Quality control measures for lowering the seroconversion rate of hemodialysis patients with hepatitis B or C virus
Hangzhou, China
Quality control measures for lowering the seroconversion rate of hemodialysis patients with hepatitis B or C virus
Jing Yuan, Yi Yang, Fei Han, Ping Zhang, Xiao-Ying Du, Hua Jiang and Jiang-Hua Chen
Hangzhou, China
BACKGROUND: Hemodialysis (HD) patients are at high risk of infection by hepatitis B virus (HBV) or hepatitis C virus (HCV). The present study was designed to determine the impact of quality control measures on the prevention of transmission of blood-borne viruses.
METHODS: A total of 6182 adult maintenance HD patients from all HD units in Zhejiang Province were recruited on January 1, 2007. The baseline demographic and clinical characteristics were recorded and all patients were followed up until death or survival at 4 years later. The Quality Control Standards of Hemodialysis were gradually implemented in HD units. The HBV or HCV seroconversion rates of the recruited patients were calculated and compared every year during the observation period.
RESULTS:The prevalence of HBV was 8.3% at the beginning of the study, and 6.6% for HCV. With the implementation of the HD quality control measures, the HBV seroconversion rate tended to decrease year by year (χ2=6.620,P=0.085), and the HCV seroconversion rate decreased significantly (χ2=10.41,P=0.015). Compared with the data in 2007, the HBV seroconversion rate (χ2=4.204,P=0.040, relative risk ratio 0.393, 95% CI 0.156-0.991) and the HCV seroconversion rate (χ2=7.373,P=0.007, relative risk ratio 0.386, 95% CI 0.189-0.787) decreased significantly in 2010.
CONCLUSION: Quality control measures for HD decreased the seroconversion rates of HBV or HCV in HD patients, showing that updated quality control measures reduce the risk for transmission of blood-borne viruses in the HD population.
(Hepatobiliary Pancreat Dis Int 2012;11:302-306)
hemodialysis; quality control measures; infection; hepatitis B virus; hepatitis C virus
Introduction
Hemodialysis (HD) is one of the main renal replacement therapies for patients with end-stage renal disease (ESRD). At present, nearly 10 000 uremic patients are receiving HD in Zhejiang Province, China. HD patients are at high-risk of nosocomial infections, including hepatitis B virus (HBV) and hepatitis C virus (HCV). HD patients are vulnerable to these infections because of the opportunity for exposure to hepatitis virus associated with the dialysis procedure.[1-4]After infection with HBV, HD patients are at greater risk of becoming chronic carriers compared with the general population.[5,6]Furthermore, seroprotection rates after standard vaccination of current recombinant HBV vaccine are poor in dialysis patients (32%-80%), in contrast to the general population (>95%).[7-9]In contrast to HBV, no vaccine is available for HCV.[10]Moreover, patients infected with HCV often have minimal clinical evidence of the disease, and accurate testing is not feasible.[10,11]HBV/HCV infection is related to higher morbidity and mortality in HD patients even after they have received a kidney transplant.[12,13]
Quality control measures for HD have been developed to reduce the risk of transmission of blood-borne viruses in the ESRD population. These measures include protocols for handling body fluids, isolation policies, use of erythropoietin to minimize blood transfusion, and vaccination against HBV. Such measures have undoubtedly contributed to the subsequent dramatic decrease of HBV or HCV incidence or prevalence in HD patients as well as the HD staff.[14-17]However, HBV or HCV persists within HD units and nosocomial virustransmission remains a concern.[17,18]Thus quality control measures should be updated and executed to control HBV or HCV transmission to dialysis patients. Unfortunately, data about quality control measures for HD have been rare in China.
The Quality Control Standards of Hemodialysis in Zhejiang Province were gradually implemented at 150 HD units after the establishment of the provincial Hemodialysis Quality Control Center in 2007. In the present study, we retrospectively calculated the yearly HBV and HCV seroconversion rates of HD patients in Zhejiang Province to quantify the impact of the quality control standards on the prevention of transmission of blood-borne viruses.
Methods
Patients
A total of 6182 adult maintenance HD patients from all HD units in Zhejiang Province were recruited in the study on January 1, 2007. The patients were selected via the provincial hemodialysis registration system. Their baseline demographic and clinical characteristics were recorded and all the patients were followed up until death or survival at 4 years later. This study was approved by the Institutional Ethics Committee of Zhejiang University, and informed consent was obtained from guardians and/or patients.
Quality control measures for HD
The Quality Control Standards of Hemodialysis of Zhejiang Province include the following aspects:
Infection control: Dialysis units should be divided into clean, semi-contaminated, and contaminated areas according to the passage of patients and the HD staff. Dialysis units should set side a special area for patients with infectious diseases (HBV or HCV) and provide isolation care. Reuse of a dialyzer is not permitted for HBV or HCV patients. The staff of HD units should carry out standard prophylaxis including disinfection and isolation, and use the single-use HD care package. Patient management and hygiene of the staff's hands are emphasized. The policies of single-use of bedclothes and a one-way flow of supplies should be followed. The staff of HD units must be subjected to test of liver function and hepatitis screening every year, and individuals with negative HBV antibody should receive HBV vaccination. New patients should be subjected to test of liver function and hepatitis screening before the first dialysis, and be re-tested at a 6-month interval. The results of hepatitis screening are recorded. Disinfection of dialysis machines including the surfaces of facilities should be performed between shifts, and disinfection of the floor and ultraviolet disinfection should be performed daily.
Staff training: The staff of HD units should know the quality control standards including the rules of disinfection, isolation and control of nosocomial infections, and the operating procedures. They are certificated persons.
Data reporting: An HD Quality Control Database in each HD unit is connected to the provincial Quality Control Center. HD units should report regularly to the Quality Control Center on the execution of the standards in terms of the positive rate of HBV or HCV infection and new cases.
Regular inspection: The Quality Control Center inspects regularly the implementation of the standards including disinfection and isolation, and gives instructions to HD units within the province.
Status of HBV and HCV
A case of HBV was defined as a patient who was diagnosed with HBV or who was positive for HBsAg at the time of entering the study. A case of HCV was defined as a patient who was diagnosed with HCV or who was HCV antibody-positive at the time of entry. After the patient entered the study, HBV and HCV statuses were investigated every 6 months. A new case of HBV infection was defined as a patient with seroconversion from HBsAg negative at the time of entry to HBsAg positive during the study period. Similarly, a new case of HCV infection was defined as a patient with seroconversion from HCV antibody-negative at initial testing to HCV antibodypositive during the study period.
Variables and statistical methods
The main variables of interest were HBV and HCV prevalence and seroconversion rates. The prevalence represented a cross-section taken at the beginning of the study. HBV and HCV seroconversion rates were calculated as the number of conversions in 100 patients per year. Yearly HBV and HCV seroconversion rates were defined as annual new onset seroconversions and calculated at the end of each year.
The isolation status in HD units was divided into three conditions: absolute isolation, in which patients with infectious diseases received HD on isolated machines and in an isolated area; semi-isolation, in which patients with infectious diseases received HD on isolated machines but not in an isolated area; and non-isolation, in which patients with infectious diseases received HD neither on isolated machines nor in an isolated area.
The differences between groups were compared using the Chi-square test. The analyses were performed with SPSS 11.5 software (SPSS Inc., USA).P<0.05 was considered statistically significant.
Results
The baseline demographic and clinical characteristics of the patients are summarized in Table 1. Their mean age was 54.6±15.4 years, and 3692 (59.72%) were male. Their mean dialysis duration was 29.1±32.5 months at the time of recruitment. In all the patients, 4007 patients were followed up. During the observation period, 769 patients received renal transplantation, 61 switched to peritoneal dialysis, 844 were lost to follow-up, and 501 died. The prevalence of HBV and HCV was 8.3% and 6.6% respectively at the beginning of the study.
Quality control measures for HD
The quality control measures for HD were improved in the HD units. During the observation period, 2167 HD staff members, including physicians, nurses, engineers and administrators were trained and certified. Regular and randomized qualification inspections were organized by the Quality Control Center to supervise the implementation of the measures. According to theQuality Control Standards, the satisfactory rate of disinfection and isolation in the HD units rose from 50% to 100%. During the observation period, the disinfection rate of dialysis machines and the surfaces of facilities between shifts rose from 20% to 100%, including the disinfection rate of the floor, and daily ultraviolet disinfection rose from almost 0% to 68%. The percentage of absolutely isolated units increased from 21% at the end of 2007 to 64% at the end of 2010, while the percentage of non-isolated units decreased from 25% to zero (Table 2).
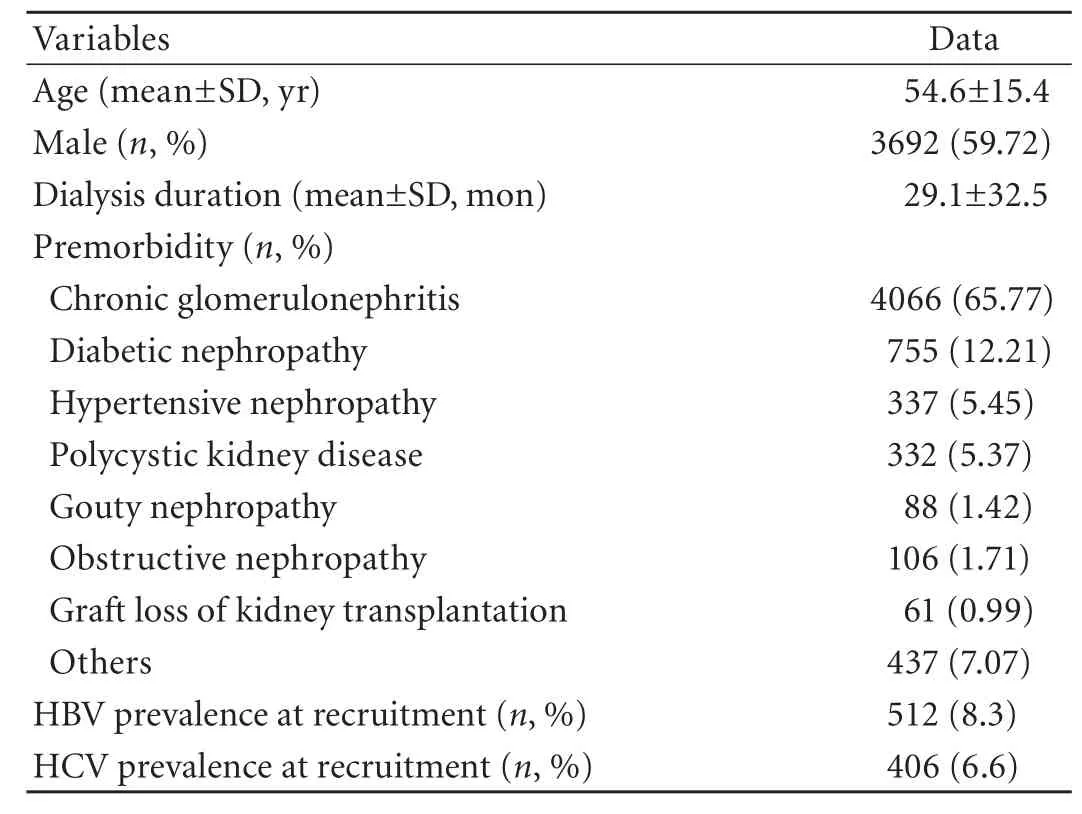
Table 1. Baseline demographic and clinical characteristics of HD patients
HBV and HCV seroconversion rates
The HBV seroconversion rate was 0.39 per 100 patients in 2007, 0.23 per 100 patients in 2008, 0.17 per 100 patients in 2009, and 0.16 per 100 patients in 2010. This rate tended to decrease year by year with the implementation of HD quality control measures (χ2=6.620,P=0.085) (Table 3). The HCV seroconversion rate was 0.66 per 100 patients in 2007, 0.43 per 100 patients in 2008, 0.31 per 100 patients in 2009, and 0.26 per 100 patients in 2010. This rate decreased significantly year by year with the implementation of the HD quality control measures (χ2=10.41,P=0.015) (Table 3). Compared with the data in 2007, the HBV seroconversion rate (χ2=4.204,P=0.040, relative risk ratio 0.393, 95% CI 0.156-0.991) and the HCV seroconversion rate (χ2=7.373,P=0.007, relative risk ratio 0.386, 95% CI 0.189-0.787) significantly decreased in 2010.
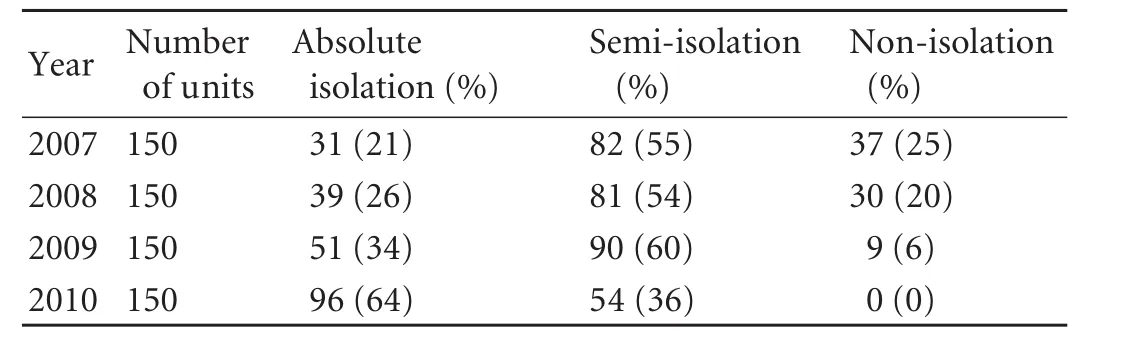
Table 2. Changes of isolation status in HD units in Zhejiang Province
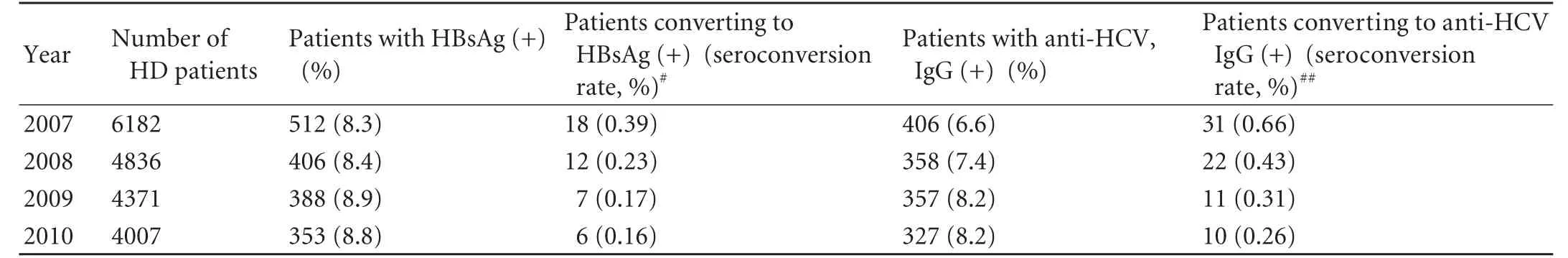
Table 3. Seroconversion rates of HBV and HCV*
Discussion
HD is one of the main renal replacement therapies for ESRD patients. At present, there are about 2 000 000 ESRD patients surviving on HD worldwide and the number is increasing dramatically. Recently, the prevaleace of ESRD has reached 100 to 200 per million population in Zhejiang Province, China. From the provincial HD database, the HD population reaches 11 060 in the province in 2010 and more than 2000 new cases are reported each year. Nowadays there are over 170 HD centers equipped with more than 2800 HD machines. Hence an updated HD quality control measures are needed for the HD patients and HD units.
HD patients are at high risk of nosocomial infections, especially HBV and HCV infections. Before 2007, the incidence of these infections differed greatly between HD centers in the province. To control the quality of HD, we established the Hemodialysis Quality Control Center and set up the Quality Control Standards of Hemodialysis, which includes strict disinfection rules, regular physical examinations for patients and medical staff, a training system for HD staff and regular inspections.
The present study was based on the data from the HD quality control database. From 2007 to 2010, the prophylactic status of HBV or HCV was improved and the new onset infection rate decreased significantly. The positive rate of anti-HCV IgG in the HD population was 6.6% at the beginning of the present study, similar to that reported in the USA (5.5%-9.8%).[15-17]With the implementation of the HD quality control measures, the seroconversion rate of anti-HCV IgG decreased to 0.26 per 100 patients in 2010, which was better than those reported in China[16,17]and in developed countries (1.1-3.6 per 100 patients).[19]We found that HD quality control measures reduced the risk of transmission of bloodborne viruses in the patients. This finding is consistent with that of a number of preliminary studies.[14-17]Indeed, the impact of infection control measures on HCV transmission in HD units is positive[20-22]although some current guidelines do not recommend isolation with dedicated machines for HCV-infected patients.
The positive rate of HBsAg in the HD population in Zhejiang is 8.3%-8.8%, similar to that in the general population (9.1%).[23]The incidence of HBV infection in the present study was lower than those reported in other parts of China (11.9%).[24]However, it is higher than that in the HD population in Western countries (3.3%).[6]We found HD quality control measures lowered the HBV seroconversion rate from 0.39 per 100 patients in 2007 to 0.16 per 100 in 2010. Interestingly, statistical analysis indicated that the impact of HD quality control measures is more marked on the control of HCV infection than HBV infection. HBV vaccination may at least partly have contributed to this result. Since HBV vaccination was recommended in 2001,[2]many individuals have been vaccinated, including the general and HD populations. Therefore, the HD population may have a higher HBsAb level gaining natural resistance to HBV infection, while there is no protective antibody against HCV.
In conclusion, the updated HD quality control measures decrease the seroconversion rate of HBV/HCV as well as reduce the risk of transmission of blood-borne viruses in HD patients.
Contributors: YJ and CJH proposed the study. JH and DXY collected and analyzed the data. YJ wrote the main body of the article. All authors contributed to further drafts. JH is the guarantor.
Funding: This study was supported by grants from Zhejiang Health Bureau (2008B073) and Zhejiang Education Bureau (Y200909640).
Ethical approval: This study was approved by the Institutional Ethics Committee of Zhejiang University.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lok AS. Chronic hepatitis B. N Engl J Med 2002;346:1682-1683.
2 No authors listed. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001;50:1-43.
3 Almroth G, Ekermo B, M?nsson AS, Svensson G, Widell A. Detection and prevention of hepatitis C in dialysis patients and renal transplant recipients. A long-term follow up (1989-January 1997). J Intern Med 2002;251:119-128.
4 Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology 2002;36: 3-10.
5 Charest AF, McDougall J, Goldstein MB. A randomized comparison of intradermal and intramuscular vaccination against hepatitis B virus in incident chronic hemodialysis patients. Am J Kidney Dis 2000;36:976-982.
6 Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, et al. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int 2003;63:2222-2229.
7 Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis 2002;35:1368-1375.
8 Schroth RJ, Hitchon CA, Uhanova J, Noreddin A, Taback SP, Moffatt ME, et al. Hepatitis B vaccination for patients with chronic renal failure. Cochrane Database Syst Rev 2004;3: CD003775.
9 Lim WH, Kireta S, Russ GR, Coates PT. Uremia impairs blood dendritic cell function in hemodialysis patients. Kidney Int 2007;71:1122-1131.
10 Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41-52.
11 Bassit L, Van Heuverswyn H, De Bosschere K, Nishiya AS, Carrilho FJ, Moraes CR, et al. Comparative study of twoanti-HCV screening tests in a large genotyped population of Brazilian dialysis patients. Eur J Clin Microbiol Infect Dis 2002;21:404-406.
12 Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol 2000;11:1896-1902.
13 Pol S, Samuel D, Cadranel J, Legendre C, Bismuth H, Bréchot C, et al. Hepatitis and solid organ transplantation. Transplant Proc 2000;32:454-457.
14 KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008;109:S1-99.
15 Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis 2010;56:371-378.
16 Crowley ST. What is the risk of hepatitis C spread within dialysis units and how can we curtail it? Semin Dial 2011;24: 436-438.
17 Li BC, Lu J, Xu J, Ji YF, Cui RL, Su H, et al. HCV Infection in Hemodialysis Patients: Investigation and Prevention. Chin J Nosocomiol 2003;13:329-331.
18 Lanini S, Puro V, Lauria FN, Fusco FM, Nisii C, Ippolito G. Patient to patient transmission of hepatitis B virus: a systematic review of reports on outbreaks between 1992 and 2007. BMC Med 2009;7:15.
19 Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int 2004;65:2335-2342.
20 Elamin S, Abu-Aisha H. Prevention of hepatitis B virus and hepatitis C virus transmission in hemodialysis centers: review of current international recommendations. Arab J Nephrol Transplant 2011;4:35-47.
21 Rahnavardi M, Hosseini Moghaddam SM, Alavian SM. Hepatitis C in hemodialysis patients: current global magnitude, natural history, diagnostic difficulties, and preventive measures. Am J Nephrol 2008;28:628-640.
22 Hussein MM, Mooij JM. Methods used to reduce the prevalence of hepatitis C in a dialysis unit. Saudi J Kidney Dis Transpl 2010;21:909-913.
23 Liang XF, Chen YS, Wang XJ, He X, Chen LJ, Wang J, et al. A study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old. Zhonghua Liu Xing Bing Xue Za Zhi 2005;26:655-658.
24 Wang C, Sun J, Zhu B, Larsen S, Yu R, Wu J, et al. Hepatitis B virus infection and related factors in hemodialysis patients in China-systematic review and meta-analysis. Ren Fail 2010;32:1255-1264.
October 7, 2011
Accepted after revision January 14, 2012
Author Affiliations: The Kidney Disease Center, First Affiliated Hospital, Zhejiang University School of Medicine (Yuan J, Yang Y, Han F, Zhang P, Du XY, Jiang H and Chen JH); and Hemodialysis Quality Control Center of Zhejiang Province (Yuan J, Zhang P, Du XY and Chen JH), Hangzhou 310003, China
Hua Jiang, MD, The Kidney Disease Center, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236189, Fax: 86-571-87236188; Email: zyjianghua@163.com)
? 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60164-7
 Hepatobiliary & Pancreatic Diseases International2012年3期
Hepatobiliary & Pancreatic Diseases International2012年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Management of splenic artery aneurysm associated with extrahepatic portal vein obstruction
- Pancreas-preserving segmental duodenectomy for gastrointestinal stromal tumor of the duodenum and splenectomy for splenic angiosarcoma
- Induction, modulation and potential targets of miR-210 in pancreatic cancer cells
- Quantitative analysis of intestinal gas in patients with acute pancreatitis
- Can the biliary enhancement of Gd-EOB-DTPA predict the degree of liver function?
- Effect of recombinant human growth hormone and interferon gamma on hepatic collagen synthesis and proliferation of hepatic stellate cells in cirrhotic rats
