preparations as a universal remedy, so it is worth considering in the investigation for new natural ingredients for treatment of aging-related diseases.Extensive preclinical studies have demonstrated that DNLA plays a role in delaying the development of AD. This review focuses on the mechanisms underlying DNLA-mediated neuroprotection in AD. We discuss the effects of DNLA in AD animal models and highlighted potential therapeutic targets to be investigated in future studies.
Search Strategy
This review focused on the effects of DNLA in various models of Alzheimer’s diseasein vivoandin vitro. We also summarized the main potential mechanisms underlying DNLAmediated neuroprotection that ameliorate the pathological changes in Alzheimer’s disease models. Studies were retrieved from the PubMed and Google Scholar databases using the following search terms: Alzheimer’s disease, amyloid β plaques,Dendrobiumnobile Lindl alkaloid, tau protein,neuroinflammation, neurofibrillary tangle, neuroprotection,and various possible combinations of these terms. Retrievaltime: from inception to January 2021.
Structural Characterization of Dendrobium Nobile Lindl Alkaloid
Dendrobium, the second largest orchid genus, is widely distributed in Southeast Asia, Europe, and Australia.Dendrobiumis a sympo dial epiphytic plant that has been used for a thousand years as a first-rate herb and prized folk medicine in India and China (Cakova et al., 2017). About 70Dendrobiumspecies are found in China, but only two monographs are found in Chinese Pharmacopoeia (2010 edition) (Lam et al., 2015). “Shihu” is a common name forDendrobiumspecies. In traditional Chinese medicine,Dendrobiumnobile Lindl has been used as a medicinal herb to treat a variety of disorders, such as nourishing the stomach,enhancing body fluid production, or replenishing vital essence.A systems pharmacology approach has shown that the active ingredients ofDendrobiumnobile Lindl, including alkaloids,glycosides phenanthrenes and bibenzils, have several pharmacological effects, including tonic, astringent, analgesic,anti-pyretic, and anti-inflammatory actions (Xu et al., 2013).
Chemical composition of dendrobium nobile Lindl alkaloid
The chemical constituents ofDendrobiumnobile Lindl include alkaloids, polysaccharide, phenanthrene, dibenzyl and others.DNLA is the active ingredient extracted from the stem ofDendrobiumnobile Lindl (Piccinelli et al., 2013). The total content of alkaloids in the dried stems ofDendrobiumnobile Lindl is about 0.3–0.5%. To extract DNLA, the dried stems ofDendrobiumnobile Lindl are boiled in ethanol, then dissolved with hydrochloric acid, treated with petroleum ether and then a chloroform mixture, and finally separated through cation exchange resin. Alkaloids are obtained by freeze-drying under vacuum and analyzed by liquid chromatography-tandem mass spectrometry (Nie et al., 2020). The chemical structures,names, formulas, and percentages of the total in the plant or from all alkaloids are provided inTable 1.
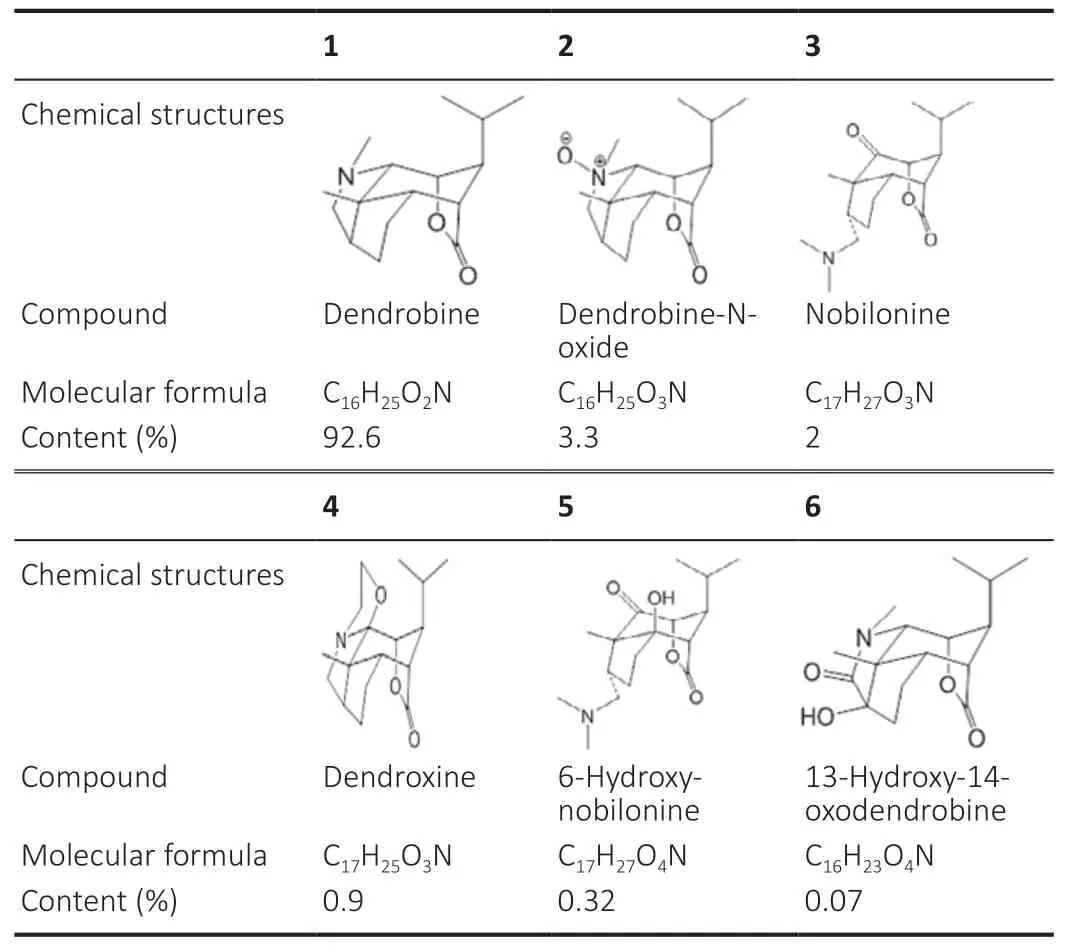
Table 1|Chemical structures of Dendrobium nobile Lindl alkaloid
Pharmacokinetics and tissue distribution
In a previous study, super-high performance liquid chromatography-mass spectrometry was applied to measure Dendrobine in plasma of rats or mice, and to explore the pharmacokinetics and tissue distribution ofDendrobiumnobile Lindl alkaloid (Du et al., 2018). Animals were given Dendrobine by intragastric administration, and their plasma was collected and analyzed 1 hour later. Dendrobine was detected in the rat plasma and the plasma concentration peaked at 11.71 minutes after gavage. As a result, Cmaxand t1/2were 212.17 μg/L and 351.95 minutes, respectively.The pharmacokinetics provides a reference for determining the dose and administration interval of DNLA and aids investigations of safe and effective drug concentrations in animal models.
The low bioavailability of drugs and the difficulty to cross the blood-brain barrier (BBB) remain the major obstacles for the development of new therapies for neurodegenerative diseases. Dendrobine is widely distributed in C57 mice (Lu et al., 2018b). The distribution of Dendrobine in the brain indicates that Dendrobine can penetrate the BBB, DNLA has the ability as a neuroprotective compound.
The metabolites in drug metabolic pathways are largely different between different species. Therefore, appropriate animal models are needed to mimic human drug metabolization as closely as possible and to provide reliable experimental data for preclinical studies on efficacy and toxicology. A previous study reported that the metabolites of Dendrobine in human liver microsomes are similar to those in mouse and dog liver microsomes (Lu et al., 2018a). Thus,mouse and dog are ideal models for preclinical studies to investigate the effects of DNLA.
Proposed Mechanisms of Dendrobium Nobile Lindl Alkaloid-Mediated Neuroprotection in Alzheimer’s Disease
Neuroprotection involves the preservation of neuronal structure or function. It is necessary to identify promising neuroprotective agents to prevent neuron loss and thereby prevent or slow AD progression. AD is characterized by the buildup of two aberrant protein aggregates in the brain, Aβ plaques and NFTs. Common mechanisms of the disease include increased levels of protein aggregation,hyperphosphorylation, inflammatory processes (Ransohoff,2016) or apoptosis and autophagy (Selkoe, 2001; Wang et al.,2010; Yang et al., 2014). Increasing evidence has indicated that DNLA may be beneficial for AD. Different models and their responses have provided insights into the mechanisms of DNLA’s neuroprotective effects. DNLA-mediated mechanisms are shown inTable 2.
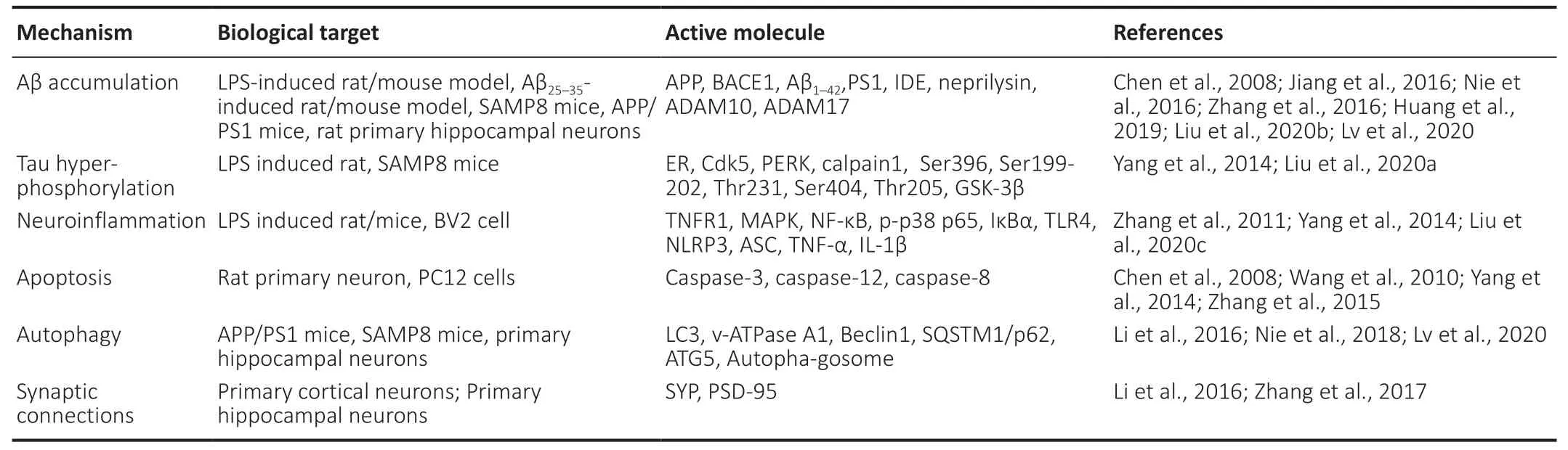
Table 2|Features of DNLA-mediated mechanisms
Dendrobium nobile Lindl alkaloid in amyloid β neurotoxicity
Aβ plaque is the major characteristic in AD patients, and the Aβ peptide cascade is the most influential pathogenesis of AD(Serrano-Pozo et al., 2011). The formation of Aβ plaques are affected by amyloid precursor protein (APP) metabolism. Inbrief, α-, β-, and γ-secretase are essential for endoproteolysis of APP. Disintegrin and metalloprotease17 (ADAM17) and ADAM10 are important α-secretases involved in the nonamyloidogenic APP pathway. In the amyloidogenic pathway,β-secretase activity cleaving enzyme (BACE1) catalyzes at the β site of APP to produce an APP C-terminal fragment, which is then subjected to sequential intra-membrane processing by γ-secretase at residue 40 or 42, leading to the release of different length Aβ peptides Aβ40and Aβ42(Jho et al.,2010; Κoelsch, 2017). Measurement of the Aβ42/Aβ40ratio in cerebrospinal fluid has been shown to improve AD diagnosis in clinical practice, which might increase the confidence of AD diagnoses in memory centers (Lehmann et al., 2018,2020). Because APP increases BACE1 activity toward the APP substrate, BACE1 has been implicated as the APP β-secretase.Several BACE1 inhibitor drugs have been developed to thwart Aβ production and AD progression.
The effects of DNLA on counteracting Aβ deposition and Aβ-induced neurotoxicity have been well investigated. The first study to investigate its effects examined DNLA in rats challenged with liposaccharide (LPS) (Chen et al., 2008). DNLA(40 mg/kg) administered for 14 days significantly attenuated the cognitive deficits in LPS-treated rats and decreased Aβ1–42in the hippocampus. These findings were confirmed in a different AD model. Aβ25–35(10 μg) was injected into the bilateral ventricles of male mice, followed by an oral administration of DNLA (40 mg/kg) for 19 days. The results showed that DNLA ameliorated Aβ25–35-induced spatial learning and memory impairments in mice, prevented neuronal loss in the hippocampus and cortex, and increased the number of Nissl bodies and synapses in neurons (Nie et al., 2016). In a rat AD model, DNLA decreased APP and BACE1 protein expression and the generation of Aβ1–42in the hippocampus (Zhang et al.,2016). Another study reported that DNLA improved the spatial learning and memory function of APP/PS1 mice and increased the numbers of neurons and Nissl bodies (Jiang et al., 2016).The senescence accelerated mouse-prone 8 (SAMP8) model is an ideal model to study AD characterized by age-related learning and memory disorders (Liu et al., 2020b). In a recent study, DNLA decreased expressions of Aβ1–42, APP, PS1, and BACE1, and increased insulin-degrading enzyme and neprilysin for Aβ clearance (Lv et al., 2020). Inin vitrostudies, a similar effect of DNLA was observed in primary hippocampal neurons,in which DNLA (350 ng/mL) reduced the protein expressions of APP, ADAM10, BACE1 and Aβ1–42, and increased the protein expression of ADAM17 (Huang et al., 2019). Together, these published findings indicate that DNLA protects hippocampal neurons against LPS- and Aβ-induced neurotoxicity bothin vivoandin vitro. These actions might be closely associated with the inhibition of Aβ deposition.
Dendrobium nobile Lindl alkaloid in Tau hyperphosphorylation
Tau is an unfolded, highly soluble, and multifaceted neuronal protein that stabilizes microtubules, thereby promoting normal function of neurons (Duan et al., 2017). In AD,hyperphosphorylated tau protein aggregation leads to NFTs that are postulated to follow from the imbalance between Aβ production and clearance (Chong et al., 2018; Twohig et al., 2018; Shi et al., 2020). When tau is hyperphosphorylated,the phosphorylation of tau induces a net charge to affect the conformation of the microtubule-binding region, thereby causing the formation of NFTs in the brain (Chong et al., 2018).In contrast to Aβ plaques, tau pathology has shown a closer correlation with declining cognitive performance based on longitudinal pathological and imaging studies (Aschenbrenner et al., 2018; Vergallo et al., 2018).
The effects of DNLA on counteracting hyperphosphorylated tau protein aggregation have been investigated. DNLA (40 mg/kg) treatment for 6 months downregulated endoplasmic reticulum stress-related protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway, sequential inhibition of calpain1, glycogen synthase kinase-3 beta (GSΚ-3β) and cyclin-dependent kinase 5 activities, and eventually reduced the hyperphosphorylation of tau in SAMP8 mice(Liu et al., 2020a). The study reported that DNLA significantly reduced tau hyperphosphorylation, significantly attenuated neuronal loss, and subsequently improved memory function.
Dendrobium nobile Lindl alkaloid in neuroinflammation
The other recognized pathological feature of AD is neuroinflammation (Sofroniew, 2014; Fakhoury, 2018).Microglia and astrocytes are the main glial cells involved in the immune responses of the central nervous system,and react to a diverse range of pro- and anti-inflammatory agents (Zilka et al., 2006). Several studies have demonstrated that inflammatory processes might promote neuronal loss and cognitive decline (Cai et al., 2014; Webers et al., 2020).Microglia play a crucial role in Aβ homeostasis. Activated microglia initiate phagocytosis to clear Aβ from the brain.But in AD, Aβ accumulation has been shown to cause inflammation (Calsolaro and Edison, 2016). Microglia are activated by Aβ and APP, leading to microglial activation around Aβ plaques (Regen et al., 2017). Activation of microglia sustains release of proinflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6(Chen and Zhong, 2017; Rajendran and Paolicelli, 2018). The accumulation of these inflammatory factors further stimulates immune reactions and contributes to the degeneration of neurons, including the progressive loss of neurons, which results in cognitive decline and dementia.
LPS, an inflammation inducer, has been reported to influence Aβ deposition. LPS injection into the mouse brain ventricle can cause memory deficiency and Aβ accumulation. DNLA treatment has been reported to protect rat brain against LPS-induced neuroinflammation and cognitive dysfunction;this effect appeared to be mediated by suppression of LPSinduced overexpression of tumor necrosis factor receptor 1, and inhibition phosphorylated p38 mitogen-activated protein kinases (p-p38 MAPK) expression (Zhang et al.,2011). In addition, DNLA has been shown to suppress LPS-induced microglial activation and decrease nuclear factor-Κb (NF-κB) p65, inhibitor of NF-κB (IκBα) and their phosphorylative products in the cellular nucleus and cytosol of BV2 microglia, and the expression of Toll-like receptor 4(TLR4), NLR family pyrin domain-containing protein 3 (NLRP3),apoptosis-associated Speck-like protein containing CARD and caspase-1 (Liu et al., 2020c). Together, these findings suggest that DNLA protects neurons against LPS-induced neuroinflammation via the attenuation of glial cell activation,reduced proinflammatory factor production, and inhibition of p-p38 MAPΚ and the downstream NF-κB and NLRP3 signaling pathway.
Dendrobium nobile Lindl alkaloid in apoptosis
Another common mechanism of AD is apoptosis (Obulesu and Lakshmi, 2014). Apoptosis is preprogrammed cell death(Fleisher, 1997). Programmed cell death is an essential biological process in the development and functional maintenance of the human body (Tower, 2015). Pathological apoptosis is associated with various disorders, including neurodegenerative diseases and cancer. Apoptosis can lead to neurodegeneration and might contribute to AD (Radi et al., 2014). Apoptosis is activated by many mediators, such as caspases 2, 3, 8, and 9 (Friedlander, 2003), MAPK (Sun et al., 2015; Aghaei et al., 2020), p53 (Wang et al., 2015),Bax (Aghaei et al., 2020) and Aβ. Although Aβ contributes to the development of AD and induces neuronal apoptosis,the underlying mechanisms are elusive (Li et al., 2018). Aβinduced synthesis of GD3 has been reported to contribute to apoptosis in cortical neurons (Κim et al., 2010). Consequently,the inhibition of apoptosis is considered to be a promising approach to prevent AD.
A previous study reported that DNLA exhibits protective effects against PC12 cell injury induced by Aβ25–35through attenuating apoptosis, as evidenced by increased cell viability and decreased cell morphology impairmentin vitro(Zhang et al., 2015). Also, DNLA (2.5 mg/mL) has demonstrated neuroprotective effects against oxygen-glucose deprivation/reperfusion (OGD/RP)-induced neuronal damage in rat primary neuron cultures by stabilizing the mitochondrial membrane potential, inhibiting intracellular free calcium overload, and lessening neuronal apoptosis mediated by downregulating mRNA expression of caspase-3 and caspase-12 (Wang et al., 2010). Furthermore, DNLA has been shown to attenuate LPS-induced cognitive deficits in rats,and the effect might be related to the downregulation of caspase 3/8 mRNA expression and the decrease of Aβ1–42in the hippocampus (Chen et al., 2008). DNLA inhibited neuronal apoptosis and further ameliorated the dementia symptoms in AD models, which might be associated with the inhibition of hyperphosphorylation of tau protein. Additionally, DNLA ameliorated LPS-induced memory and cognitive impairments in rats; the mechanism was closely associated with decreased number of apoptotic cells, decreased expression of hyperphosphorylated tau protein at serine 396 (Ser396),Ser199-202, Ser404, tyrosine 231 (Tyr231), Thr205 sites and increased expression of GSΚ-3β (Yang et al., 2014). In summary, published reports suggest that DNLA is beneficial for the dementia symptoms in AD models via reducing Aβ accumulation and inhibiting hyperphosphorylation of tau protein. The underlying mechanism might be related to suppressing neural apoptosis.
Dendrobium nobile Lindl alkaloid in autophagy
The basal autophagy pathway is essential for neuronal degradation (Funderburk et al., 2010). Activated autophagy participates in various physiological processes and pathological conditions, including cell death, removal of microorganisms invading the cell, and tumor suppression (Glick et al., 2010).A recent study has demonstrated that autophagy is closely linked to aging (Madalina et al., 2017). Various autophagy dysfunctions might contribute to neurodegeneration, including inhibition of autophagosome-lysosome fusion (Tammineni and Cai, 2017), reduction of lysosomal acidification (Tanaka et al., 2013) or accumulation of proteins in neuronal cells(Menzies et al., 2017). In parallel, autophagy is a key regulator of Aβ accumulation and clearance (Li et al., 2017). In AD,autophagosome fusion with lysosomes and their retrograde passage toward the neuronal body are hindered (Uddin et al.,2018). These reports suggest that autophagy mechanisms are critical for AD progression.
DNLA has been studied for its protective effects on autophagy as a potential mechanism involved in AD. DNLA improved learning and memory impairment in APP/PS1 mice, and the effect was reported to be mediated by the promotion of intracellular Aβ degradation by increasing v-ATPase A1 protein levels and then improving autolysosomal acidification and proteolysis (Nie et al., 2018). Furthermore, in the SAMP8 model, after 6 months of treatment, DNLA enhanced autophagy activity by increasing the expression of autophagy marker light chain 3 (LC3), autophagy-related protein Beclin1 and Klotho, and decreasing nucleoporin p62 in the hippocampus and cortex (Lv et al., 2020). In anin vitrostudy,a similar effect of DNLA was observed in primary hippocampal neurons. DNLA pretreatment significantly suppressed axonal degeneration induced by Aβ25–35cytotoxicity; the authors reported that the effect might be associated with enhanced autophagic flux by promoting the formation and degradation of autophagosomes in axonal degeneration of hippocampal neurons (Li et al., 2016). Based on these findings, we draw a reliable conclusion that DNLA protects against neuronal degeneration via activation of autophagy.
Dendrobium nobile Lindl alkaloid in neuronal synaptic connection
Unfortunately, numerous new therapeutic drugs prepared based on traditional hypotheses have encountered disappointing outcomes in clinical trials; they have not been able to halt disease progression or stimulate neuronal regeneration (Alipour et al., 2019). Adult neurogenesis carries the potential of brain self-repair by the endogenous formation of new neurons in the adult brain. However, it also declines with age. Pharmacological strategies to improve the symptoms of AD have included different approaches to stimulate neurogenesis. Therefore, a deeper understanding of the regulatory mechanism underlying stem cell neurogenesis or a functional integration of newborn neurons may contribute to the development of novel and effective AD therapies (Vasic et al., 2019).
Our previous studies have demonstrated that DNLA protects primary cortical neurons against Aβ25–35-induced neurotoxicity and synaptic damage. DNLA reversed Aβ25–35-induced decreases in synaptophysin (SYP) and postsynaptic density 95 (PSD-95) (Zhang et al., 2017). SYP, PSD-95 and other synapse-associated proteins are essential factors to maintain synaptic morphology and function. DNLA might play a key role in upregulating neurogenesis-related synapse-associated proteins to improve synaptic transmission in the nervous system.
Conclusion
Nutraceuticals have become promising new compounds to prevent or treat AD. As reported, substantial preclinical studies indicate that DNLA is a promising molecule to counteract various pathophysiological processes of AD, thereby improving cognitive functions and inhibiting neurodegeneration. As shown inFigure 1.
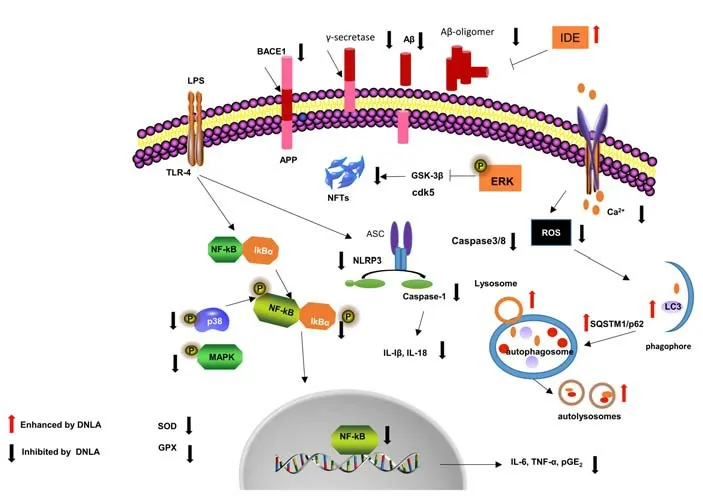
Figure 1|Potential mechanisms underlying DNLA-mediated neuroprotection in Alzheimer’s disease (AD) models.
The mechanisms underlying DNLA’s effects might be related to inhibiting Aβ plaque production and tau protein hyperphosphorylation, reducing neuroinflammation and apoptosis, activating autophagy, and enhancing synaptic connections. Studies investigating the mechanisms of DNLA are ongoing in animal disease models. At this point, clinical studies have not been performed. Therefore, comprehensive clinical investigations are needed to elucidate the multiple practical and theoretical issues of DNLA-mediated neuroprotection in AD.
Author contributions:JSS conceived and designed the review. DDL and CQZ conducted a literature search. DDL wrote the manuscript. FZ substantially edited and improved the manuscript. All authors contributed to critical comments and manuscript revision and approved the final manuscript.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was supported by Shijingshan’s Tutor Studio of Pharmacology? No. GZS-2016-07 (to JSS); the Construction of National First Class Pharmacy Disciplineb? No. GESR-2017-85 (to JSS); the Master Start Foundation of Zunyi Medical University? No. F-839 (to DDL); and a grant from Guizhou Chinese Medicine Administration? No. QZYY-2018-025(to DDL). The funding sources had no role in study conception and design?data analysis or interpretation? paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal? and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License? which allows others to remix?tweak? and build upon the work non-commercially? as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Hans-Gert Bernstein? Otto-von-Guericke University?Germany; Paulina Carriba? Cardiff University? UK.
Additional file:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- From regenerative strategies to pharmacological approaches: can we fine-tune treatment for Parkinson’s disease?
- Glymphatic imaging and modulation of the optic nerve
- Time-to-enrollment in clinical trials investigating neurological recovery in chronic spinal cord injury:observations from a systematic review and ClinicalTrials.gov database
- Semaphorin7A: its role in the control of serotonergic circuits and functional recovery following spinal cord injury
- Application value of biofluid-based biomarkers for the diagnosis and treatment of spinal cord injury
- Diffusion tensor tractography characteristics of axonal injury in concussion/mild traumatic brain injury

