Anti-aging performance improvement and enhanced combustion efficiency of boron via the coating of PDA
Shui M , Qinghi Shu , Mengyng Zhng , Hongyu Hung , Ynsong Shi ,*,Xijun Lv , Shui Zho b,**
a School of Materials Science and Engineering, Beijing Institute of Technology, Beijing,100081, China
b Key Lab of Advanced Optoelectronic Quantum Architecture and Measurement (MOE), Beijing Key Lab of Nanophotonics & Ultrafine Optoelectronic Systems, School of Physics, Beijing Institute of Technology, Beijing,100081, China
c Tangshan Research Institute of Beijing Institute of Technology, Tangshan, 063000, Hebei, China
Keywords:Boron particles Polydopamine Anti-aging performance improvement Heat release
ABSTRACT Boron is an ambitious fuel in energetic materials since its high heat release values, but its application is prohibited by low combustion efficiency and oxidization during storage.The polydopamine (PDA) was introduced into boron particles, investigating the impact of PDA content on the energetic behavior of boron.The results indicated that the PDA coating formed a fishing net structure on the surface of boron particles.The heat release results showed that the combustion calorific value of B@PDA was higher than that of the raw boron.Specifically, the actual combustion heat of boron powder in B@10%PDA increased by 38.08%.Meanwhile, the DSC peak temperature decreased by 100.65 °C under similar oxidation rate compared to raw boron.Simultaneously,the B@PDA@AP and B@AP composites were prepared,and their combustion properties were evaluated.It was demonstrated that B@10%PDA@AP exhibited superior performance in terms of peak pressure and burning time, respectively.The peak pressure is 12.43 kPa more than B@AP and burning time is 2.22 times higher than B@AP.Therefore, the coating of PDA effectively inhibits the oxidization of boron during storage and enhances the energetic behavior of boron and corresponding composites.
1.Introduction
Metal fuels are promising candidates for energetic materials owing to their high heat release.Boron(B)has been considered as the most attractive metal fuel additive due to its high mass heat of combustion(58.30 kJ/g)and volume heat of combustion(136.44 kJ/cm3), which is higher than that of commonly used aluminum (Al,31.02 kJ/g) [1,2].However, the low combustion efficiency of B prevents its further application in energetic materials.The high melting point of boron around 2177°C makes gas-phase reaction difficult to occur [3].Despite the lower melting point of B2O3compared to Al2O3,the expansion coefficient of elemental boron is inferior to that of the oxide, which prevents the inner boron nucleus from penetrating its shell and reacting with surrounding oxidizing materials.Especially,in the initial stage of boron powder reaction,the formation rate of boron oxide exceeds its consumption rate,leading to the accumulation of a dense film of boron oxide on the surface of boron,which further impedes the reaction between boron and surrounding oxidizing substances,which delays ignition and combustion[4,5].In addition to the formation of an oxide layer during ignition and combustion of boron,it is challenging to avoid the formation of an oxide layer on its surface during commercial preparation[6,7].Furthermore,even if there is no initial oxide layer present,reoxidation occurs rapidly upon exposure to air and water[8], leading to a continuous increase in oxide content during storage[9,10].W.G.Shin et al.revealed that the thickness of the oxide layer on fresh produced boron reaches 2 nm after two days[11],and the presence of initial oxide layer is also detrimental to boron ignition and combustion [12,13].The anti-aging ability not only plays a crucial role in the production and storage of boron particles but also significantly impacts the energy behavior of boron particles.Consequently, the incomplete combustion of boron particles results in an unrealized energy advantage.
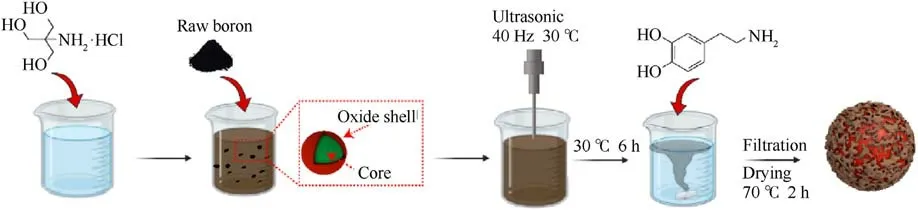
Fig.1.Preparation process of B@PDA.
In order to investigate the energy release mechanism and improve the ignition and combustion performance of boron powder, researchers have conducted various methods such as purification and reducing pre-oxidation [14], modification [15,16],coating [17,18], incorporation of energetic components [19-21] to address the challenges associated with ignition difficulty and low combustion efficiency in boron particles.The organic fluoride,which has been modified as mentioned above,is widely recognized as a highly promising material for surface modification of boron powder due to its exothermic combustion in various oxidation environments and the small molecules generated from the decomposition of fluoride can react with boron oxide, forming boron fluoride and mitigating the impact of the oxide film on energy release [22,23].However, the application of fluoride often poses challenges such as synthetic hazards and environmental contamination.Therefore, the development of boron powder coating materials that are facile to synthesize and environmentally friendly has become an urgent research topic.Since its initial discovery in 2007, polydopamine (PDA) has found widespread application across various fields including biomedicine and industry[24,25].In recent years, PDA has been introduced by many researchers into the field of energetic materials[26-28].W.Ao et al.found that a thin layer of PDA can provide some protection to n-Al[29].However, increasing the thickness of the PDA coating can enhance the reaction between n-Al and active components.Compared with uncoated n-Al/kerosene and other PDA-coated nanofluid fuels, n-Al@PDA(2h)/kerosene exhibits superior ignition and combustion characteristics.W.He et al.revealed that compared to traditional n-Al/CuO MICs, the n-Al@PDA@CuO showed improved initial reaction temperature (528.4°C),enhanced energy release (2.934 kJ/g), and lower combustion temperature(1606°C) due to superior nanoscale contact between the n-Al core and CuO shell, resulting in higher combustion efficiency[30].Additional,W.He et al.proposed that compared to traditional n-Al/PTFE MICs, the n-Al@PDA/PTFE exhibits an enhanced energy release, which makes the tunable reactivity of n-Al@PDA/PTFE possible and results in a higher onset thermal decomposition temperature [31].As to increases, a more complete reaction between PTFE and Al nanoparticles can be achieved.
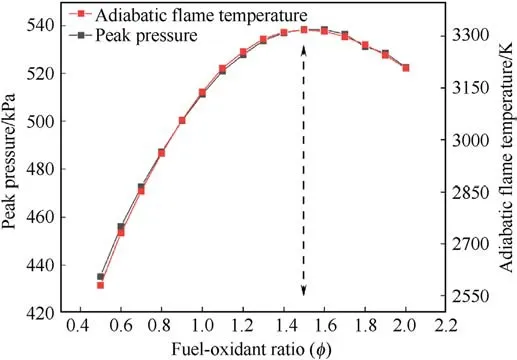
Fig.2.Energy parameters of B/AP composite system at different Φ values.
In this study, we conducted experimental investigations to examine the impact of varying PDA quality percentages on the heat release characteristics of boron particles,aiming to address ignition difficulties and low combustion efficiency while exploring new possibilities for its application in energetic materials fields.
2.Experimental section
2.1.Chemicals
Raw boron (2-5 μm, purity ~90%) was supplied by Tianyuan Aviation Materials Yingkou,China Technology Co.,LTD.Ammonium perchlorate (99%), dopamine hydrochloride (98%), trishydrochloride buffer (pH = 8.5), ethyl acetate (Analytical Reagent,AR), and dimethylformamide (AR) were purchased from Shanghai Aladdin Biochemical Technology Co., LTD.
2.2.Samples preparation
2.2.1.Preparation of B@PDA
The B@PDA composite was synthesized via an oxidationinduced self-polymerization assembly.As shown in Fig.1, boron powders and tris-hydrochloride buffer were measured in corresponding amounts and mixed up in the beaker.The dispersion was subjected to ultrasonic treatment, followed by the addition of dopamine hydrochloride.Mechanical stirring at room temperature(25°C) was carried out for 6 h to induce oxidative selfpolymerization with different mass percentages of 10 wt%,15 wt%,and 20 wt%dopamine hydrochloride.Finally,the B@PDA particle samples were obtained by varying the content of the PDA coating.
2.2.2.Preparation of B@PDA@AP
The REAL software was employed to determine the fuel-oxidizer ratio(Φ)of the B@AP composite system by evaluating the pressure peak(P)and adiabatic flame temperature(AFT)at various Φ based on the principle of minimum free energy [32-34].During the calculation, the specific volume was set up at 1.65 m3/kg since 200 mg composites were ignited in a sealed constant-volume(330 cm3) crucible.The results in Fig.2 demonstrated that both P and AFT values reach their maximums as the Φ =1.5 in B@AP andB@PDA@AP composite systems.Thus, the formulation used in this work is determined,which is shown in Table 1.
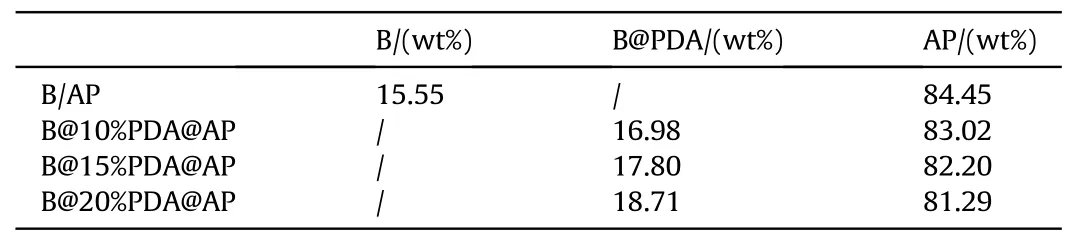
Table 1 Formulations of B@PDA@AP composites.
When it comes to the preparation of B@PDA@AP, the N-N dimethylformamide solution of AP was added dropwise into the ethyl acetate solution of B@PDA under a controlled stirring speed of 150 r/min and with a system temperature of 55°C.As the continuous solvent volatilization, the B@PDA@AP composite was obtained ultimately by vacuum drying and grinding.
2.3.Characterization
The Scanning Electron Microscopy (SEM, ZEISS Gemini 300)with the Energy Dispersive Spectrometer (EDS, OXFORD XPLORE30),and Transmission Electron Microscope(TEM,FEI Talos F200x G2) with the EDS (super-x) were used to characterize the morphology and element distributions of the raw boron and B@PDA particles,respectively.Quantitative analysis was carried out via X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI).The X-Ray Diffraction (XRD, X'Pert PRO MPD) was used to characterize the structures with scanning rate 2°/min.
2.4.Aging treatment
The raw boron and B@PDA samples were subjected to an accelerated aging treatment following the compatibility test conditions specified in GJB772A.The samples were weighed and placed into a glass tube, and then the two-week aging process at 60°C and 85% humidity were applied.
2.5.Characterization of energetic behavior
2.5.1.Heat release measurement
The sample was ignited by nickel-chromium wire in an oxygen atmosphere of 3 MPa.The heat release of each sample has been measured by three times.The average values with error bars have been displayed and compared.
2.5.2.Thermal analysis
Thermal analysis was carried out to investigate the oxidation process of samples with a heating rate of 10°C/min under the air flow with a rate of 50 mL/min.Differential scanning calorimetric(DSC)-Thermogravimetry (TG) was performed with Germany NETZSCH STA 449 F3 instrument (NETZSCH Co.) from room temperature to 1000°C.
2.5.3.Pressure cell test
Closed bomb pressure was measured in a combustion cell(330 mL free volume).The mass of the samples was fixed at 0.200±0.001 g.A nichrome wire with the voltage of 60 V was placed on top of the loose powder sample to propagate the reaction downwards upon ignition.The pressure curves with time were recorded by a piezo-electric pressure sensor with the collection frequency of 1000 points/s.The peak pressure, maximum pressurization rate could be obtained.The peak pressure was defined as the maximum value of the pressure curve and the pressurization rate was calculated by taking the ratio of 95%of maximum pressure to the rise time [35].
2.5.4.Ignition and combustion test
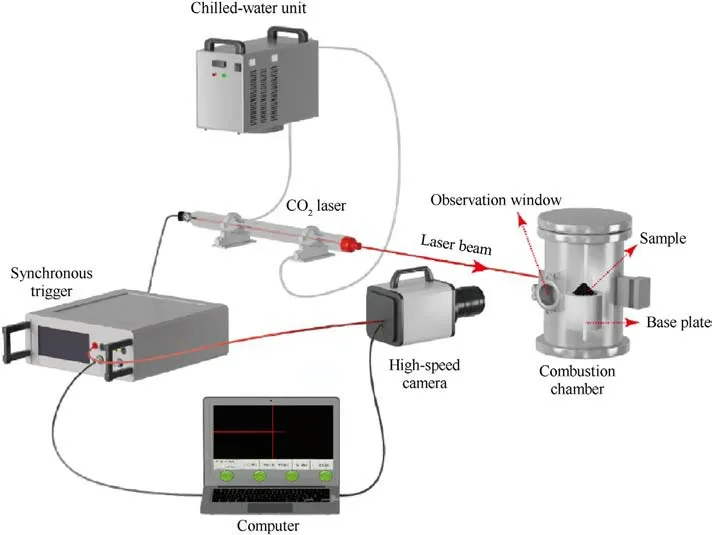
Fig.3.Schematic diagram of laser ignition and combustion experimental system.
As shown in Fig.3, an experimental system was used to investigate the combustion characteristics of powders.A fixed mass(0.20±0.01 g) of the samples that kept them loose was placed in a closed combustion chamber that was with a natural atmosphere.During the experiments,a CO2laser facility with a power setting of 60 W was performed to ignite the samples in the combustion chamber with windows.A high-speed camera was used to record the combustion dynamic process, which was triggered synchronously with the laser using a synchronous trigger.The frame rate was set to 1000 fps and the image resolution was 1280×860 pixels.
3.Results and discussion
3.1.Morphology of B@PDA particles
The morphology of the raw boron and B@PDA samples with varying contents of PDA were characterized by SEM and TEMHAADF.As shown in Fig.4(a), the raw boron particles exhibited irregular morphology and severe agglomeration.Fig.5(a) revealed the presence of a discernible nano-sized oxide layer on the surface of raw boron particles, while B, Mg, and O elements were distributed across their exterior.When it was coated by the PDA to form the B@PDA,the EDS results of the B@PDA showed that B,C,N,and O elements were uniformly distributed into the B@PDA composites surface,demonstrating that PDA film adhered to the boron particles forming B@PDA composites.As can be seen from Fig.5(b), we proposed the PDA film adhered to the surface of boron in a porous coating.Moreover, the net aperture of the mesh structure decreased with increasing PDA content(as depicted in Figs.S2 and S3.).
Fig.6 displayed high-resolution XPS spectra of O 1s peaks for raw boron and B@PDA particles.The presence of B2O3(~532.5 eV)[36] in Fig.6(a) proved the partial oxidation of boron particles,which was consistent with the TEM image displayed in Fig.5(a).For the B@PDA particles (Figs.6(b), (c), 6(d)), hydroxyl(~531.3 eV)and carbonyl(~532.2 eV)groups proved the existence of C-O and C=O bonds [31].The binding energy associated with the B2O3group increased to 532.9 eV, 532.8 eV, and 532.9 eV respectively, further demonstrating PDA attachment to boron particles.
3.2.Characterization of energetic performance
3.2.1.Combustion calorific value analysis
To investigate the impact of the PDA layer on the energy release and anti-aging properties of boron, oxygen bomb tests were conducted on both B and B@PDA samples pre- and post-aging.The results shown in Fig.7 indicated that the heat release of B@PDA composites increased significantly after PDA coating, which was attributed to the heat released during PDA decomposition, which provided a high-temperature environment around the boron particles and then promoted the heat release of boron.
However, the combustion calorific value of B@PDA composites decreased slightly as the PDA content increased.The calorific value of PDA is lower than that of B, thus indicating a decrease in the caloric value of B@PDA with increasing PDA content.However,this is attributed to a higher PDA content causing greater heat release during the initial stage of boron particle reaction, leading to an increased rate of formation for the outer boron oxide shell compared to its consumption rate.This accelerates the accumulation of the boron pre-oxide shell,further impeding the heat release of boron.Meanwhile, as the temperature increases in the system,the PDA absorbs heat and melts, enveloping the boron particles.Consequently, a higher PDA content results in greater heat absorption and reduced overall heat release.
Furthermore, we also noticed the large spatter of combustion products.The phenomenon of boron particles splashing can be found in the Supplementary Materials.Figs.S1(b) and S1(d)showed that the splash residue mainly consisted of H3BO3, B2O3,and un-sputtered combustion products mainly consisted of BO6.The formation of H3BO3is likely attributed to the reaction between B2O3and water added before conducting the oxygen bomb test to prevent scorching particles from causing any damage to the oxygen bomb.The BO6, an intermediate product of boron oxidation [37],and the combustion calorific value of raw boron,B@PDA,and aged B@PDA were significantly lower than the theoretical combustion heat value (59.3 kJ/g) of boron illustrated in Fig.7, suggesting incomplete reaction and inferior energy release efficiency of these materials in the oxygen bomb of oxygen atmosphere.
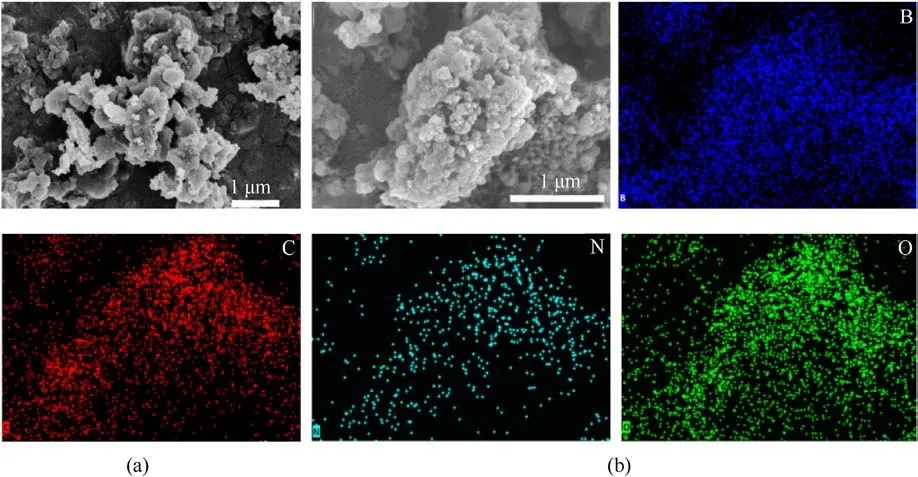
Fig.4.SEM image of the samples: (a) Raw boron; (b) B@20%PDA with EDS mapping.

Fig.5.TEM-HAADF images of (a) boron particles; (b) B@20%PDA.
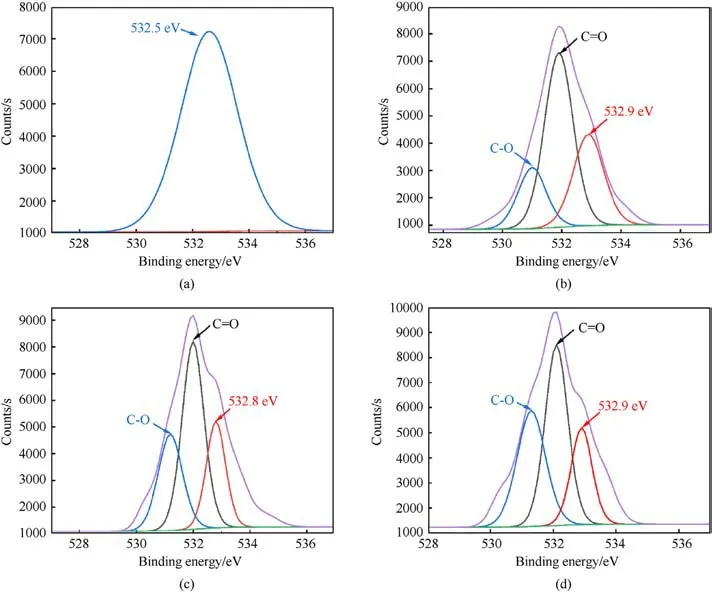
Fig.6.High-resolution XPS spectra of O 1 s peaks for (a) raw boron particles (b) B@20%PDA (c) B@15%PDA, and (d) B@10%PDA.Deconvolution of the O 1s signal for the B@PDA particles shows the presence of B2O3 (~532.5 eV), C-O (hydroxyl, ~531.3 eV), and C-O (carbonyl, ~532.2 eV) groups.

Fig.7.Heat release of B, B@PDA composites, and corresponding samples after aging.
In the meanwhile, we found that PDA coating can effectively prevent the oxidization of boron when supposed to high temperature and humidity,which can simulate the aging of boron during storage.The combustion calorific value of boron decreased by approximately 20.07%, from 17.145 kJ/g to 13.703 kJ/g after aging.However, the combustion calorific value of B@PDA composites decreased by 9.08%, 11.05%, and 3.13%, respectively.Since PDA coatings can isolate boron particles from high-temperature and high-humidity environments during storage,thus reducing contact between boron particles and oxidizing substances,which prevents the oxidation of boron.The results demonstrated that PDA coating not only enhanced the heat release of boron but also effectively inhibited the absorption of water and self-oxidation, presenting a novel approach for the subsequent storage and transportation of boron powders.
3.2.2.Thermal behavior analysis
To further study the effect of PDA on the thermal behavior of boron powders, TG and DSC tests have been performed.Fig.8(a)presented the DSC curves of boron and B@PDA composites,and the characteristic parameters of the DSC curves were summarized in Table 2.It showed that the B@PDA composites exhibited both endothermic and exothermic peaks, whereas raw boron only displayed a single exothermic peak.
The endothermic peak of the B@PDA is predominantly attributed to the melting of PDA [38-40], while the exothermic peak arises from the oxidation of boron.The exothermic peak temperature of B@PDA composite particles advanced significantly compared to raw boron,moving forward by 108.38°C,31.29°C,and 25.04°C,respectively.Since the PDA layer facilitated the reaction to occur at a lower temperature owing to mitigating heat dissipation surrounding the boron powder,expediting heat accumulation,thus promoting boron oxidation.Meanwhile, as the PDA content increased, the exothermic peak temperature approached that of raw boron powder more closely.Because the thermal effect of PDA around boron powder is positively correlated with PDA concentration, the enhancement of the thermal effect is advantageous to the oxidation of boron, forming a boron oxide shell.However, the boron oxide shell formed during the initial stages of boron oxidation is difficult to evaporate and subsequent gas-phase reactions,thereby the thickness of the boron oxide shell gradually increases and hinders further contact between boron powder and oxidizing substances, so that the obstruction effect of the boron oxide shell enhances but the PDA thermal effects decreases.Ultimately, the exothermal peaks increased as the PDA content increases.
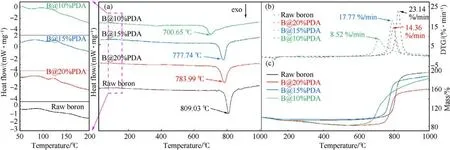
Fig.8.(a) DSC, (b) DTG, and (c) TG curves for boron and B@PDA composites.

Table 2 DSC characteristic parameters for boron and B@PDA composites.
Interestingly, it can be observed in Fig.8(b) that the DTG peak value of the B@PDA composite was lower than that of raw boron.PDA coating retards the thermal conductivity of boron powder and air, and absorbs heat during the early melting stage, thereby reducing the reaction rate between boron powder and surrounding oxidizing substances.Meanwhile,when the PDA content increased from 10% to 20%, the peak value of DTG initially exhibited an increase followed by a subsequent decrease.This phenomenon can be attributed to the synergistic effect between the decomposition heat effect of PDA and the obstructive effect of boron oxide.Specifically,as the PDA content increases from 10% to 15%, there is an augmentation in its thermal effect leading to an elevation in the peak value of DTG.However, when the PDA content further increases from 15%to 20%,it intensifies the obstruction effect caused by boron oxide, resulting in a decline in the peak value of DTG.
Fig.8(c)illustrated that the mass gain of raw boron particles and B@PDA composites underwent two stages.For boron, the impurities lead to a small amount of decrease in mass during the initial stage.When the temperature raised to above ~784°C, boron underwent significant oxidation, resulting in exothermic heat generation and main weight gain.For B@PDA composites,the initial stage of weight loss is attributed to PDA decomposition.While in the subsequent stage, the weight gain results from boron oxidation.The TG curves indicated that PDA can promote the oxidation reaction of boron, specially, the promotion effect on the oxidation reaction is more significant under lower content of PDA,consisting with the DSC results.Although the B@PDA composites exhibited lower oxidation weight gain compared to raw boron, B@10%PDA demonstrated that the oxidative weight gain per unit mass is nearly identical to that of raw boron(shown in Table 2).We proposed that the boron is enveloped by a PDA fishing net on the exterior.When the PDA content reaches 20%, the aperture of the outer PDA net becomes smaller, which prohibits the diffusion of oxygen to the boron core, thereby impeding the complete reaction of boron.However,the outer layer of boron powder forms a larger and more uniform aperture for the PDA net with the 10% content of PDA,which not only raises the surrounding temperature high for the oxidation of boron but also facilitates the diffusion of oxygen to boron core.Therefore, B@10%PDA exhibited higher reaction performance than raw boron.
3.2.3.Combustion performance analysis
To further reveal the influence of PDA on the boron-based system, boron, and prepared B@PDA composites were applied in energetic materials with AP as the oxidizer.The ignition and combustion of energetic composites were tested by using a CO2laser igniter.The typical combustion images of B@AP and B@PDA@AP under the atmosphere obtained by the high-speed camera were illustrated in Fig.9.The time of the first occurrence of the laser upon the sample is 0 s.Overall, the combustion intensity of the B@AP samples was higher than that of the B@PDA@AP samples, because the thermal conductivity of PDA was inferior to that of boron, resulting in a reduction in the heat transfer rate among boron particles and surrounding oxidizing substances.
For B@10%PDA@AP composites, before 283 ms the combustion growth stage, the flame was primarily composed of external the green flame and internal yellow flame,with the former occupying a larger area than the latter.The green flame is the characteristic flame of the intermediate product (BO)nof boron powder combustion[37].Then,the intensity of green flame gradually increased,indicating that a significant amount of B2O3generated and released a substantial amount of heat to form a heat flow, resulting in the outer flame taking on a distinct mushroom shape [41].With the temperature increases, the yellow flame generated [42], which is predominantly concentrated at the base of the flame,and then the flame progressed to the stable combustion stage,the concentrated yellow flame transformed into a blinding white flame and dispersed yellow particles flame.The white flame indicated a more intense combustion phenomenon of B@PDA@AP samples [37].Accompanying the continuous progression of combustion, smoke gradually separated with flame,leading to a progressive decrease in system temperature, the bright white flame transitioned into a dimmed flame finally, meaning the combustion entered the extinguishing stage.

Fig.9.Combustion snapshot of raw boron and boron-based composite energetic materials: (a) B@AP; (b) B@10%PDA@AP; (c) B@15%PDA@AP; (d) B@20%PDA@AP.
Meanwhile, with the relative increase of PDA content, we observed a distinct difference in the flame color distribution compared to the B@10%PDA@AP.During combustion growth and stable combustion stages, the distribution area and combustion intensity of white flames were obviously larger and stronger than that of green flames.And the grainy yellow flame is also distributed around the perimeter of the flame, which is formed by the combustion of the decomposition products of the PDA.Summarizing,the combustion state of composites varies with the content of PDA,resulting in significant differences in flame color distribution during the combustion growth stage.This phenomenon can be explained by the fact that while the B@10%PDA@AP system mainly undergoes oxidation combustion of relatively bare raw boron, the B@20%PDA@AP system experiences both oxidation of raw boron and combustion of PDA attached to its surface.

Fig.10.(a) Peak pressure; (b) Pressurization rate; (c) The pressure curves of samples with time.
Based on the above typical combustion images and Subsection 3.1 mentioned,the surface aperture size of the B@PDA composites is dependent on the content of PDA.There are two contact modes including the direct and indirect between B and AP in the B@PDA@AP composites.Particularly, in the B@10%PDA@AP composites, the proportion of direct contact between boron and AP is higher than that in the B@20%PDA@AP composites.
The contact mode between boron and AP significantly impacts the subsequent combustion and heat release of boron.In the B@AP sample, AP is directly deposited onto the surface of B or agglomerates around it.When AP exposed to high-energy laser radiation undergoes a violent decomposition reaction, a portion of the gas generated reacts with B, while another portion fails to react with boron particles due to the impact and collision.Consequently,these incompletely burned boron particles are ejected from the system,rendering the combustion state unstable and hindering the full utilization the advantages of boron.
Although the combustion intensity of B@PDA@AP composite particles exhibited a slight reduction compared with B@AP,the PDA can mitigate particle splashing during combustion owing to the effective adhesion of the PDA, thereby enabling stable and continuous burning of the system.However, with the increasing content of PDA, the aperture size at the periphery of B@PDA decreased.This reduction in aperture size is not conducive to the contact between boron and oxidizing substances, leading to an increased ignition delay and then affecting the heat release of boron powder.When the PDA content reached 10%, the B@10%PDA@AP exhibited superior performance, including shortened ignition delay time, less particle spattering and persistent combustion.
3.2.4.P-t curves analysis
Pressure cell test was applied to B@AP and B@PDA@AP samples to characterize the combustion behavior to obtain the peak pressure and pressurization rate.As shown in Fig.10,it was found that as the PDA content increased from 10% to 15%, the peak pressure decreased by 56.88% from 281.53 kPa to 121.39 kPa, and the pressurization rate decreased by 86.02% from 0.93 kPa/ms to 0.13 kPa/ms.However, as the PDA content increased to 20%, there was a slight increase in the peak pressure,reaching 144.86 kPa with a rise of 19.33%, and the peak pressurization rate was 0.33 kPa/ms, representing an increase of 153.84%.The results above indicated that the peak pressure and pressurization rate exhibit a non-monotonic trend,but increase first and then decrease with the increase of PDA content.Both the gas generated from PDA decomposition and the heat released by boron combustion can enhance the peak pressure of the system.However, an increase in gas production is often accompanied by a decrease in boron content within boron-based composites.These two influencing factors interact with each other, resulting in non-nonmonotonic change for composite material pressure peaks.The pressurization rate of B@AP exceeded that of B@10%PDA@AP,yet the peak pressure was lower than that of B@10%PDA@AP.In the case of B@AP,AP,and B are in direct contact,facilitating rapid heat and mass transfer within the system to achieve peak pressure quickly.However, excessive fast reaction rates may cause particles to splash out before complete reaction,thereby affecting overall energy release.For the B@10%PDA@AP,the contact mode between B and AP is altered by PDA,which affects the heat and mass transfer of the interface layer.Despite a decrease in pressurization rate, PDA facilitates strong bonding between B and AP.It not only reduces heat loss around the boron powder to establish a high-temperature environment but also facilitates AP decomposition and enhances combustion pressure.Meanwhile,the subsequent decomposition and combustion of PDA results in the release of gas,which further elevates the peak combustion pressure of the system.
When it comes to the contact mode between boron and AP,which was influenced by the aperture size of the PDA structure.In the B@AP,boron particles were in direct contact with AP;however,in B@PDA@AP,there was not only direct contact between boron and AP but also indirect contact via the adhesion of the PDA.When the PDA content reached 15%, the aperture size of PDA coating decreased, hindering contact between oxidizing substances and boron, thereby reducing the reactivity of boron and resulting in decreased heat release in the system,which is not conducive to an increase in final peak pressure.However, when the PDA content increased to 20%, the thermal effect of PDA decomposition was enhanced,which facilitated the combustion reaction and released a significant amount of heat, leading to an increase in final peak pressure.Finally, in consideration of the thermal accumulation effect of the PDA and the impact of PDA aperture size on B and AP contact mode, B@10%PDA@AP exhibited superior performance than that of the B@AP sample.Additionally, the peak pressures measured were lower than the theoretical values calculated by the first free energy principle showed in Fig.2, because a portion of heat energy being consumed for wall heating through heat transfer during combustion of boron-based composites in the closed bomb.
3.3.Proposed mechanism of PDA on boron-based composites
The XRD and DSC technique are employed here to investigate the combustion mechanism of B@PDA@AP composites with results showed in Figs.S1(a) and S7 presented that a novel structure is formed between B and PDA after PDA treatment, demonstrating that the PDA is coated onto the surface of boron,which is because a plethora of hydroxyl and amine functional groups are introduced onto the surface of the material [43] that is conducive to the anchoring of boron oxide and PDA.As illustrated in Fig.S1(c), the XRD curves of B@PDA@AP samples exhibited identical profiles,with all characteristic peaks corresponding to AP crystal planes and no characteristic peak of boron, meaning the successful coating of AP onto the surface of B@PDA.Meanwhile,the characteristic peaks of the oxygen bomb combustion residues were matched to H3BO3,indicating the sufficient burning of boron.The PDA has two endothermic peaks at 73.47°C and 228.21°C,as shown in Fig.S6,while the AP displays a single endothermic peak at 241.45°C [44].The emergence of the new peak at 246°C in the DSC curve of the B@PDA@AP(Fig.S7)is due to the endothermic effect of PDA and AP.Fig.S7 also showed that the peak temperature of B@PDA@AP was higher than that of B@PDA,but not significantly different from that of B(809.03°C),which can be attributed to the synergistic coupling between the endothermic and exothermic effects of PDA and AP.
Thus,we propose a mechanism of PDA action in the combustion of B@PDA@AP,as illustrated in Fig.11.PDA plays the role of a bridge for grafting B and AP together due to the abundance of active sites and strong adhesion properties of PDA [45], resulting in the formation of a B@PDA@AP core-shell structure.Upon heating,B@PDA@AP undergoes the change where PDA and AP absorb heat and melt at low temperatures.Simultaneously, the PDA wraps around B to reduce heat loss,allowing for slow oxidation of boron,and the primary reaction process is illustrated by Eq.(1).
As the temperature increases, AP undergoes thermal decomposition,the documented literatures demonstrated that the partial decomposition of AP into HClO4and NH3due to proton transfer occurs between NH+4and ClO-4in the AP crystal [46-48].HClO4is unstable and easily decomposes into oxychlorides such as HClO3,ClO2,HClO,and ClO,which may directly leave the surface of the AP crystal[47-49].When the temperature further rises (300-450°C,illustrated in Fig.S7),the AP can be completely decomposed to form Cl2and O2.During the period, boron will occur the reaction as depicted in Eq.(2).And then the NH3can be fully oxidized by chlorine oxides to form N2O, NO2, etc.As the reaction progresses,PDA undergo decomposition to produce-CH3,NO2,NO and release a significant amount of heat[29],promoting the decomposition of AP and accelerating the reaction of boron oxidation.
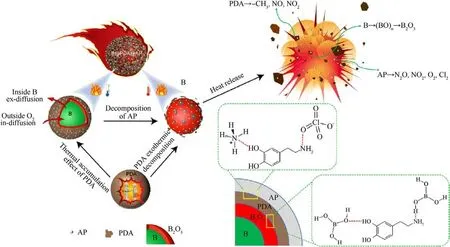
Fig.11.Diagram of reaction mechanism of B@PDA@AP composites.
Throughout the reaction process, PDA not only facilitates combustion by oxidative decomposition heat release but also induces heat accumulation around boron powder and AP during endothermic melting, thereby promoting early pre-oxidation of boron powder.The PDA film operates in the function to that of doublesided tape, binding the boron and AP together, thereby retarding the violent decomposition of AP to prevent plentiful particles from splashing away from the energy source and maintaining a more stable combustion of the system.In summary, the PDA can significantly impact the energy release of boron and the content of the PDA can be optimized to regulate the energy release of boron powder in the future, which provides novel ideas for the engineering application of boron powder.
4.Conclusions
In this study, we have investigated the influence of PDA on the energetic behavior of boron and boron-based composites.The main conclusions are as follows:
(1) Heat release measurement results revealed that the heat release of B@PDA composites was higher than that of boron.Because PDA is anchored to the oxide layer on the surface of B,which affects both heat and mass transfer between B and its surroundings, resulting to mitigate the impact of boron oxide on the combustion process of B.Meanwhile, the application of PDA coating can mitigate the deleterious impact of peroxidation on the heat release performance of boron particles, improving the antioxidant properties of B that the rate of combustion calorific value loss has reduced from 20.07%to 3.13% for B@20%PDA composites.
(2) DSC results demonstrated that when boron particles were coated with PDA, the exothermic peak temperature decreased by 108.38°C-25.04°C.This phenomenon can be attributed to the heat accumulation effect exerted by PDA,which effectively relieves heat dissipation around B and consequently advances its reaction.
(3) The B@AP exhibited stronger combustion intensity than the B@PDA@AP composites.However, the B@10%PDA@AP sample shown the best performance, the peak pressure is 12.43 kPa more than B@AP.And the burning time of the system increased to 2320 ms,which was 2.22 times than that of B@AP.The introduction of PDA not only facilitated the exothermic decomposition to promote the reaction but also effectively bound B and AP together to minimize heat loss caused by particle splashing,thereby enhancing the stability of the combustion reaction.
(4) The energy release of boron can be tailored by varying the content of the PDA, which provides new ideas for the application of boron powder in the field of energetic materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.10.007.
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

