Quantitative analysis of intestinal gas in patients with acute pancreatitis
Wuhan, China
Quantitative analysis of intestinal gas in patients with acute pancreatitis
Ying Liu and He-Sheng Luo
Wuhan, China
BACKGROUND: Disturbance of gastrointestinal function is a common complication in the early phase of acute pancreatitis (AP). Intestinal gas may reflect the function of the gut. Using plain abdominal radiographs, we investigated whether intestinal gas volume is related to AP.
METHODS: Plain abdominal radiographs of 68 patients with AP within 24 hours after admission and 21 normal controls were digitized and transmitted to a computer. The region of intestinal gas was identified by an image manipulation software and the gas volume score (GVS) was calculated. The relationships between the GVS values and various clinical factors of AP were analyzed.
RESULTS: The GVS in the AP group was 0.084±0.016, in the mild AP (MAP) group 0.070±0.005, and in the severe AP (SAP) group 0.094±0.013; all values were higher than that in the control group (P<0.01). The GVS in the SAP group was higher than that in the MAP group. The GVSs were correlated to the Ranson's scores (r=0.762,P<0.01) and the acute physiology and chronic health evaluation II (APACHE II) scores (r=0.801,P<0.01). In addition, the GVS in patients with secondary pancreatic and/or peripancreatic infection was 0.107±0.014, higher than that in patients without secondary infection (P<0.01). GVS was not related to gender, age, etiology or clinical outcome of AP.
CONCLUSIONS: Intestinal gas volume is significantly elevated in patients with AP. It is closely related to Ranson's and APACHE II score and secondary pancreatic and/or peripancreatic infection. GVS may be a new prognostic tool for assessing the severity of AP in the early course of the disease.
(Hepatobiliary Pancreat Dis Int 2012;11:314-318)
acute pancreatitis; gas volume score; intestinal gas; gut motility; bacterial overgrowth
Introduction
Disturbances of gastrointestinal function such as intestinal ileus, gut barrier dysfunction, bacterial overgrowth and bacterial translocation are common complications in the early phase of acute pancreatitis (AP)[1-3]and play critical roles in initiating the development of pancreatitis-associated sepsis and multiple organ dysfunction syndrome.[2-5]
Most intestinal gas is produced by the fermentation of unabsorbed carbohydrates by anaerobic organisms in the colon,[6-8]and the gases of bacterial origin include hydrogen, methane, hydrogen sulfide and mainly carbon dioxide.[7-9]Intestinal gas is easily visualized on plain abdominal radiographs. The quantity of intestinal gas, determined by the pixel value on images and standardized by physique, is defined as gas volume score (GVS).[10]The GVS is an objective estimate of intestinal gas volume and computed tomography is more accurate for volume estimation.[11]
The volume of intestinal gas depends on the rate of gas production and excretion. Conceivably, many pathological factors, such as disturbed gut motility and intestinal bacterial overgrowth, can increase the volume of gas retention. This suggests that intestinal gas itself may reflect bowel function to some extent and is potentially related to AP in the early course of this disease.
To clarify the association between intestinal gas volume and AP in the present study, we analyzed GVSs using plain abdominal radiographs and assessed the relationships between these findings and various clinical factors of AP.
Methods
Patients
Medical charts and computerized records for all patients with AP treated at Renmin Hospital of Wuhan University located in central China from January 2004 to January 2010 were retrospectively reviewed. The diagnosis and classification of AP were made accordingto the guide for diagnosis and treatment of AP (draft, 2004) formulated by the Pancreatology Unit, Division of Gastroenterology, Chinese Medical Association.
Eligible patients were at least 18 years of age, had undergone a supine abdominal radiographic examination within 24 hours after admission, and had received a diagnosis and classification of AP. Exclusion criteria were a history of inflammatory bowel disease, constipation, chronic diarrhea, diabetes, thyroid disease, previous abdominal surgery, and renal or hepatic disease. Patients were excluded if they had taken antibiotics, tegaserod, antispasmodics, or antidiarrheal agents in the previous 28 days. Patients who had a history of intestinal infection, colonoscopic examination, and large bowel enemas in the previous 28 days were also excluded.
Among the 68 patients finally enrolled in the study, 44 were male and 24 female. Their median age was 53 years (range 18-75). Twenty-seven of these patients were classified as having mild AP (MAP), and 41 as having severe AP (SAP). For the control group, 21 healthy subjects recruited from the medical and nursing staff of the center were enrolled.
Data extraction
The following data were extracted: demographic data, etiological factors, secondary pancreatic and/or peripancreatic infection, and clinical outcome. The Ranson's score and the acute physiology and chronic health evaluation II (APACHE II) score were calculated using data from the first 48 hours after admission.
Radiography
Taking into account that some patients were not orthostatic and the measurements should be unified, supine abdominal radiographs were chosen. Images that were stored as soft copies in a picture archival and communication system were converted to JPG format. The number of pixels in each area was determined by an image software (Adobe Photoshop version 7.0, Adobe Systems, Inc., San Jose, CA, USA). GVS was calculated as the number of pixels of gas-filled area divided by the number of pixels in a rectangle drawn by a horizontal line tangential to the superior margin of the pubic symphysis, a horizontal line tangential to the inferior margin of the tenth dorsal vertebra and vertical lines tangential to the left and right anterio-superior iliac crests.[10,11]
Statistical analysis
Statistical analysis was performed with SPSS version 13.0 (SPSS Inc., Chicago, IL., USA). Data are expressed as mean±SEM. Differences between or among the groups were compared by Student'sttest or one-way ANOVA. Correlations were assessed by Pearson's productmoment correlation coefficient test. APvalue <0.05 was considered statistically significant.
Results
GVS in patients with AP
The GVS in the AP group was 0.084±0.016, which was higher than that in the control group (0.057±0.006) (P<0.01). The GVSs in the MAP (0.070±0.005) and SAP (0.094±0.013) groups were both higher than that in the control group (P<0.01). The GVS in the SAP group was higher than that in the MAP group (P<0.01) (Fig. 1).
Relationship between demographics and GVS
While the GVS in male patients (0.083±0.013;n=44) was lower than that in female patients (0.086±0.021;n=24), the difference was not significant (P=0.387).
The GVS of AP patients who were under 40 years of age was 0.084±0.021 (n=11), between 40 and 60 years was 0.083±0.013 (n=36), and over 60 years was 0.086± 0.019 (n=21). Hence the GVS was not correlated with age in AP patients (P=0.890).
Relationship between etiology and GVS
The GVSs in patients with AP caused by biliary (0.085±0.015;n=44), alcoholic (0.082±0.018;n=17), and other etiological factors (0.087±0.020;n=7) were not significantly different (P=0.771).
Correlations between Ranson's score, APACHE II score and GVS
The GVSs were correlated with the Ranson's scores (r=0.762,P<0.01) and the APACHE II scores (r=0.801,P<0.01) (Fig. 2). The coefficients of determination were 0.581 and 0.641, respectively.
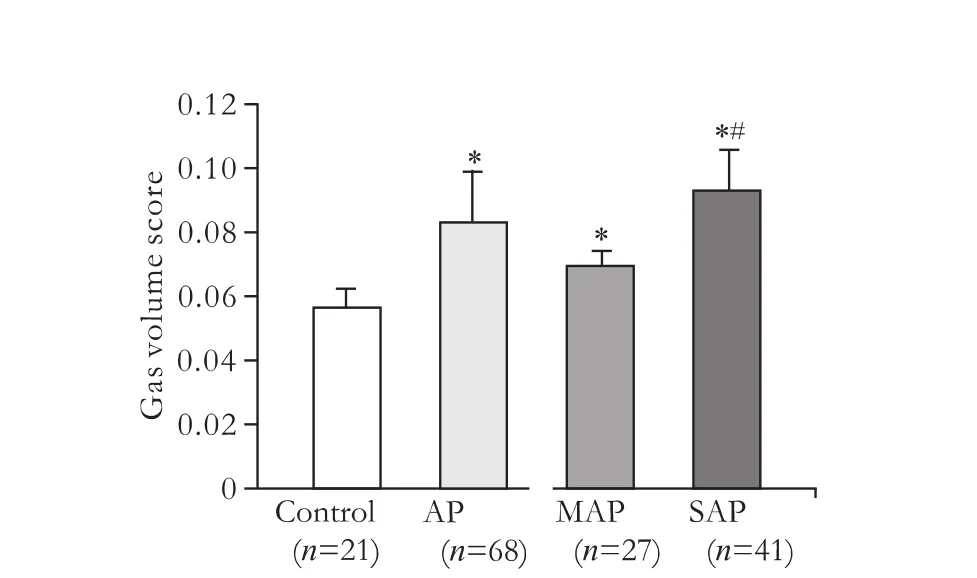
Fig. 1. GVSs in patients with AP. *:P<0.01, compared to the control group, #:P<0.01, compared to the MAP group.
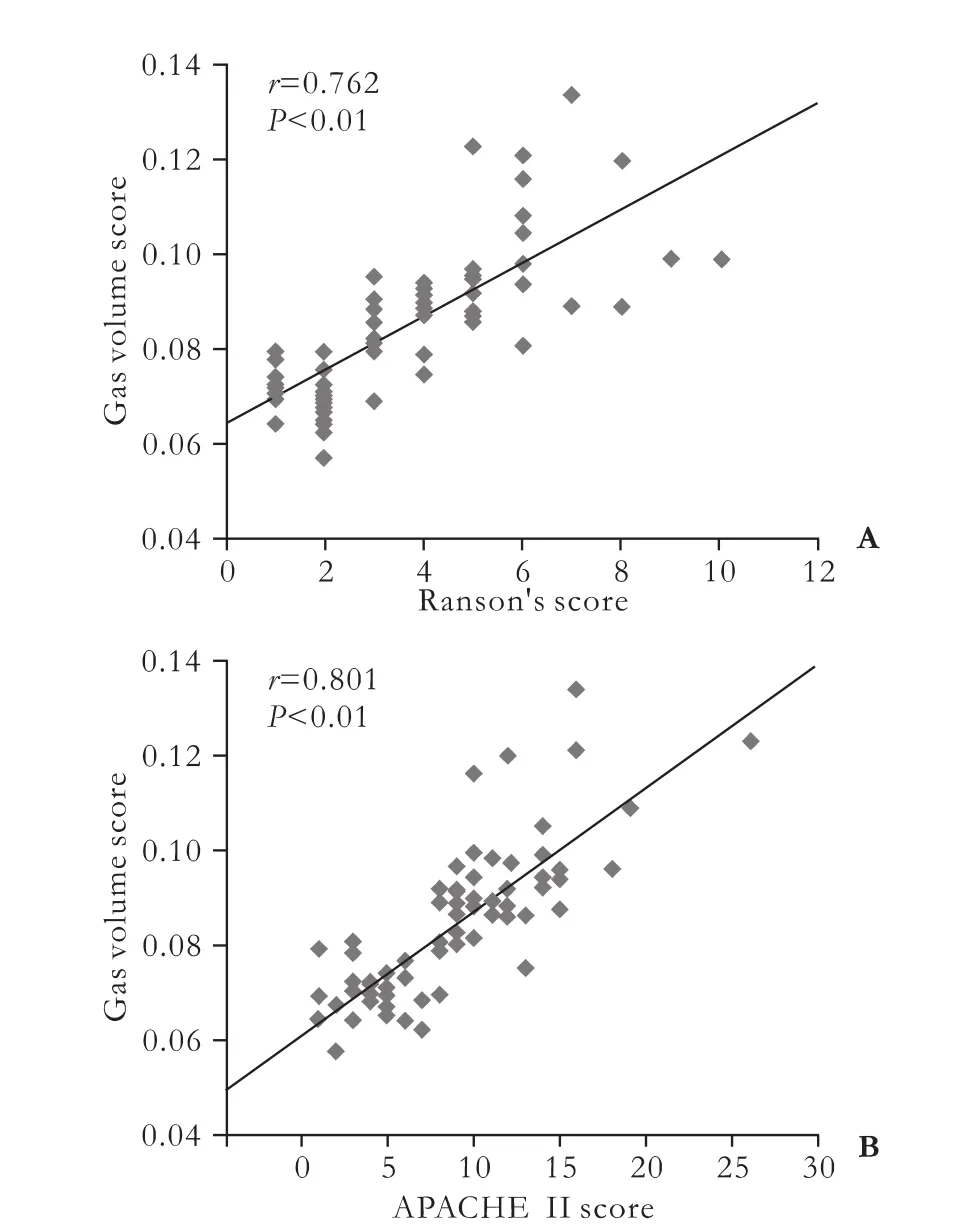
Fig. 2. Correlations of GVSs with Ranson's (A) and APACHE II (B) scores.
Relationship between secondary pancreatic and/or peripancreatic infection during hospitalization and GVS
Thirty patients developed secondary pancreatic and/ or peripancreatic infection during hospitalization. The GVS in these patients (0.107±0.014;n=30) was higher than that in patients without secondary infection (0.079± 0.011;n=38) (P<0.01)
Relationship between clinical outcome and GVS
The GVS in non-survivors was 0.098±0.009 (n=4), which was higher than that in survivors (0.083±0.016;n=64), but the difference was not significant (P=0.082).
Discussion
In this study, the GVS in patients with AP was significantly elevated. The plain abdominal radiographs of these patients showed sentinel loops, colon cutoff sign, and bowel distention with multiple air-fluid levels.
The cause of the increased level of intestinal gas in patients with AP remains unexplained, but several mechanisms can account for it. First, soon after the onset of AP, various factors such as hormones, neurotransmitters, inflammatory mediators and endotoxin can alter gastrointestinal motility by a direct effect on smooth muscle cells or an indirect effect by influencing the neuronal circuits involved in peristalsis.[12,13]The reduced peristaltic activity may result in abnormal gas retention. Second, intestinal motility serves as a normal cleansing mechanism of the intestine.[14]Delayed motility with systemic and local immunosuppression from the early phase of AP can cause small intestinal bacterial overgrowth.[15-21]The contaminating flora in small intestinal bacterial overgrowth commonly feature colonic-type bacteria,[18]and gases of bacterial origin account for almost 3/4 of flatulence.[22,23]Therefore, bacterial overgrowth and intestinal content retention can create the potential for large quantities of gas within the intestinal lumen. GVS, reflecting gastrointestinal motility and intestinal bacterial overgrowth, appears to be a practical tool to assess gastrointestinal function. The significantly higher GVS in the SAP group demonstrates that these patients have greater gastrointestinal disturbance.
The Ranson's and APACHE II scores are the "traditional" multifactorial scoring systems in predicting the severity, pancreatic necrosis, and mortality of AP.[24]The data showed that the GVSs within 24 hours of admission were significantly correlated with these scores, suggesting that GVS may be a new and useful parameter for early prediction of the prognosis of AP. Intraabdominal hypertension and abdominal compartment syndrome due to the accumulation of gas within the intestinal lumen[25]may be some of the reasons for these strong correlations between GVS and these scores.
Secondary infection supervenes in 30%-70% of patients with pancreatic necrosis and is significantly associated with increased late death.[2,5,26]Enteric Gramnegative organisms account for most infections of pancreatic necrosis and subsequent sepsis,[27-29]suggesting the gut itself acts as a source of infection. The small bowel seems to be the major source of enteral bacteria in infected pancreatic necrosis.[30]In our study, the GVS in patients with secondary pancreatic and/or peripancreatic infection was significantly higher than that in patients without secondary infection. This implies that the increased level of intestinal gas may reflect the development of bacterial translocation. Although pathology of the intestine may be the initial cause of intra-abdominal hypertension, further intra-abdominal pressure can, in turn, result in intestinal mucosal ischemia and ischemia/reperfusion injury,[25]and then increase intestinal permeability to bacteria and endotoxin.[2]The development of abnormal bacterial overgrowth and an increase in intestinal permeability may partly explain why patients with secondary pancreatic and/or peripancreatic infection had higher GVSs.
Interestingly, the GVS in survivors was lower than that in non-survivors, but the difference was not significant. Perhaps the improvement of conservative management, surgical intervention and interventional intensive care account for this result.
AP is a dynamic and evolving process that involves the risk of multiple organ complications.[24]Many factors, such as extensive inflammatory processes in the retroperitoneum, visceral edema, increased capillary permeability, massive fluid resuscitation, electrolyte imbalance, formation of pancreatic ascites and pharmacological stimulation of intestinal propulsion appear to affect GVS. Whether GVS performs with high accuracy in the prediction of the severity and clinical outcome of AP over 24 hours after admission is still unknown. Moreover, the GVSs were not completely correlated with the Ranson's and APACHE II scores. For these reasons, additional structured analysis may be needed to determine the impact of multiple factors to accurately predict specific outcomes on a patient-bypatient basis.
In conclusion, intestinal gas volume is significantly elevated in patients with AP within 24 hours after admission and is closely related to Ranson's score, APACHE II score and secondary pancreatic and/or peripancreatic infection. GVS may be a new prognostic tool to assess the severity of AP in the early course of this disease. However, as GVS appears to be affected by many factors, other pathological indexes may be taken into account in the accurate prediction of the severity and outcome of AP.
Contributors: LY and LHS proposed the study. LY wrote the first draft. Both authors contributed to the design and interpretation of the study and to further drafts. LHS is the guarantor.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (81070297).
Ethical approval: Not needed.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery 2007;141:470-480.
2 Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg 1999;3:252-262.
3 Seerden TC, De Winter BY, Van Den Bossche RM, Herman AG, Pelckmans PA, De Man JG. Regional differences in gastrointestinal motility disturbances during acute necrotising pancreatitis. Neurogastroenterol Motil 2005;17: 671-679.
4 Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Matsumura N, et al. Treatment outcome of selective digestive decontamination and enteral nutrition in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Surg 2007;14: 503-508.
5 Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003; 7:26-36.
6 Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984;86:174-193.
7 Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci 2010;55:2135-2143.
8 Kurbel S, Kurbel B, Vcev A. Intestinal gases and flatulence: possible causes of occurrence. Med Hypotheses 2006;67:235-239.
9 Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet 1983;1:1206-1209.
10 Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, et al. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol 2000;95:1735-1741.
11 Morken MH, Berstad AE, Nysaeter G, Berstad A. Intestinal gas in plain abdominal radiographs does not correlate with symptoms after lactulose challenge. Eur J Gastroenterol Hepatol 2007;19:589-593.
12 Seerden TC, De Man JG, Holzer P, Van den Bossche RM, Herman AG, Pelckmans PA, et al. Experimental pancreatitis disturbs gastrointestinal and colonic motility in mice: effect of the prokinetic agent tegaserod. Neurogastroenterol Motil 2007;19:856-864.
13 van Minnen LP, Blom M, Timmerman HM, Visser MR, Gooszen HG, Akkermans LM. The use of animal models to study bacterial translocation during acute pancreatitis. J Gastrointest Surg 2007;11:682-689.
14 Gangarosa EJ. Recent developments in diarrheal diseases. Postgrad Med 1977;62:113-117.
15 Leveau P, Wang X, Soltesz V, Ihse I, Andersson R. Alterations in intestinal motility and microflora in experimental acute pancreatitis. Int J Pancreatol 1996;20:119-125.
16 Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, et al. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg 1998;228:188-193.
17 Nieuwenhuijs VB, van Duijvenbode-Beumer H, Verheem A, Visser MR, Verhoef J, Gooszen HG, et al. The effects of ABT-229 and octreotide on interdigestive small bowel motility, bacterial overgrowth and bacterial translocation in rats. Eur J Clin Invest 1999;29:33-40.
18 Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology 2006;130:S78-90.
19 Stotzer PO, Bj?rnsson ES, Abrahamsson H. Interdigestive and postprandial motility in small-intestinal bacterialovergrowth. Scand J Gastroenterol 1996;31:875-880.
20 Husebye E, Skar V, H?verstad T, Iversen T, Melby K. Abnormal intestinal motor patterns explain enteric colonization with gram-negative bacilli in late radiation enteropathy. Gastroenterology 1995;109:1078-1089.
21 Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, doubleblind, placebo-controlled trial. Lancet 2008;371:651-659.
22 Suarez F, Furne J, Springfield J, Levitt M. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol 1997;272:G1028-1033.
23 Tomlin J, Lowis C, Read NW. Investigation of normal flatus production in healthy volunteers. Gut 1991;32:665-669.
24 Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105:435-442.
25 Gallagher JJ. Intra-abdominal hypertension: detecting and managing a lethal complication of critical illness. AACN Adv Crit Care 2010;21:205-219.
26 Bradley EL 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 1991;161:19-25.
27 Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003;26:122-129.
28 Wu XM, Ji KQ, Wang HY, Li GF, Zang B, Chen WM. Total enteral nutrition in prevention of pancreatic necrotic infection in severe acute pancreatitis. Pancreas 2010;39:248-251.
29 Sakorafas GH, Lappas C, Mastoraki A, Delis SG, Safioleas M. Current trends in the management of infected necrotizing pancreatitis. Infect Disord Drug Targets 2010;10:9-14.
30 Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg 2010; 200:111-117.
June 17, 2011
Accepted after revision December 4, 2011
Correction
10.1016/S1499-3872(12)60167-2)
Author Affiliations: Department of Gastroenterology, Renmin Hospital, Wuhan University, Wuhan 430060, China (Liu Y and Luo HS)
He-Sheng Luo, MD, Department of Gastroenterology, Renmin Hospital, Wuhan University, Wuhan 430060, China (Tel: 86-27-88041911; Fax: 86-27-88042292; Email: luotang@public.wh.hb.cn)
? 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
doi: 10.1016/S1499-3872(12)60166-0
The article entitled "Tumor ablation with nanosecond pulsed electric fields" by Chen et al (Hepatobiliary Pancreat Dis Int2012;11:122-124.) contains an error. The 3rd to last paragraph equates nanosecond pulsed electric fields with the Nanoknife. This is in error. Nanosecond pulsed electric fields (nsPEFs) use pulse durations in the nanosecond range while the Nanoknife uses microsecond pulses. Thus, really the term "Nanoknife" is an unfortunate, but established misnomer. The two technologies are distinctly different in the levels of pulse power applied and in cell and tissue responses to them. At least part of the death processes induced by nsPEFs is due to apoptosis. The Nanoknife induces necrosis, as best understood at this time. The full extent of cell response differences between nsPEFs and the Nanoknife remain to be determined.
 Hepatobiliary & Pancreatic Diseases International2012年3期
Hepatobiliary & Pancreatic Diseases International2012年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Management of splenic artery aneurysm associated with extrahepatic portal vein obstruction
- Pancreas-preserving segmental duodenectomy for gastrointestinal stromal tumor of the duodenum and splenectomy for splenic angiosarcoma
- Induction, modulation and potential targets of miR-210 in pancreatic cancer cells
- Can the biliary enhancement of Gd-EOB-DTPA predict the degree of liver function?
- Quality control measures for lowering the seroconversion rate of hemodialysis patients with hepatitis B or C virus
- Effect of recombinant human growth hormone and interferon gamma on hepatic collagen synthesis and proliferation of hepatic stellate cells in cirrhotic rats
