Using Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to analyze differentially expressed brain polypeptides in scrapie strain 22L-infected BALB/c mice***☆
Xiangyu Liao , Jiayu Wan, Wensen Liu, Xin Tang, Wufei Zhu , Na Xu Jing Xu, Nan Li,Yaping Chang Chuanjing Ju
1Department of Immunology, Norman Bethune College of Medical Science, Jilin University, Changchun 130021, Jilin Province, China
2The First Clinical Medical College of Three Gorges University, Yichang Central Hospital, Yichang 443003, Hubei Province, China
3Institute of Military Veterinary Sciences, Academy of Military Medical Sciences, Changchun 130122, Jilin Province, China
4College of Animal Science and Veterinary Medicine, Jilin University, Changchun 130062, Jilin Province, China
5The Fourth Hospital of Jilin University, Changchun 130011, Jilin Province, China
INTRODUCTION
Prion diseases, or transmissible spongiform encephalopathies, are a group of fatal neurodegenerative diseases affecting the central nerve system in both humans and animals. The misfolded prion proteins are transmissible particles which are devoid of nucleic acid[1-2]. There are two forms of prion protein: one is cellular prion protein (PrPc),which is normal; the other is misfolded prion protein (PrPsc), which transmits the disease.
Conversion of PrPcto PrPscand accumulation of PrPscin the brain leads to prion disease[3-5]. The mechanism underlying prion disease is poorly understood. Investigators have tried to use transcriptomic and proteomic methods such as microarrays,two-dimensional polyacrylamide gel electrophoresis, and liquid chromatography-mass spectrometry to uncover mechanisms underlying neurodegenerative disease[6-15]. However, extraction, separation and identification are essential for sample preparation and these processes subsequently destroy tissue structure, complicating understanding of the relationship between tissue and the proteins characteristic of that tissue[16].
In recent years, a new technique named imaging mass spectrometry (IMS) has appeared which is based on biological mass spectrometry, proteomics, and metabolomics. Biological tissue samples coated with matrix can be directly scanned and analyzed by IMS, for example, by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS). Using a combination of data processing and image reconstruction, MALDI-TOF/MS is used to define the position and relative amount of specific molecules in different regions of diseased and normal tissues, and to determine the distribution of polypeptides. There is no need to extract protein or peptides from the tissues of interest, or to stain with drugs, because IMS can correlate the region,process and molecules related to the disease with the region. IMS also shows good potential for evaluating the pathogenesis,development, prognostic value, and selection of drug. It is reported that IMS has been widely used to observe protein distribution in diseased versus normal tissues for different diseases. For instance, it has been applied to evaluate the distribution of protein in mouse brain, colon and testis,changes in protein expression in
Parkinson’s and Alzheimer’s disease, and differentially expressed proteins in breast cancer, prostatic carcinoma and lung cancer[17-29]. However, there is little data regarding application of IMS to prion disease for finding differentially expressed peptides or proteins.
In this study, a model of scrapie infection was established in BALB/c mice intracerebrally using the 22L strain of scrapie. In the final stage of disease, mice were sacrificed, brains harvested and frozen sections prepared.
Hematoxylin and eosin staining and IMS were performed in parallel to observe pathological changes and differentially expressed peptides. This approach provides a new way of diagnosing prion disease.
RESULTS
Quantitative analysis of experimental animals
Ten female BALB/c mice were randomly divided into two groups, with five mice in each group. The test group was inoculated intraperitoneally with 22L-infected brain homogenate, while the control group was inoculated with normal homogenate. No animals died during the experiment and all 10 mice were analyzed.
Behavioral changes and incubation period of 22L-infected BALB/c mice
To investigate the incubation period of 22L-infected BALB/c mice, the survival rate and clinical manifestation were measured. At 120 days post inoculation, five experimental mice exhibited clinical symptoms such as rough coat, hunched back, hyperresponsiveness, ataxia and weight loss, which lasted for 2 weeks. At 140 days post inoculation, experimental mice were depressed,cachectic, and moribund (supplementary Figure 1 online).
Control mice did not appear to have these symptoms.The average incubation period of 22L-infected mice was 140.8 ± 4.1 days (Figure 1).
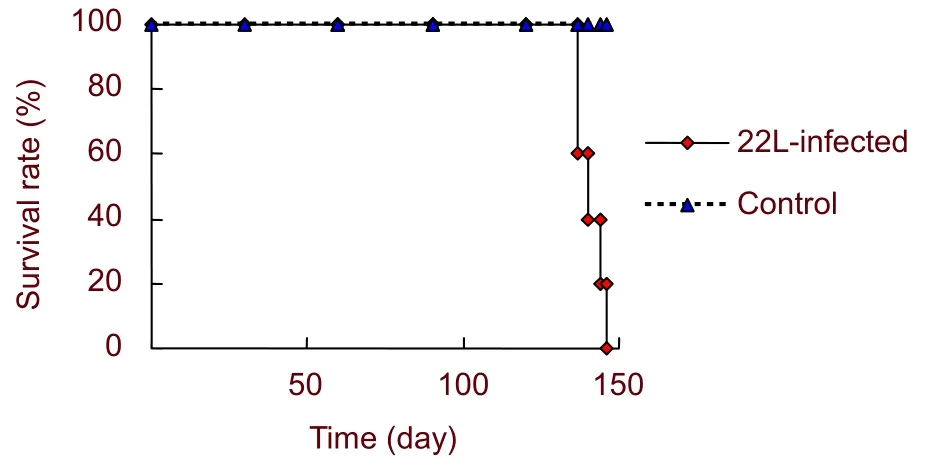
Figure 1 Survival curves of 22L-infected and control mice. The average incubation period was 140.8 ± 4.1 days.
Brain pathology of 22L-infected mice at the terminal stage
Hematoxylin-eosin staining was used to assess the changes in brain morphology of 22L-infected mice. The cortex and hippocampus of infected mice showed vacuolation to different extents (Figure 2). This indicated that 22L-infected mice appear to have typical pathological changes associated with prion disease, such as vacuolation of neurons at the terminal stage, and suggested establishment of a prion mouse model.
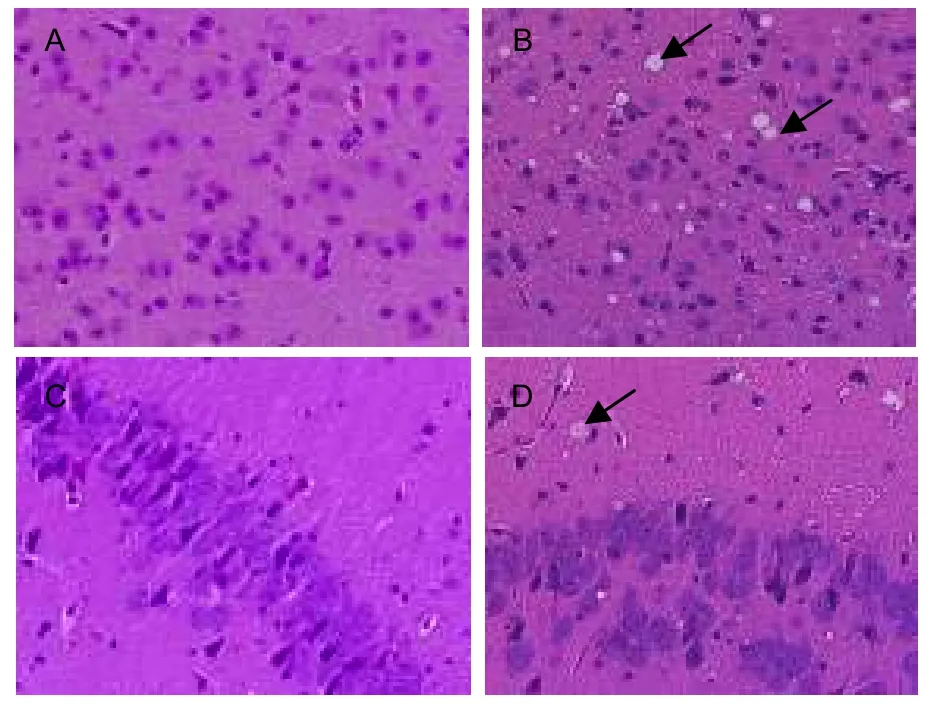
Figure 2 Pathological changes in the brain of 22L-infected mice at terminal stage disease (hematoxylineosin staining, × 400). Vacuoles were detected in the cortex and hippocampus of 22L-infected mice (arrows in B and D). No vacuoles were detected in control group (A and C).
Differentially expressed polypeptides in brain of 22L-infected mice analyzed by MALDI-TOF IMS
To explore differentially expressed polypeptides in prion disease, brain of 22L-infected mice at end-stage disease was analyzed by IMS. In preliminary experiments, we found that 7 mg/mL alpha-cyano-4-hydroxycinnamic acid(CHCA) in 0.1% TFA and 50% acetonitrile allows high quality mass spectra to be obtained from intact tissues.MALDI mass spectrum were obtained from direct analysis of brain sections of 22L-infected and control mice, in the mass-to-charge ratio (m/z) range from 1 000-20 000 Da (Figure 3).
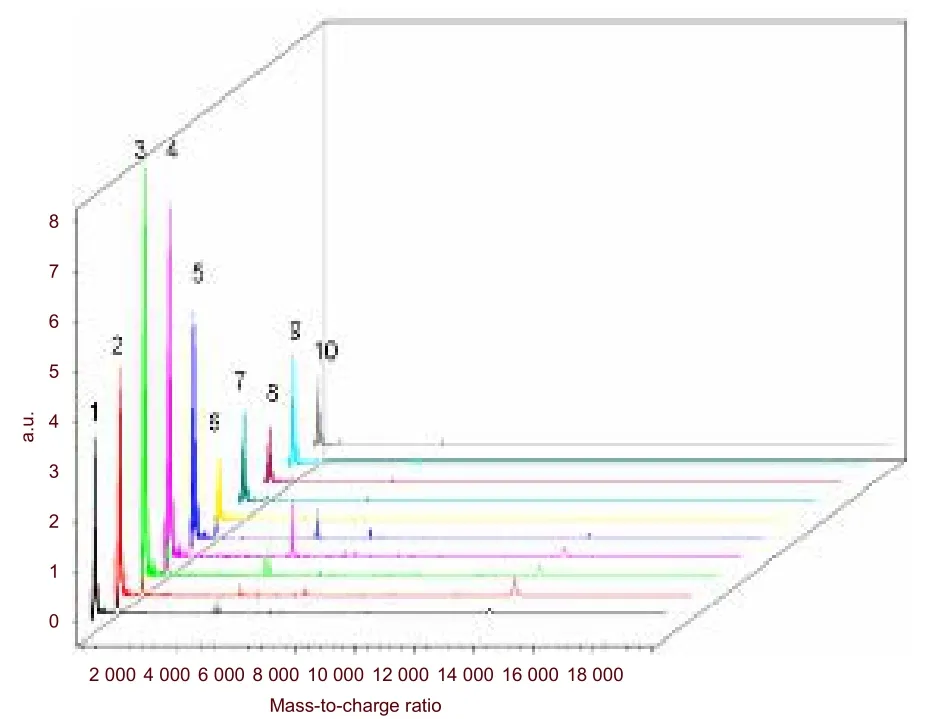
Figure 3 Mass spectrometry of 22L-infected and control mouse brain. 1-5: Mass spectrometry of control mice;6-10: mass spectrometry of 22L-infected mice.
Peptides that had the same m/z were downregulated in 22L-infected mice. By applying FlexAnalysis 3.0, Fleximaging 2.0 and ClinProtools 3.0, we were able to identify 21 peptides that were downregulated. The m/z of these peptides was: 758.772 5, 894.891 9,1 167.130 8, 1 507.429 4, 1 983.847 5, 2 029.220 6,3 345.042, 4 796.982 8, 4 933.102 2, 5 205.341 1,5 432.206 9, 6 702.655 1, 6 271.610 2, 7 065.640 3,7 542.058 4, 7 836.783 8, 8 562.954 2, 8 948.626,9 946.835 3, 12 147.433 and 14 121.165. Peptides 758.772 5 and 5 432.206 9 were downregulated in test mice relative to controls (P < 0.05). The spatial distribution of these two peptides in brain sections is shown in Figure 4. Both m/z 758.772 5 and m/z 5 432.206 9 were expressed in brain sections of control mice, but were expressed at a low level in 22L-infected mice.
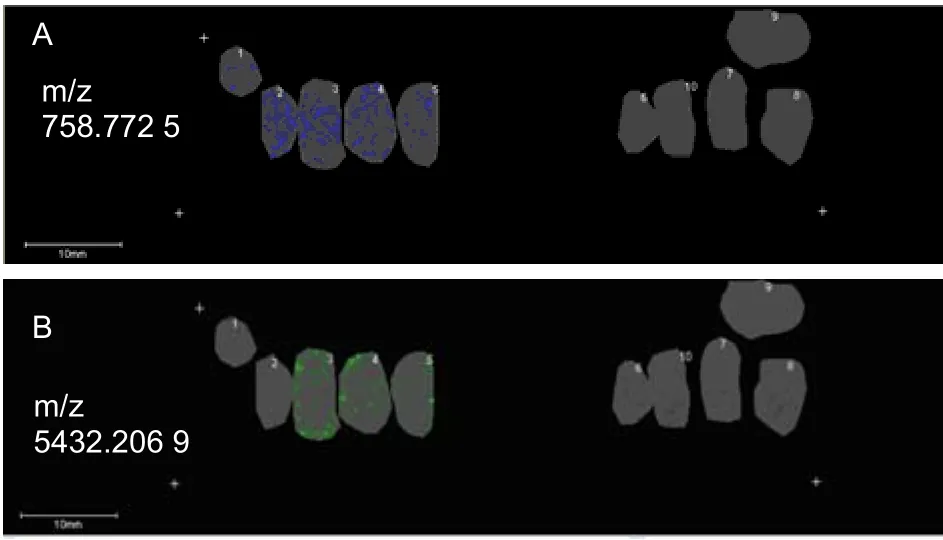
Figure 4 Distribution of polypeptides (m/z 758.772 5 and m/z 5 432.206 9) in the brain of 22L-infected and control mice. Section 1-5: control mice; 6-10: 22L-infected mice.Scale bars: 10 mm. m/z: Mass-to-charge ratio.
DISCUSSION
It has been reported that prion disease can propagate between species such as monkey, cavia, hamster and mouse. Diseased animals showed similar pathological changes when infected with prion disease[30]. As mice are small and easy to obtain and raise, they are good choices to establish models of prion disease. In addition,many prion strains have been isolated from different mouse hosts. Because they are easily transmitted,scrapie strains are considered as a good choice to establish models of prion disease[31]. In this study, we used scrapie strain 22L to establish a BALB/c mouse model of prion disease. At 120 days post inoculation, the 22L-infected mice presented clinical symptoms such as a rough coat, hunched back, ataxia, and weight loss. The average incubation period was 140.8 ± 4.1 days. Hematoxylin-eosin staining of brain sections revealed that the cortex and hippocampus showed differential vacuolation, indicative of typical prion pathology.
MALDI-IMS is a new technology that allows for simultaneous profiling of hundreds of peptides and proteins present in thin tissue sections. The key steps of IMS are preparation of tissue sections, coating them in matrix,followed by mass spectrometric analysis[32]. Matrix is crucial in IMS experiments. Depending on the properties of the samples, different matrices are selected. The commonly used matrices are CHCA, 2,5-dihydroxybenzoic acid (DHB), and sinapic acid (SA).
The selection of matrix depends on the analyte. SA is usually used for high molecular range compounds such as proteins, while CHCA is for low-molecular range species such as lipids and peptides. DHB is for small molecules in metabolites and pharmaceuticals[32-34]. In addition, the matrix solvent is also very important. Since the aim of this study was to observe expression changes in polypeptides in prion disease, CHCA was selected. In preliminary experiments, we found that 7 mg/mL CHCA in 0.1% TFA and 50% acetonitrile allowed high-quality mass spectra to be obtained from intact tissues.
Rohner et al[35]analyzed brain tissue sections of Alzheimer’s disease by IMS and observed a specific distribution of m/z 4545.1. Onoue et al[36]used IMS to analyze heart tissue from patients with Fabry’s disease,wherein polypeptide m/z 782.5 showed unique peaks and elevated levels relative to controls. This suggested m/z 782.5 might be a biomarker for Fabry’s disease.
Stoeckli et al[20]analyzed 1 mm lung cancer biopsies by IMS. More than 1 600 mass spectra peaks were acquired and 15 of them were correlated to lung cancer.
Brains of five 22L-infected mice at terminal disease, and five controls, were analyzed by MALDI-TOF/IMS. We first reported the downregulation of 21 polypeptides in infected mice. The peptides with m/z 758.772 5 and 5 432.206 9 were statistically significantly down-regulated in test mice relative to controls (P < 0.05). This indicated that these peptides might play an important role in prion disease and may serve as biomarkers. This study established 22L-infected BALB/c mice as a model of prion disease. At a range of 1 000- 20 000 Da, 21 polypeptides were down-regulated, with peptides m/z 758.772 5 and m/z 5 432.206 9 showing statistically significant differences. This suggests that they may play an important role in prion disease and are potential diagnostic biomarkers.
MATERIALS AND METHODS
Design
The study was a randomized, controlled experiment.
Time and setting
It was performed at the Institute of Military Veterinary Sciences, Academy of Military Medical Sciences, China,and the Department of Immunology, Norman Bethune College of Medical Science, Jilin University, China, from September 2009 to February 2010.
Materials
Ten female BALB/c mice, aged 4-6 weeks, weighing 18-22 g, of clean grade were purchased from the Experimental Animal Center of Jilin University, China. All mice were housed under controlled lighting (12-hour light/darkness), temperature (21-22°C), and humidity(60-65%) in standard, individually ventilated cages in barrier conditions in the same animal facility, and allowed access to food and water ad libitum. The experimental protocols were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals,formulated by the Ministry of Science and Technology of the People’s Republic of China[37].
Methods
Establishment of 22L-infected BALB/c mice models
After being anesthetized by diethyl ether, mice were inoculated intraperitoneally (2-3 cm depth below cranium) with a 10-2dilution (25 μL/mouse) of 22L scrapie brain homogenates (No. 5 Laboratory, Institute of Military Veterinary Sciences, Academy of Military Medical Sciences, China), or normal brain homogenate at the right brain (isometric site between right eye and right ear), with sterile syringes. Behavior was observed once every 3 days. After the mice presented with rough coat, hunched back, weight loss, ataxia,and paralysis for 1 week, they were diagnosed as having onset of prion disease. If the mice showed rapid weight loss, hyporesponsiveness, and articulo mortis, they were considered to be at the end point of disease pathogenesis.
Tissue section preparation
Five 22L-infected and five control mice were sacrificed at end stage disease. Brains were harvested and put into brain tooting (Shenzhen Rui Wode Life Sciences Ltd,Shenzhen, China). Brains were frozen in liquid nitrogen for 1-2 minutes, and stored at -80°C for 20 minutes.
Before sectioning, each brain was divided into five parts according to the scale of the brain tooting. Each part of the brain was cut into 10-μm-thick sections using a cryostat (Leica, Solms, Germany). Sections were thaw-mounted on conductive glass slides (Bruker Daltonics, Bremen, Germany) and marked. The sections were immersed in 70% ethanol (TEDIA, Carson, CA,USA) for 30 seconds, then 100% ethanol for 30 seconds. After drying in a desiccator for 30 minutes, tissue sections were scanned using a scanner (Hewlett-Packard Development Company, PaloAlto, CA,USA). Each brain was cut into 5-μm-thick sections.The sections were subjected to routine hematoxylin-eosin staining[38].
Matrix coating
CHCA (Bruker Daltonics, Bremen, Germany) matrix solutions were prepared fresh at a concentration of 7 mg/mL in 50% acetonitrile (TEDIA) and 0.1% TFA (TEDIA). A pneumatic matrix sprayer (Bruker Daltonics) was used to apply matrix coating. Sections were then fixed onto a stainless steel MALDI plate and analyzed by MALDI-TOF.
Mass spectrometry analysis
IMS was performed using a MALDI-TOF/TOF-type III instrument (Bruker Daltonics). The data were acquired in positive ion mode using an external calibration method. In this analysis, signals over the range of m/z 1-20 kDa were measured at an accelerating voltage of 25 kV, an ion source voltage of 20 kV, and a laser energy range of 30-50%. FlexImaging v.2.0 software(Bruker Daltonics) was used for image reconstruction.
Images were captured using FlexControl 3.0 (Bruker Daltonics). The scan area was defined in Fleximaging 2.0 (Bruker Daltonics) and divided into two-dimensional lattices. The distance between two points was 150 μm, with every point matched to a mass chromatogram.
Statistical analysis
Mass spectrometry data was analyzed by ClinProtools 3.0 software (Bruker Daltonics). The data were expressed as mean ± SD, followed by t-tests. Differences were considered statistically significant at P < 0.05.
Author contributions:Xiangyu Liao provided and integrated experimental data, and wrote the manuscript. Jiayu Wan,Wensen Liu and Chuanjing Ju were responsible for the funding and authorized the study. Xin Tang, Na Xu and Wufei Zhu analyzed experimental data. Jing Xu and Nan Li acquired the IMS data. Yaping Chang designed and authorized the study.
Conflicts of interest:None declared.
Funding:The study was supported by the National Natural Science Foundation of China, No. 30972197 and 31072148,and the Science and Technology Plan Program of Jilin Province,No. 201105038.
Ethical approval:The experiment was approved by the Animals Ethics Committee of the Academy of Military Medical Sciences, China and Jilin University, China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6, No. 23, 2011 after selecting the “NRR Current Issue” button on the page.
[1]Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95(23):13363-13383.
[2]Prusiner SB. Molecular biology of prion diseases. Science. 1991;252(5012):1515-1522.
[3]Pan KM, Baldwin M, Nguyen J, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90(23):10962-10966.
[4]Pergami P, Jaffe H, Safar J. Semipreparative chromatographic method to purify the normal cellular isoform of the prion protein in nondenatured form. Anal Biochem. 1996;236(1):63-73.
[5]Kretzschmar HA, Ironside JW, DeArmond SJ, et al. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53(9):913-920.
[6]Hwang D, Lee IY, Yoo H, et al. A systems approach to prion disease. Mol Syst Biol. 2009;5(252):1-10.
[7]Tang Y, Xiang W, Hawkins SA, et al. Transcriptional changes in the brains of cattle orally infected with the bovine spongiform encephalopathy agent precede detection of infectivity. J Virol.2009;83(18):9464-9473.
[8]Cosseddu GM, Andreoletti O, Maestrale C, et al. Gene expression profiling on sheep brain reveals differential transcripts in scrapie-affected/not-affected animals. Brain Res. 2007;1142:217-222.
[9]Xiang W, Hummel M, Mitteregger G, et al. Transcriptome analysis reveals altered cholesterol metabolism during the neurodegeneration in mouse scrapie model. J Neurochem. 2007;102(3):834-847.
[10]Qualtieri A, Urso E, Le Pera M, et al. Proteomic profiling of cerebrospinal fluid in Creutzfeldt-Jakob disease. Expert Rev Proteomics. 2010;7(6):907-917.
[11]Thomas SN, Cripps D, Yang AJ. Proteomic analysis of protein phosphorylation and ubiquitination in Alzheimer's disease.Methods Mol Biol. 2009;566:109-121.
[12]Giorgi A, Di Francesco L, Principe S, et al. Proteomic profiling of PrP27-30-enriched preparations extracted from the brain of hamsters with experimental scrapie. Proteomics. 2009;9(15):3802-3814.
[13]Alberio T, Bossi AM, Milli A, et al. Proteomic analysis of dopamine and alpha-synuclein interplay in a cellular model of Parkinson's disease pathogenesis. Febs J. 2010;277(23):4909-4919.
[14]Maarouf CL, Andacht TM, Kokjohn TA, et al. Proteomic analysis of Alzheimer's disease cerebrospinal fluid from neuropathologically diagnosed subjects. Curr Alzheimer Res. 2009;6(4):399-406.
[15]Cepek L, Brechlin P, Steinacker P, et al. Proteomic analysis of the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease.Dement Geriatr Cogn Disord. 2007;23(1):22-28.
[16]Curran S, McKay JA, McLeod HL, et al. Laser capture microscopy.Mol Pathol. 2000;53(2):64-68.
[17]Walch A, Rauser S, Deininger SO, et al. MALDI imaging mass spectrometry for direct tissue analysis: a new frontier for molecular histology. Histochem Cell Biol. 2008;130(3):421-434.
[18]Chaurand P, Schwartz SA, Reyzer ML, et al. Imaging mass spectrometry: principles and potentials. Toxicol Pathol. 2005;33(1):92-101.
[19]Chaurand P, Stoeckli M, Caprioli RM. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal Chem. 1999;71(23):5263-5270.
[20]Stoeckli M, Chaurand P, Hallahan DE, et al. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001;7(4):493-496.
[21]Todd PJ, Schaaff TG, Chaurand P, et al. Organic ion imaging of biological tissue with secondary ion mass spectrometry and matrix-assisted laser desorption/ionization. J Mass Spectrom.2001;36(4):355-369.
[22]Chaurand P, Fouchecourt S, DaGue BB, et al. Profiling and imaging proteins in the mouse epididymis by imaging mass spectrometry. Proteomics. 2003;3(11):2221-2239.
[23]Pierson J, Norris JL, Aerni HR, et al. Molecular profiling of experimental Parkinson's disease: direct analysis of peptides and proteins on brain tissue sections by MALDI mass spectrometry. J Proteome Res. 2004;3(2):289-295.
[24]Stoeckli M, Staab D, Staufenbiel M, et al. Molecular imaging of amyloid beta peptides in mouse brain sections using mass spectrometry. Anal Biochem. 2002;311(1):33-39.
[25]Palmer-Toy DE, Sarracino DA, Sgroi D, et al. Direct acquisition of matrix-assisted laser Desorption/Ionization time-of-flight mass spectra from laser capture microdissected tissues. Clin Chem.2000;46(9):1513-1516.
[26]Xu BJ, Caprioli RM, Sanders ME, et al. Direct analysis of laser capture microdissected cells by MALDI mass spectrometry. J Am Soc Mass Spectrom. 2002;13(11):1292-1297.
[27]Masumori N, Thomas TZ, Chaurand P, et al. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61(5):2239-2249.
[28]Schwamborn K, Krieg RC, Reska M, et al. Identifying prostate carcinoma by MALDI-Imaging. Int J Mol Med. 2007;20(2):155-159.
[29]Bhattacharya SH, Gal AA, Murray KK. Laser capture microdissection MALDI for direct analysis of archival tissue. J Proteome Res. 2003;2(1):95-98.
[30]Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8(7):552-561.
[31]Bruce ME. Scrapie strain variation and mutation. Br Med Bull.1993;49(4):822-838.
[32]Schwartz SA, Reyzer ML, Caprioli RM. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom. 2003;38(7):699-708.
[33]Lemaire R, Desmons A, Tabet JC, et al. Direct analysis and MALDI imaging of formalin-fixed, paraffin-embedded tissue sections. J Proteome Res. 2007;6(4):1295-1305.
[34]Baluya DL, Garrett TJ, Yost RA. Automated MALDI matrix deposition method with inkjet printing for imaging mass spectrometry. Anal Chem. 2007;79(17):6862-6867.
[35]Rohner TC, Staab D, Stoeckli M. MALDI mass spectrometric imaging of biological tissue sections. Mech Ageing Dev. 2005;126(1):177-185.
[36]Onoue K, Zaima N, Sugiura Y, et al. Using imaging mass spectrometry to accurately diagnose Fabry’s disease. Circ J.2003;75(1):221-223.
[37]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[38]Tsuda H, Seki K, Hasebe T, et al. A histopathological study for evaluation of therapeutic effects of radiofrequency ablation in patients with breast cancer. Breast Cancer. 2011;18(1):24-32.
 中國(guó)神經(jīng)再生研究(英文版)2011年23期
中國(guó)神經(jīng)再生研究(英文版)2011年23期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- NIH funding for disease categories related to neurodegenerative diseases
- A case of thalamic hemorrhage-induced diaschisis☆
- Occlusion of the middle cerebral artery Guidance by screen imaging using an EDA-H portable medium-soft electronic endoscope☆
- Propofol regulates Ca2+, Mg2+, Cu2+ and Zn2+ balance in the spinal cord after ischemia/reperfusion injury***★
- Optimal velocity encoding during measurement of cerebral blood flow volume using phase-contrast magnetic resonance angiography*☆
- Evidence-based treatment for acute spinal cord injury☆
