Soil seed bank is affected by transferred soil thickness and properties in the reclaimed coal mine in the Qilian Mountains, China
YANG Jingyi, LUO Weicheng, ZHAO Wenzhi,*, LIU Jiliang, WANG Dejin, LI Guang
1 College of Forestry, Gansu Agricultural University, Lanzhou 730070, China;
2 Linze Inland River Basin Research Station, Chinese Ecosystem Research Network, Key Laboratory of Ecohydrology of Inland River Basin, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou 730000, China;
3 Faculty of Modern Agricultural Engineering, Kunming University of Science and Technology, Kunming 650500, China
Abstract: Reclamation of lands abandoned after mining in mountain areas is critical to erosion control,safety from landslides, and ecological protection of mountain ecosystems.However, little is known about alpine coal mine reclamation using the soil seed bank as a potential source for revegetation.We collected samples of persistent soil seed bank for germination experiments from nine reclaimed sites with different soil cover thicknesses and from six control sites in the Qilian Mountains of China.Soil properties of each site were determined (including soil water content, soil available potassium, soil available phosphorus, soil total nitrogen, pH, soil organic matter, soil total phosphorus, and soil total potassium, and soil alkali-hydrolyzable nitrogen), and the relationships of the characteristics of the soil seed bank with soil cover thickness and soil properties were examined.The results showed that the density, number of species, and diversity of the topsoil seed bank were significantly correlated with soil cover thickness, and all increased with the increment of soil cover thickness.Soil cover thickness controlled the soil seed bank by influencing soil properties.With the increase in soil cover thickness, soil properties (e.g., soil organic matter, soil total nitrogen, etc.) content increased while soil pH decreased.The soil seed bank had the potential to restored the pre-mining habitat at reclaimed sites with approximately 20-cm soil cover thickness.Soil properties of reclaimed sites were lower than that of natural sites.The relationship between the soil seed bank and soil cover thickness determined in this study provides a foundation for improving reclamation measures used in coal mines, as well as for the management and monitoring of reclaimed areas.
Keywords: soil seed bank; soil cover thickness; species composition; soil properties; Qilian Mountains
1 Introduction
About 70% of the energy infrastructure in China depends on coal (Guo et al., 2021).However,while coal mining promoted the development of society and economy, it led to ecological and environmental degradation (Bi et al., 2021).A stark example is the Qilian Mountains in northwestern China, which experienced severe damage from mining, prospecting, and related activities since the 1950s.More recently, as coal resources diminished, vast areas became abandoned mine lands, including abandoned mines, subsidence areas, and coal mine waste dumps(Hu et al., 2015).These areas are prone to soil erosion (i.e., topsoil stripping, nutrient loss, and insufficient soil water-holding capacity) and vegetation degradation (Yao et al., 2016; Lv et al.,2021).Toxic substances in the tailings piles polluted the surrounding land during rain and wind(De-Bashan et al., 2010).Furthermore, these areas experienced a loss of natural vegetation and a decline in soil quality, coupled with drought and low rainfall (Wang et al., 2021).A strategy of ecological engineering in these post-mining areas is critical to effectively implement an ecological restoration process (Bi et al., 2021).
To reconstruct the environment of the Qilian Mountains damaged by mining, the Chinese government conducted reclamation of the abandoned mine lands in 2017 and adopted a range of ecological engineering measures, including mine backfilling, gangue pile leveling, gentle slope repairing, soil covering (Bor?vka et al., 2012), and planting ofElymus nutansGriseb.(Hu et al.,2020).These measures are widely adopted because of their low cost, zero pollution, and other benefits (Holmes, 2001).Soil cover in particular is the foundation of mined areas reclamation as it increases soil nutrients (Bor?vka et al., 2012) and improves soil pore structure (Burr-Hersey et al.,2020).Also, the use of native topsoil secures the source of seeds, propagules, roots, and soil microbes, creating communities that closely resemble those of the original native environment(Markowicz et al., 2015; Stradic et al., 2016).In particular, the soil seed bank has been a major source of species return (Elliott et al., 2022) and an important component of post-mining recovery.
The native seed bank in the soil can offer materials for revegetation of damaged ecosystems(Luo et al., 2023).Using the native seed bank supports the idea of restoration as close to nature as possible (Crouzeilles et al., 2017).The soil seed bank serves as a dynamic system with legacies of past vegetation, present species, and seeds dispersed from adjacent communities (Zhao et al.,2021a).In the other word, the soil seed bank is affected by inputs and outputs of seeds (Yang et al., 2021).In general, the soil seed bank is replenished by seed dispersal by aboveground vegetation, and consumed by seed predation by small vertebrates and by other factors, such as germination, pathogen attack, and mechanical decay (Ma et al., 2017a; Zhao et al., 2021b).The soil seed bank is therefore a valuable natural resource for promoting plant diversity and the reintroduction of native species in mining areas (Hall et al., 2010).There is evidence that the distribution and species composition of the soil seed bank is influenced by local environmental conditions (Jaganathan et al., 2015; K?nig et al., 2023).Previous studies demonstrated that mining restoration areas were subject to biotic and abiotic influences (light, water, soil, seed dispersal, etc.) that altered seed dynamics and thus affected the soil seed bank (Li et al., 2017).This plastic response can also have important consequences for the fitness of the seedlings as they develop (Ko?odziejek, 2017).Soil pH is an important factor influencing species richness of plant communities and may indirectly affect the soil seed bank by affecting plant growth (Eycott et al.,2006; Ma et al., 2017b); it is also strongly related to soil processes such as nitrogen cycling(Anderson et al., 2018).High levels of nitrogen in the environment tend to produce seeds of greater mass that promote seed germination, thus reducing species richness of the seed bank(Ko?odziejek, 2017).In addition, soil moisture stimulates seed germination, but excessively moist soil can reduce the supply of oxygen required for seed respiration, thereby interfering with seed metabolism and leading to higher levels of seeds rotting or deteriorating (Ma et al., 2017a; Zhao et al., 2021a).
Topsoil translocation is a suitable method for obtaining seeds from multiple species rather than a single species (Kiehl et al., 2010).Moreover, topsoil translocation increases seed bank richness at receptor sites (Piqueray et al., 2020) and can be used for vegetation restoration of arable fields(Piqueray et al., 2020), savannas (Ferreira et al., 2015), forests (Hall et al., 2010), and mining areas (Ribeiro et al., 2018; Slukovskaya et al., 2019).Previous studies have shown that the most favorable method for topsoil translocation to restore vegetation in Mediterranean steppe was to use a soil cover thickness of at least 40 cm and eliminate contact with groundwater (Chenot et al.,2017).Nevertheless, another study on topsoil translocation for forest revegetation revealed that there was no significant difference in total seedling recruitment at receptor sites at two spreading depths, 10 and 30 cm (Rokich et al., 2000).This indicated that the 10 cm thickness of soil cover is sufficient for seedling establishment at receptor sites.Although topsoil translocation in the reclamation and restoration of degraded areas are commonplace (Ferreira et al., 2015), few studies to date have investigated the impact of soil translocation on soil seed bank, which are critical for ecological restoration.Elucidating the various influencing factors of the soil seed bank, especially the properties of topsoil transferred to mine areas, is important for understanding the contribution of the soil seed bank to ecological restoration.
Indeed, a previous study confirmed that the soil seed bank after topsoil stripping was reduced to about 74% of their original density (Koch et al., 1996).We hypothesize that the potential plant diversity in mining areas is related to the richness of the soil seed bank (Luo et al., 2023).Here,we selected nine reclaimed sites that had undergone topsoil translocation and six natural sites that had never been disturbed to determine the ability of the topsoil to renew the flora.The aim of this study is to compare the potential for ecological restoration of coal mine reclamation sites with different soil cover thicknesses.We predict that: (1) the success of restoration of receptor sites is proportional to the thickness of the transferred topsoil; (2) different thicknesses of the transferred topsoil exacerbate the disparities in the species composition of the soil seed bank at receptor sites,leading to different successional trajectories; and (3) soil properties of receptor sites are important factors affecting the species composition of the soil seed bank.Comparing reclaimed sites with natural sites is of great significance for guiding the ecological restoration of mined areas to achieve favorable conservation values.Furthermore, it provides a theoretical basis for the restoration and management of coal mines.
2 Materials and methods
2.1 Study area
This experiment was carried out in Xiyinghe Nature Reserve in the Qilian Mountains(37°36′-38°06′N(xiāo), 101°07′-102°12′E; 2130-3331 m a.s.l.) in Gansu Province, northwestern China(Fig.1).The area is dominated by rangelands which occupy 57% of western China (Hou et al.,2008).The climate of this study area is semi-arid alpine characterized by cool and short summers,and cold and long winters, with a short growing season.The annual average temperature is approximately -1.7°C and a total annual precipitation is 412.7 mm (Wang et al., 2023).The annual potential evaporation is 1828.5 mm, and the annual accumulated temperature (≥10.0°C) is 1630.9°C with 2200 h of sunshine duration (Gao, 2017).An earlier study showed that the soil types include chernozem, chestnut, and brown-calcium, and vegetation is mainly composed of compositae, gramineae, and legumes (Gao, 2017).We selected nine representative reclaimed sites, designated as SMG, HG, XSH1, LSG, JFG, TS, NDB1, NDB2, and XSH2, located along a west to east transect.We classified the nine sites into three types based on the soil cover thickness.Sites with about 10 cm soil cover thickness (10T) included NDB2, XSH2, and JFG;sites with about 15 cm soil cover thickness (15T) included LSG, SMG, and TS; sites with about 20 cm soil cover thickness (20T) included XSH1, HG, and NDB1.We also selected six undisturbed natural sites (NA) near the coal mines as control sites (NA1-NA6).Table 1 shows the basic information of these sites in the study area.It is noteworthy that the soil thickness at control sites was more than 30 cm.
2.2 Soil sampling
A permanently marked quadrat (10 m×10 m) was established at each site and six plots (1 m×1 m)spaced approximately 1 m apart were selected randomly in each quadrat.Soil samples were selected randomly in each plot, and fallen leaves and branches were removed before sampling.We collected soil samples at different soil depth (0-5 and 5-10 cm) and used these samples to determine the soil seed bank.In addition, in each plot, we used a soil auger to obtain soil samples(0-10 cm), which were used to measure soil properties.A total of 180 soil seed bank samples and 90 soil property samples were collected.
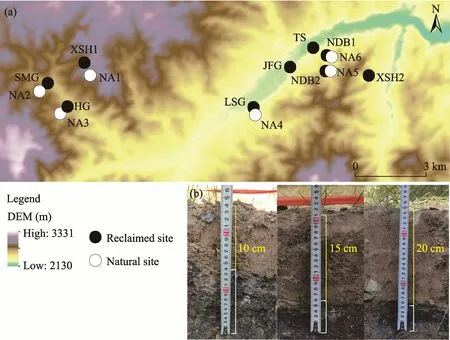
Fig.1 Spatial distribution of reclaimed and natural sites in the study area (a) and photos of different soil cover thicknesses at reclaimed sites (b).DEM, digital elevation model.
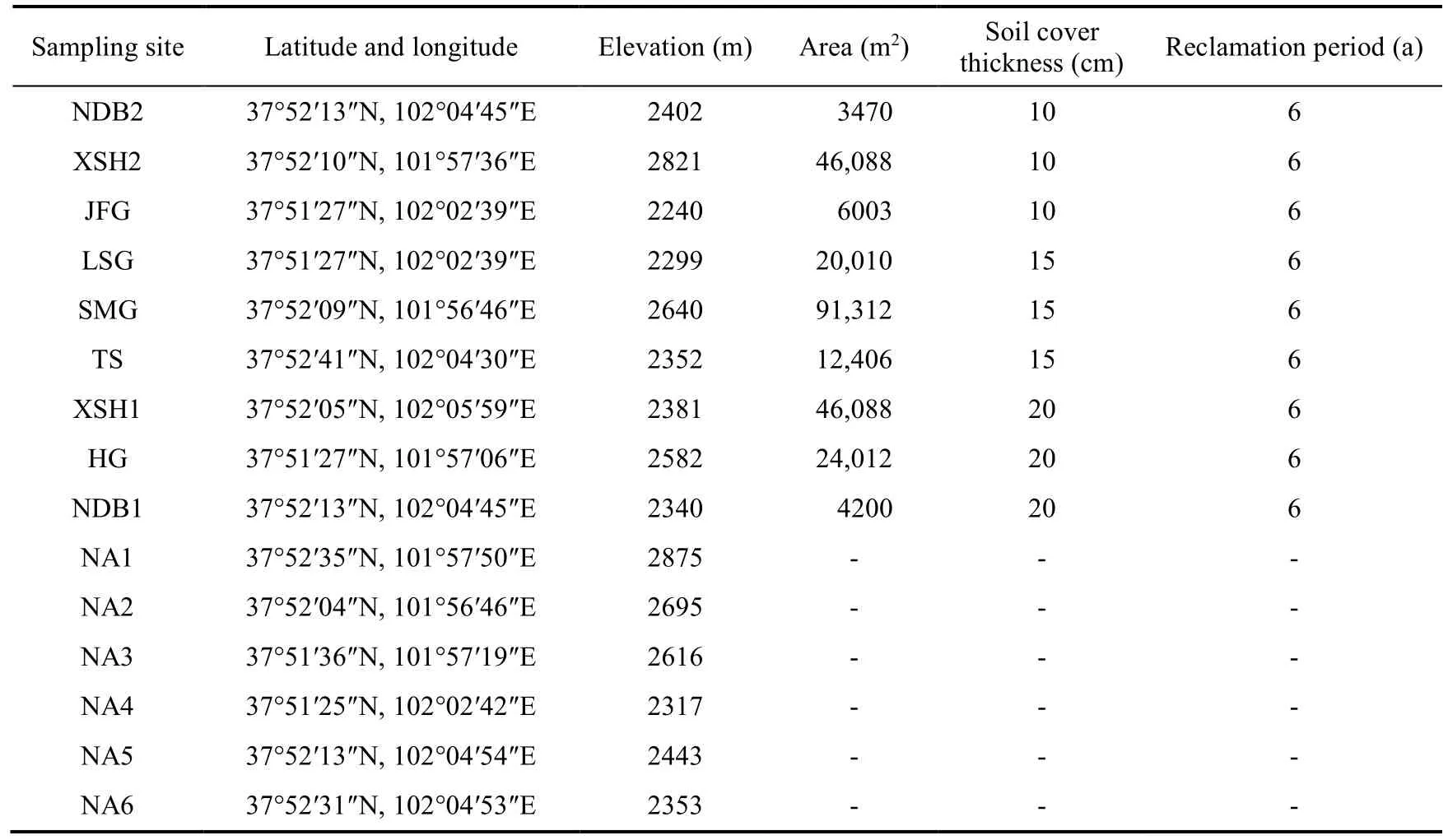
Table 1 Basic characteristics of reclaimed and natural sites in the study area
2.3 Seed germination experiment
A seed germination experiment was conducted in July 2022 at the Linze Inland River Basin Research Station, Chinese Academy of Sciences.Briefly, 180 soil seed bank samples and 90 soil property samples were dried at room temperature for 15 d and sieved through a 5 mm sieve to remove coarse fragments.Then, each soil sample was placed in a plastic pot (25 cm×25 cm) with high-temperature sterilized fine sand as a substrate.Semi-transparent gauze was used to cover the pots to prevent contamination by foreign seeds and damage from light while maintaining moisture and nutrient supply for seedling germination.After emergence (10-180 d), species and the number of germinating plants were identified, seedlings were removed, and the experiment ended when no new seedlings growing in the pots.
2.4 Soil properties
Air-dried soil samples were sieved through a 2 mm mesh.Soil water content (SWC) was calculated by subtracting the mass of oven-dried soil (dried at 105°C for 48 h) from that of collected soil.Soil available potassium (AK), soil available phosphorus (AP), and soil total nitrogen (TN) were measured using the 1.0 mol/L CH3COONH4buffer solution (pH 7.0) flame spectrometry method, Olsen method, and the modified Kjeldahl method, respectively(Kazempour-Larsary et al., 2023).Soil pH was measured with a pH meter (FE20 Plus, Mettler Toledo, Shanghai, China).Soil organic matter (SOM), soil total phosphorus (TP), and soil total potassium (TK) were measured with the potassium dichromate oxidation method, molybdenum antimony blue colorimetry, and the flame spectrophotometer method, respectively (Shao et al.,2022).Soil alkali-hydrolyzable nitrogen (AN) was determined with the alkaline hydrolysis diffusion method (Li et al., 2022).Soil properties were tested at the Academy of Agricultural Sciences, Southwest University.
2.5 Statistical analysis
One-way analysis of variance (ANOVA) combined with the least significant difference (LSD)post hoc test was used to examine the differences in the density, number of species, and diversity of the soil seed bank among different soil cover thicknesses.Correlations between species composition at different study sites were detected using Pearson correlation.Redundancy analysis(RDA) was used to ascertained the relationships between soil properties and the species composition of the soil seed bank.Statistical analysis and visualization were completed using IMB SPSS Statistics 26 (IBM, 2019) and Origin 2021 (OriginLab, 2021).The visualization of linear regression and RDA were done using R 4.2.3 software.Four popular alpha diversity indices, including Margalef richness (R), Shannon-Wiener diversity (H), Simpson dominance (D),and Pielou's evenness (E), were selected to analyze the diversity of the soil seed bank.The diversity indices were calculated as follows:
whereSrepresents the number of species in the community;Nis the total number of seeds in the sample (seeds/m2); andpiis the proportion of seeds of theispecies (wherei=1, 2, 3, …, 11)(Wang et al., 2018; Xu et al., 2022).
3 Results
3.1 Species composition of the soil seed bank
A total of 11 species from 9 families were recorded among germinating seeds, with 30.9% of annual species and 62.1% of perennial species (Table 2).Papaveraceae and Poaceae accounted for 23.4% and 22.0% of the total, respectively, among which Papaveraceae contributed the most to the density of the seed bank.Amaranthaceae, Nitrariaceae, Plantaginaceae, Brassicaceae,Rosaceae, Asteraceae, and Fabaceae together constituted 54.6% of the total; within that,Asteraceae accounted for less than 2.0%.Notably, the three most abundant species in the soil seed bank wereElymus dahuricusTurcz.(22.0%),Chenopodium glaucumL.(17.6%), andPlantagodepressawilld.(14.4%), which accounted for over 50.0% of the seeds recorded in the soil seed bank.At each site,E.dahuricus,C.glaucum,Peganum multisectum(Maxim.) Bobrov, andChelidonium majusL.were abundant, but some species, such asFragaria orientalisLozinsk.,Artemisia argyiLévl.et Van., andThermopsis lanceolataR.Br.were only present at 20T and NA.Differences in the type and number of species resulted in differences in the species composition among sampling sites.Correlation analysis showed that XSH1 and NA1 had similar species composition (R2=0.80;P<0.05), but the correlation coefficient of XSH1 and NA1 with other sampling sites was lower (Fig.2).However, the correlation coefficients of the species composition among other sampling sites were high.

Table 2 Species composition and density of the soil seed bank
3.2 Characteristics of the soil seed bank under different soil conver thicknesses
The density of the soil seed bank in 0-10 cm soil layer under four soil cover thicknesses (10T,15T, 20T, and NA) in this study were 383, 422, 811, and 892 seeds/m2, respectively, and the number of species was 5.33, 6.00, 8.67, and 8.17, respectively (Table 3; Fig.3).It is noteworthy that both the density and the number of species in the soil seed bank differed significantly among different soil cover thicknesses in the same soil layer.In 0-5 cm soil layer, the density of the soil seed bank at NA was highest (664 seeds/m2), which was significantly (P<0.001) greater than that at 10T (305 seeds/m2) and 15T (350 seeds/m2), but was not significantly different from that at 20T(664 seeds/m2).An analogous pattern was observed in 5-10 and 0-10 cm soil layers.
The number of species in the soil seed bank at 20T (8.33) was highest and significantly greater than that at 10T (5.33) and 15T (6.00) in 0-5 cm soil layer (P<0.05), but the differences between NA and other types were not significant.However, the number of species in the soil seed bank at NA was significantly higher than that at 10T and 15T in 5-10 and 0-10 cm soil layers.
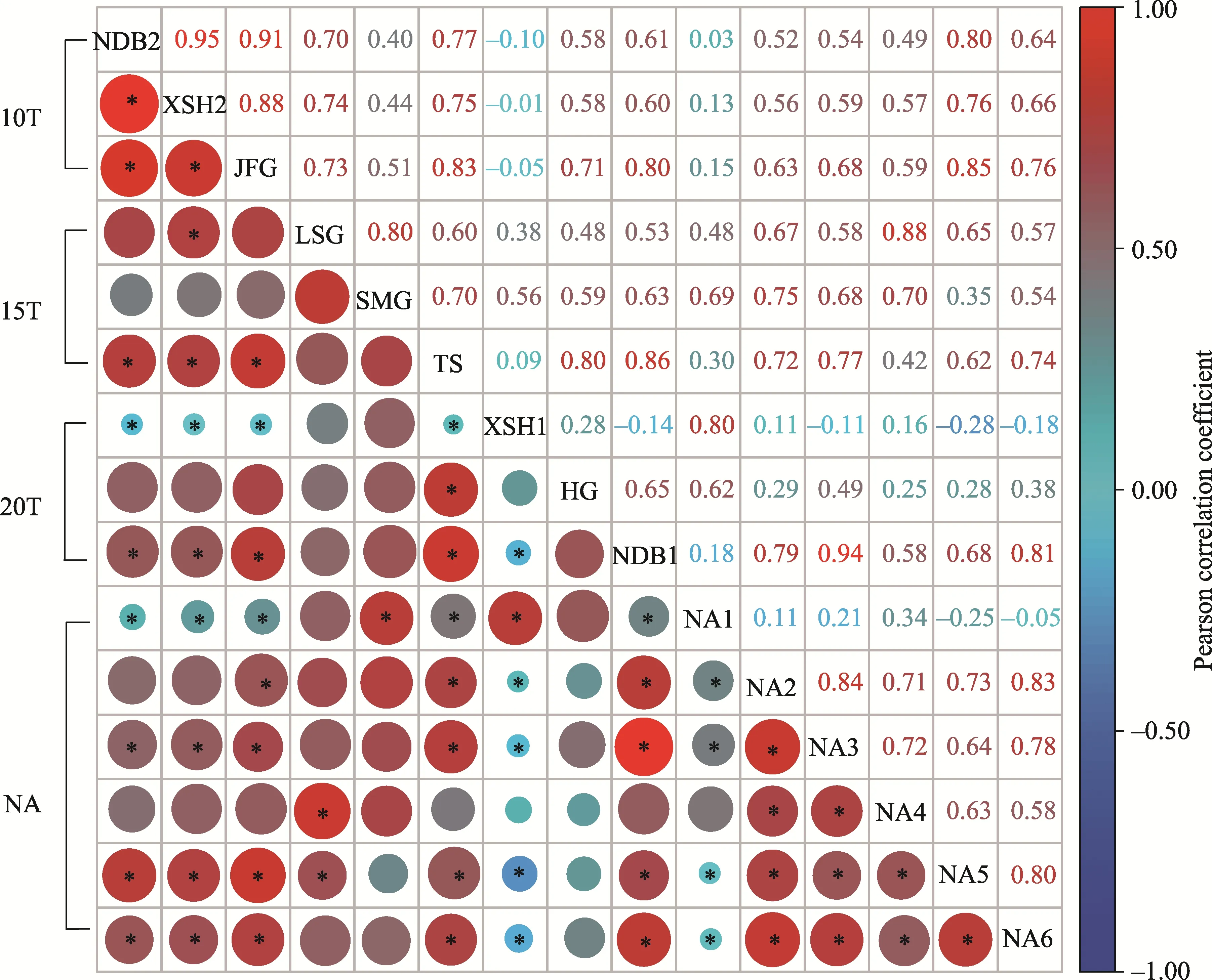
Fig.2 Pearson correlation coefficient for the species composition of the soil seed bank in 0-10 cm soil layer.10T denotes reclaimed sites with 10 cm soil cover thickness (including NDB2, XSH2, and JFG), 15T denotes reclaimed sites with 15 cm soil cover thickness (including LSG, SMG, and TS), 20T denotes reclaimed sites with 20 cm soil cover thickness (including XSH1, HG, and NDB1), and NA denotes natural sites (NA1-NA6).The circles represent correlation coefficients; the larger the circle, the larger the correlation coefficient.*, P<0.05.
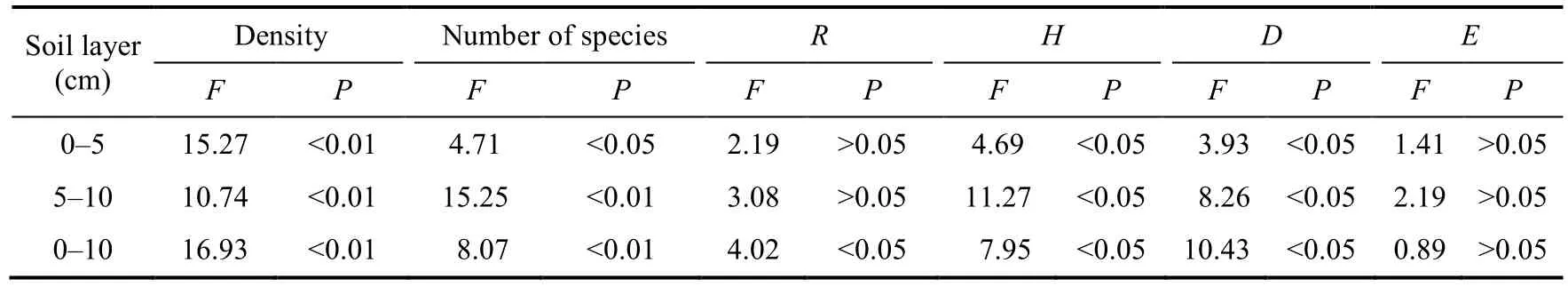
Table 3 One-way analysis of variance (ANOVA) results of the characteristics of the soil seed bank
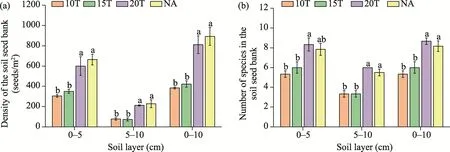
Fig.3 Distribution of the density of the soil seed bank (a) and the number of species in the soil seed bank (b) in 0-5, 5-10, and 0-10 cm soil layers.Different lowercase letters indicate significant differences among different soil cover thicknesses for the same soil layer.Bars mean standard error.
One-way ANOVA indicated that the Shannon-Wiener diversity index was significantly affected by soil cover thickness in each soil layer (P<0.05; Fig.4; Table 3).Additionally, the Margalef richness index was significantly affected by soil cover thickness in 0-10 cm soil layer, and the Simpson dominance index was significantly affected by soil cover thickness in 0-5 and 5-10 cm soil layers (P<0.05; Table 3).However, the Pielou's evenness index at 20T was not significantly differed from that at NA, but significantly higher than that at 10T and 15T in 0-10 cm soil layer.In 0-5 cm soil layer, the Shannon-Wiener diversity index at 20T was similar to that at NA (1.86), but there were no significant differences among 15T and the other soil cover thicknesses.In 5-10 and 0-10 cm soil layers, the Shannon-Wiener diversity index at 20T and NA was significantly higher than that at 10T and 15T.Similar to the Shannon-Wiener diversity index, the Simpson dominance index at 20T and NA was significantly higher than that at 10T, but the difference was not significant compared to 15T in 0-5 cm soil layer.Meanwhile, the Simpson dominance index at 20T and NA was significantly higher than that at 10T and 15T in 5-10 cm soil layer.
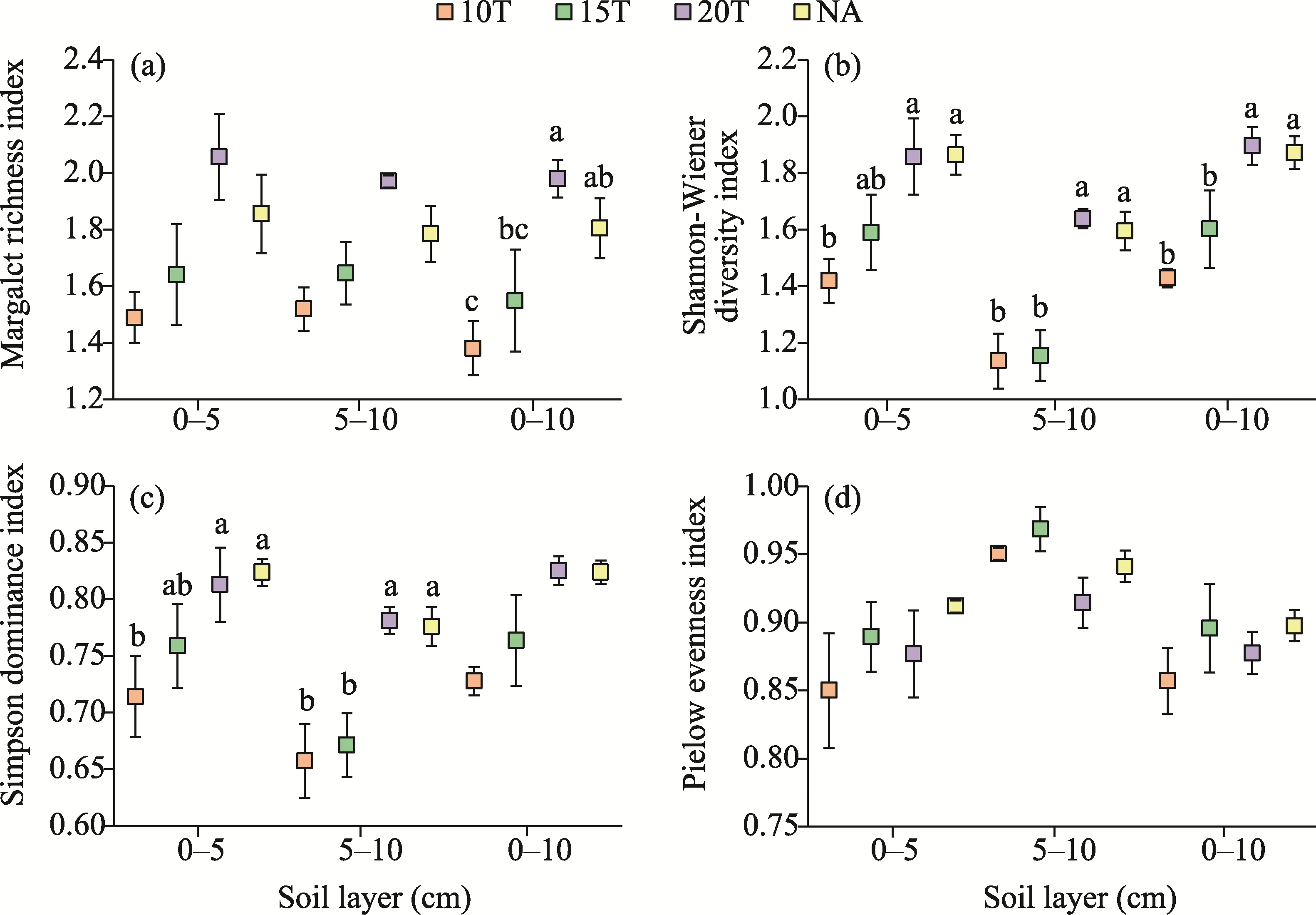
Fig.4 Margalef richness index (a), Shannon-Wiener diversity index (b), Simpson dominance index (c), and Pielou's evenness index (d) of the soil seed bank in 0-5, 5-10, and 0-10 cm soil layers.Different lowercase letters indicate significant differences among different soil cover thicknesses for the same soil layer.Bars mean standard error.
3.3 Soil properties in different soil cover thicknesses
The pH of NA (7.76) was significantly lower than that of the reclaimed sites, and pH decreased with increasing soil cover thickness at reclaimed sites (Fig.5).SWC of NA (21.9%) was higher than that of reclaimed sites, and SWC of 20T (18.2%) was higher than that of 10T (13.2%) and 15T (13.0%).SOM ranged from 15.53 to 56.98 g/kg and was significantly different among reclaimed sites and between reclaimed sites and NA.TN and TP of NA were significantly higher than that of reclaimed sites, and there was a significant difference in TN between 20T and 10T,but no significant difference in TP among reclaimed sites.TK of 10T was significantly lower than that of other sites, and there was no significant difference in TK among other sites.AN was also significantly higher at NA than that at reclaimed sites, and there were no significant differences among sites with different soil cover thicknesses.AP and AK varied significantly with soil cover thickness and were 3.19-5.91 and 242.45-393.45 mg/kg, respectively; AP and AK increased with increasing soil cover thickness at reclaimed sites.

Fig.5 Soil properties of natural sites and reclaimed sites with different soil cover thicknesses.(a), pH; (b), soil water content (SWC); (c), soil organic matter (SOM); (d), soil total nitrogen (TN); (e), soil total phosphorus (TP);(f), soil total potassium (TK); (g), soil Alkali-hydrolysable nitrogen (AN); (h), soil available phosphorus (AP); (i),soil available potassium (AK).Different lowercase letters indicate significant differences among natural sites and reclaimed sites with different soil cover thicknesses.Bars mean standard error.
3.4 Correlation of the characteristics of soil seed bank with soil properties
Soil properties explained 48.46% of RDA1 and 22.27% of RDA2 (Fig.6).Monte Carlo test for RDA1 and RDA2 was significant (P<0.05), indicating that soil properties significantly influenced the species density and composition of the soil seed bank.Each soil property contributed markedly to the species density of the soil seed bank (P<0.05); the species density of the soil seed bank was negatively correlated with pH and positively correlated with other soil properties (Fig.6a).E.dahuricus,C.glaucum,P.depressa,C.majus,A.argyi, andT.lanceolatawere more strongly influenced by soil properties.The distance between sampling sites with different soil cover thicknesses reflected their similarity in the species composition of the soil seed bank (Fig.6b).NA2-NA6 sites were more clustered, indicating that species density and composition were similar at these sites.However, XSH1 and NA1 were far away from other sampling sites and close to each other, indicating that the two sampling sites were less influenced by soil properties than other sampling sites.
4 Discussion
Healthy soil is one of the most important environmental factors influencing plant growth and stress resistance (Li et al., 2020).Low nutrient content and poor structure of abandoned mine soil hinder vegetation growth and increase the risk of soil erosion (Hayes et al., 2011).Soil cover is widely used in mining areas because of its ability to provide plant seeds and nutrients that seeds depend on for germination (Li et al., 2020).
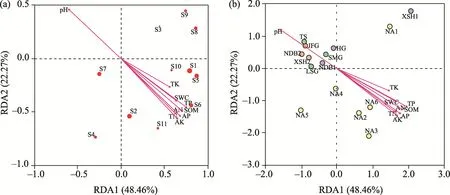
Fig.6 Redundancy analysis (RDA) of the species density (a) and composition of the soil seed bank (b).The red arrows represent soil properties (including pH, SOM, TN, TP, TK, AN, AP, AK, and SWC).The red solid circles in Figure 6a indicate the species of the soil seed bank, and the size of the solid circles is directly proportional to species density (i.e., the larger the circle, the greater the species density).The circles with different colors in Figure 6b denote the sampling sites.S1, Elymus dahuricus Turcz.; S2, Chenopodium glaucum L.; S3, Dysphania schraderiana (Roemer & Schultes) Mosyakin & Clemants; S4, Peganum multisectum (Maxim.) Bobrov; S5,Plantago depressa Willd.; S6, Chelidonium majus L.; S7, Hypecoum leptocarpum Hook.f.et Thoms.; S8,Lepidium apetalum Willd.; S9, Fragaria orientalis Lozinsk.; S10, Artemisia argyi Lévl.et Van.; S11, Thermopsis lanceolata R.Br..
Reclamation of coal mines in the Qilian Mountains provided seeds for revegetation by relocating soil from natural areas to reclaimed area.However, during soil transfer, a soil dilution effect (soil layer mixing) occurs (Fowler et al., 2015), disrupting the original vertical soil structure (Fan et al., 2018) and altering the distribution pattern of the soil seed bank.Additionally,differences in soil cover thickness exacerbate the heterogeneity of seed distribution.The seed accumulates in the shallowest soil horizons (Benvenuti and Mazzoncini, 2021), resulting in the topsoil seed bank density approaching total soil seed density (Hyatt and Casper, 2000; Traba et al., 2004).Therefore, we explored the characteristics of the topsoil (0-10 cm) seed bank in natural and reclaimed areas after six years of natural succession.The 15 sampling sites in this study demonstrated the wide variation of soil seed bank density, indicating that soil cover thickness significantly limits topsoil seed density.However, this restriction apparently did not involve the species composition of seeds.Pearson correlation coefficient was high for species composition of the soil seed bank among sampling sites (Fig.2).Indeed, compared to above-ground vegetation,the species composition of the soil seed bank is more stable (Zhao et al., 2021a) and practically undisturbed during early succession (He et al., 2020).The study area is located in an alpine mountainous region with a short growing season (Bueno et al., 2011), which facilitates storage of seeds in a dormant form in the soil by inhibiting the seed cell cycle (Ahmad et al., 2021;Benvenuti and Mazzoncini, 2021).Environmental factors drive seed dormancy and germination(Kildisheva et al., 2020).In semi-arid grasslands with limited water resources, rainfall and evapotranspiration cycles regulate seed dormancy and germination by altering acquisition and utilization of water over space and time (López et al., 2021).However, different soil cover thicknesses in reclaimed sites change the stability of these cycles in the vertical dimension and significantly affect the density and number of species in the soil seed bank (Fig.4; Table 3).Seeds in thinner soils are more susceptible to moisture limitation than seeds in thicker soils (Ribeiro et al., 2021), resulting in the conversion of some active seeds in thinner soils to inactive seeds,reducing germination rate and affecting the density and diversity of the soil seed bank (Traba et al., 2004).On the other hand, thicker soil cover provides higher water availability, which facilitates seed survival (Cueva-Ortiz et al., 2020).
The effect of soil cover thickness on the density of the soil seed bank inevitably leads to differences in seed bank diversity.Our results showed that species diversity at reclaimed sites increased with increasing soil cover thickness, except for Pielou's evenness index (Fig.4; Table 3).Reclaimed sites with soil cover thicknesses of 10 and 15 cm had lower soil seed bank diversity.We observed that not all species present at natural sites migrated successfully.For instance,Lepidium apetalumWilld., which is widespread at NA, was only found at 15T and 20T.We hypothesize that the soil cover thickness at receptor sites may prevent the establishment of some species.Soil cover thickness influenced seed germination, but soil nutrients also played an important role (Cueva-Ortiz et al., 2020).Our results showed that there were significant differences in soil properties between NA and reclaimed sites, except for TK (Fig.5; Table 4), and these differences contributed to the variability of the soil seed bank among different soil cover thicknesses.Seed germination, seed production, and longevity are influenced by the level of macronutrients in the soil (Ko?odziejek, 2017; Steidinger et al., 2019; Ma et al., 2021).Meanwhile, the high levels of SOM are beneficial for maintaining soil moisture, further promoting seed germination (Du et al., 2023).
The results of RDA showed that the effects of soil properties on species composition of the soil seed bank could be divided into two types (Fig.6).On the one hand, pH was negatively correlated with seeds and other soil properties.Yang et al.(2021) showed that there is a hump-shaped relationship between the species composition of the global seed bank and soil pH, with the highest diversity at pH 6.0-7.0; this is identical to our findings.Evidence indicates that soil pH could directly affect the survival and germination of particular species (Ma et al., 2017b).However, soil pH was frequently correlated with other environmental factors that indirectly affect the characteristics of the soil seed bank (Du et al., 2023).For example, high soil pH reduced the effectiveness of phosphorus in the soil (Fort et al., 2015), and AK increased in alkaline soil with high levels of calcium (Gattward et al., 2012).Soil pH indirectly affects the characteristics of the soil seed bank by modulating soil microbial community composition to alter mineral nutrient utilization (Zhang et al., 2016).Malik et al.(2018) found that microbial communities exhibited an increased ability to store carbon in soils near neutral pH.On the other hand, soil properties, such as SOM, TN, TP, etc., were positively correlated with the species composition of the soil seed bank.Mohammed and Denboba (2020) suggested that most species in the soil seed bank promoted seed germination by encouraging environmental conditions such as increased SOM,SWC, and soil nutrients.
Du et al.(2023) also indicated that the soil seed germination rate increased with increasing soil organic carbon and soil nitrogen, but decreased with increasing soil pH, which was similar to our findings.Moreover, we found that the soil seed bank was significantly influenced by TP and AP.The concentration of AP affects the accumulation of starch and protein in seeds by affecting carbon and nitrogen-containing metabolites in the leaves of some species, thereby limiting seed yield (Hou et al., 2018; Yuan et al., 2019).The distribution of each sampling site in RDA indicated that 20T was close to NA, but the reclamation objective (restoration to natural areas)had not been reached in full.We conclude that the soil cover thickness influences species composition of future vegetation by limiting the density and diversity of the topsoil seed bank.Meanwhile, the dilution effect of soil transfer affects vegetation growth and seed production and germination via changes in soil properties.
The results of this study are limited to some extent.Although topsoil significantly affects vegetation recovery (Piqueray et al., 2020; Rokich et al., 2000; Luo et al., 2023), our study did not use the original coal mine that was not covered with soil.As a result, the impact of soil cover thickness on coal mine is not fully reflected.Next, soil cover thickness gradient was limited.Further research is needed to refine our results.We believe that further research into the effect of soil cover thickness on the soil seed bank is warranted in order to provide a more general theoretical basis for coal mine reclamation.
Overall, the characteristics of the soil seed bank in coal mine reclamation areas are complex and determined by a combination of soil cover thickness and soil properties.A large number of researches elucidated the relationship between the soil seed bank and soil properties (Pérez et al.,2014; Ma et al., 2017a; Ribeiro et al., 2021).However, less research is available on the soil seed bank in mining areas, and the effect of soil cover thickness on the soil seed bank was poorly understood.This study focused on the effects of soil cover thickness on the soil seed bank in coal mining areas in the semi-arid steppe of the Qilian Mountains.The findings of this study provided a reference for coal mine reclamation.Based on the results of this study, we recommend the following measures to achieve the goals of coal mine reclamation in the Qilian Mountains: (1)moderate fertilization should be applied to the reclamation areas with a soil cover thickness of about 20 cm to increase soil fertility, nourish the soil seed bank, and increase seed germination rates; and (2) for reclamation areas with a soil cover thickness less than 20 cm, external seed sources, fertilization, seed sowing, increasing ecological niches, and closely monitoring of vegetation should be used.In conclusion, a variety of techniques can be used to manage and monitor reclaimed areas following soil transfer, offering the prospect for improved restoration.
5 Conclusions
Our study demonstrated that soil cover thickness had a significant effect on the topsoil seed bank and soil properties.We found that the density, number of species, and diversity of the soil seed bank at sampling sites with a soil cover thickness of 20 cm were similar to those at natural sites and significantly higher than those at other sampling sites.However, there was a significant difference in soil properties between reclaimed sites and NA, suggesting that soil property is an important factor in successful restoration.Further, soil properties at reclaimed sites increased with the soil cover thickness.Therefore, soil cover thickness is a critical element in achieving revegetation in coal mining areas.Overall, a soil cover thickness of about 20 cm provides adequate seeds for vegetation regeneration, while seed germination is limited by soil properties.This study provides another perspective on coal mine reclamation.However, due to the relatively limited data collected, further research is needed to fully assess the potential of utilizing soil seed bank for coal mine reclamation.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2019YFC0507400).We thank the editors and anonymous reviewers for their valuable comments on this manuscript.We would like to thank Dr.Kathryn B PIATEK for her assistance with English language editing and valuable comments on this article, and gratefully acknowledge Ms.FENG Yilin, a student at the Ningxia University, for his contribution to fieldwork and the administration of Gansu Qilian Mountains National Nature Reserve for their support of our study.
Author contributions
Conceptualization: ZHAO Wenzhi; Data curation and software: YANG Jingyi; Methodology: YANG Jingyi,LUO Weicheng, LI Guang.Investigation and formal analysis: YANG Jingyi, LUO Weicheng, LIU Jiliang;Writing-original draft preparation: YANG Jingyi; Writing-review and editing: YANG Jingyi, LUO Weicheng,ZHAO Wenzhi, WANG Deijin; Funding acquisition: ZHAO Wenzhi; Resources: LUO Weicheng, LIU Jiliang;Supervision: ZHAO Wenzhi.
- Journal of Arid Land的其它文章
- Integrating stable isotopes and factor analysis to delineate the groundwater provenance and pollution sources in the northwestern part of the Amman-Al Zarqa Basin, Jordan
- Monitoring vegetation drought in the nine major river basins of China based on a new developed Vegetation Drought Condition Index
- Estimation and inter-comparison of infiltration models in the agricultural area of the Mitidja Plain,Algeria
- Evaluation of the water conservation function in the Ili River Delta of Central Asia based on the InVEST model
- Effects of degradation and species composition on soil seed density in the alpine grasslands, China
- Analyzing environmental flow supply in the semi-arid area through integrating drought analysis and optimal operation of reservoir

