Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial
Amr H. Yehia, Magdy H. Koleib, Ibrahim A. Abdelazim,2, Ahmed Atik
1Department of Obstetrics & Gynecology, Ain Shams University, Cairo, Egypt
2Ahmadi Hospital, Kuwait Oil Company (KOC), Ahmadi, Kuwait.
Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial
Amr H. Yehia1*, Magdy H. Koleib1, Ibrahim A. Abdelazim1,2, Ahmed Atik1
1Department of Obstetrics & Gynecology, Ain Shams University, Cairo, Egypt
2Ahmadi Hospital, Kuwait Oil Company (KOC), Ahmadi, Kuwait.
ARTICLE INFO
Article history:
Received 5 December 2013
Received in revised form 15 December 2013
Accepted 20 December 2013
Available online 20 January 2014
Tranexamic acid
Objective: To evaluate the efficacy of tranexamic acid in reduction of blood loss during and after cesarean section. Methods: Women included in the current double blinded, randomized, controlled trial were recruited from women attending for elective cesarean section and randomized into two groups; study group: received tranexamic acid with induction of anesthesia plus 10 IU of oxytocin injection after delivery of the baby. Control group: received only oxytocin 10 IU injection after delivery of the baby. Results: Twenty four hours post-operative hemoglobin level was significantly higher in study group (11.2 ± 1.5 mg/dL) compared to control (9.6 ± 1.2 mg/dL), also 24 hours post-operative hematocrit was significantly higher in study group (30.2 ± 6.6) compared to control (29.2 ± 2.8). Calculated total blood loss from placental delivery till end of cesarean section was significantly less in study group compared to control (369.5 ± 198.0 versus 606.8 ± 193.0 mL; respectively), also, calculated vaginal bleeding during first 6 hours post-operative was significantly less in study group compared to control (85.0 ± 30.7 mL versus130.8 ± 49.3 mL, respectively). The incidence of post-partum hemorrhage was significantly less in study group compared to control (31.1% versus 63.2%; respectively), also the need for iron replacement therapy was significantly less frequent in study group compared to control (0.9% versus 6.6%, respectively). Conclusions: Tranexamic acid can be used safely to reduce blood loss during cesarean section. Reduced blood loss after tranexamic acid was associated with improvement of post-operative hemoglobin, hematocrit and with reduction of post-partum need for iron replacement.
1. Introduction
Obstetric hemorrhage remains one of the major determinants of maternal death in both developed and developing countries. Because of its weight as a leading cause of maternal mortality and morbidity, obstetric hemorrhage (ante-partum and postpartum hemorrhages) must be investigated for national guideline development [1]. Haemostatic drugs are not usually used as first-line treatment in post-partum hemorrhage (PPH) [2]. It was authenticated that extensive tissue injury can direct the haemostatic equilibrium toward increased fibrinolysis, leading to coagulopathy and bleeding [3]. Antifibrinolytic drugs, namely tranexamic acid (TXA) have been recognized to decrease blood loss and transfusion needs in various elective surgeries [4]. Furthermore, the Clinical Randomization of an Anti-fibrinolytic in Significant Haemorrhage (CRASH-2) study concluded that tranexamic acid decreases the risk of death in bleeding trauma patients [5]. Tranexamic acid decreases post-partum blood loss after vaginal birth and after cesarean section based on two randomized controlled trials (RCTs) [6].This study wasdesigned to evaluate the efficacy of TXA in reduction of blood loss during and after cesarean section (CS).
2. Materials and methods
This double blinded, randomized, controlled trial was conducted at Ain Shams University, Maternity Hospital, from September 2012 to March 2013, after approval of the study by institute ethical committee.
Two hundred and twenty three (223) women attending the labor ward for elective CS were included in the study after informed consent and randomized into 2 groups; group 1 (study group): women who received 1 gram of TXA (kapron?, Amoun, Egypt) with induction of anesthesia by slow intravenous injection over 2 minutes in addition to 10 IU of oxytocin (syntocinon?, NOVARTIS, Egypt) injection after delivery of the baby were included. Group 2 (control group): women who received only oxytocin 10 IU of oxytocin injection after delivery of the baby were included. Six women in group I and five women in group II were excluded from this study (either due to uterine contraction before elective CS or no available suction set in the operative room), so 106 women were finally analyzed in each group. All CS were done under spinal anesthesia, by lecturer of the causality (lecturer of the causality; who had passed the residency program for 3 years and having an experience for 3 years as assistant lecturer, with MD degree), assisted by a registrar of the causality. A plan of interventions was sealed in closed envelops, numbered in accordance with the randomization tables. Packing, sealing and numbering were all performed by two independent doctors other than the investigator. Surgeons and investigators were not aware whether patient received TXA or not (double-blinding). Randomization coding tables were concealed from investigators till the end of the study. Women who had medical disorders with pregnancy, bleeding tendency, risk of thromboembolism, known allergy to TXA, ante-partum hemorrhage, abnormal site of the placenta (detected by ultrasound), macrosomic baby, twin pregnancy, polyhydramnios were excluded from this study. All women were subjected to history taking, general, abdominal examination and pre-operative investigations (complete blood count, prothrombin time, activated partial thromboplastin time, liver and kidney function tests).
2.1. Primary outcome measures
Blood loss during CS after delivery of placenta; which was estimated by using soaked towels and suction bottle and was estimated by anesthesiologists attended the cesarean section to avoid potential surgeon bias. Blood loss from uterine incision and soaked towels before placental delivery were not added to blood loss measurements. Soaked towel = 150 mL. while semi-soaked towel = 75 mL. [7].
2.2. Secondary outcome measures
(1).Vital data during first 2 post-operative hours; (2). Vaginal bleeding during first 6 post-operative hours (amount of vaginal bleeding was calculated according to number of soaked pads used after cesarean section - each soaked pad = 50 mL); (3). 24 hours post-operative hemoglobin and hematocrit values (4). Need for other surgical measures to stop bleeding (B-lynch, uterine artery ligation, internal iliac artery ligation, hysterectomy and/or transfusion of blood or blood products) (5). Maternal and neonatal side effects of medications given.
2.3. Sample size justification
Sample size was calculated using data from previous studies [8, 9], data from Cochrane systematic review that showed the risk of post-partum blood loss > 400 mL was 14.44% in women who received TXA, in contrast to 32.38% in women who did not [6], and EpiInfo version 7.0, setting the power at 80%, the two-sided confidence level at 95% and 10% patients drop rate. Calculation according to these values, the minimal number of women needed to produce a statistically acceptable figure was 98 in each group. Therefore, two hundred and twenty women (223) were recruited in the beginning of the current study to be randomized into two groups.
2.4. Statistical analysis
Data were collected, tabulated then statistically analysed using the Statistical Package for Social Sciences (SPSS) computer software version 18. Numerical variables were presented as mean and standard deviation (±SD), while categorical variables were presented as number and percentage. Chi-square test (Χ2) was used for comparison between groups as regard qualitative variables. Student t-test was used for comparison between groups as regard quantitative variables. A difference with a P value <0.05 was considered statistically significant.
3. Results
The two studied groups were matched with no significant difference between the study and control groups regarding; mean age, body mass index (BMI) and gestational age (Table 1).
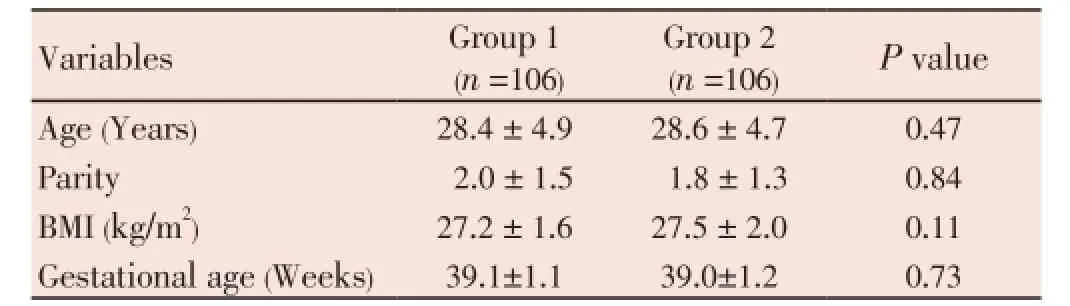
Table 1Demographic data of the two studied groups.
No significant difference regarding pre-operative hemoglobin and pre-operative hematocrit value was found, but the 24 hours post-operative hemoglobin was significantly higher in study group (11.2 ± 1.5 mg/dL) compared to control group (9.6 ± 1.2 mg/dL, P<0.05), also, 24 hours post-operative hematocrit value was significantly higher in study group (30.2 ± 6.6) compared to control (29.2 ± 2.8, P<0.05) (Table 2).
Calculated total blood loss from placental delivery till end of CS was significantly less in study group compared to control group (369.5 ±198.0 versus 606.8 ± 193.0 mL, respectively), also, calculated vaginal bleeding during first 6 hours post-operative was significantly less in study group compared to control (85.0 ± 30.7 versus 130.8 ± 49.3 mL, respectively), and calculated total blood loss from placental delivery till 6 hours post-operative was significantly less in study group compared to control (454.5 ± 201.0 versus 737.6 ± 217.0 mL, respectively) (Table 2)
The incidence of post-partum hemorrhage was significantly lower in study group compared to control group (31.1% versus 63.2%, respectively), also, the need for iron replacement was significantly less frequent in study group compared to control (0.9% versus 6.6%, respectively, P<0.05). No blood transfusion was needed in study group, while it was needed in two cases in control group (this difference was not statistically significant), no other surgical measures were needed to stop post-partum bleeding in both studied groups, no side effects or thrombotic events or neonatal adverse outcomes were recorded in both studied groups.
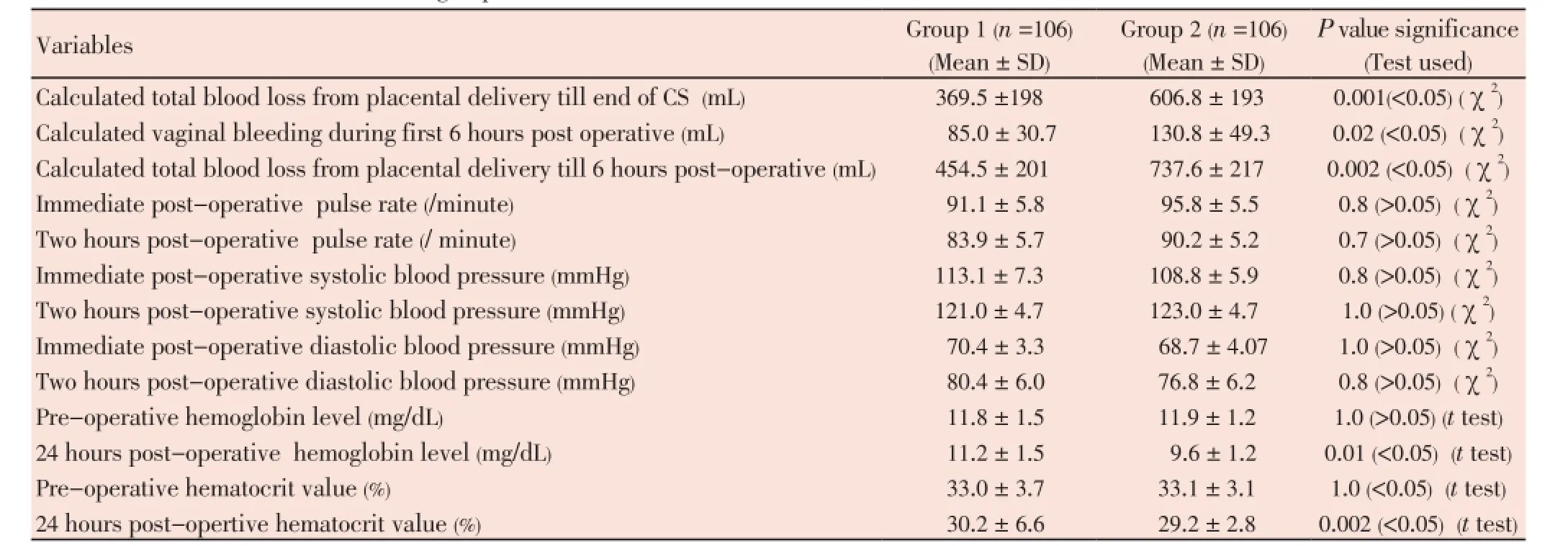
Table 2The measured outcome in both studied groups.
4. Discussion
Pregnant women with singleton fetus planned to have elective CS at ≥37 weeks gestation were randomized to receive 1 g TXA intravenously before elective CS group or not and blood loss was measured during and for two hours after operation by Abdel-Aleem and colleagues [8]. Abdel-Aleem and colleagues; concluded that the pre-operative use of TXA is associated with reduced blood loss during and after elective CS [8]. Also, women undergoing lower segment cesarean section (LSCS) were randomized to receive either TXA or distilled water just before the surgery in Shahid et al study [9]. Shahid et al, found that TXA significantly reduced the quantity of blood loss from placental delivery to the end of LSCS and it also reduced the quantity of blood loss from the end of LSCS to 2 hours post-partum. Shahid et al, concluded that TXA can be used safely and effectively in women undergoing LSCS to reduce intra-operative blood loss [9].
Six hundred and sixty women (660) women who underwent elective CS were included in Gungorduk and colleagues study to determine the efficacy and safety of TXA in reducing blood loss during elective CS [10]. Gungorduk and colleagues found that TXA significantly reduced bleeding during CS and reduced the need for additional uterotonicagents [10]. Another randomized, double-blind, casecontrolled study was conducted on 174 primipara undergoing CS by Xu et al (88 given 10 mg/kg TXA immediately before CS were compared with 86 others to whom TXA was not given) to determine the efficacy of TXA in reducing blood loss in patients after CS [11]. Xu et al, found that the blood loss in the period between the end of CS and 2 hours postpartum was significantly lower in TXA group than in the control group and they concluded that TXA is effective in reducing blood loss in patients undergoing CS [11].
In this study; the incidence of post-partum hemorrhage was significantly less in study group compared to control group, also, the need for iron replacement was significantly less frequent in study group compared to control, and no blood transfusion was needed in study group, while it was needed in two cases in control group (this difference was not statistically significant). No side effects or thrombotic events or adverse neonatal outcomes were recorded in both studied groups.
Gungorduk and colleagues, concluded that TXA can be used safely and effectively to reduce CS bleeding and to reduce the need for uterotonic agents [10]. Also, One hundred and eighty (180) primiparas were randomized into two groups; 91 in study group (received TXA immediately before CS) and 89 in control group (did not receive TXA) by Gai et al, to explore the efficacy and safety of TXA at CS [12]. Gai at al concluded that TXA can be used safely and effectively to reduce bleeding resulting from CS [12].
Hundred and twenty-three (223) women (101 study group & 122 control) were included in Sentürk & colleagues double-blind, controlled, randomized clinical trial, to detect the effect of TXA on blood loss during and after CS [13]. Sentürk & colleagues found that TXA reduced intra-operative and post-operative blood loss and they did not observe any complications caused by TXA such as venous thromboembolism, gastrointestinal problems and hypersensitivity [13]. Also, Yang et al, concluded that TXA can be used safely and effectively to reduce post-partum blood loss following vaginal delivery [14].
The current double-blind randomized trial concluded that TXA can be used safely without any maternal or neonatal side effects to reduce blood loss during and after CS. The reduced blood loss during and after CS following TXA administration was associated with improvement of postoperative hemoglobin, hematocrit values and with reduction of post-partum need for iron replacement.
Conflict of interest statement
No actual or potential conflict of interest in relation to this article exists.
Acknowledgements
I would like to express my appreciation acknowledgment to Professor Doctor; Ibrahim Anwar Abdelazim for his continuous advice for publication of this manuscript.
[1] Brace V, Kernaghan D, Penney G. Learning from adverse clinical outcomes: major obstetric haemorrhage in Scotland, 2003-05. BJOG 2007; 114(11): 1388-1396.
[2] Pfanner G, Kilgert K. Hemorrhagic complications in obstetrics. Hamostaseologie 2006; 26(3 Suppl 1): S56-63. [Article in German].
[3] Levy JH, Dutton RP, Hemphill JC, Shander A, Cooper D, Paidas MJ, et al. Hemostasis summit participants: Multidisciplinary approach to the challenge of hemostasis. Anesth Analg 2010; 110: 354-364.
[4] Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, McClelland B, et al. Anti-fibrinolytic use for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2007; 4: CD001886.
[5] CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebocontrolled trial. Lancet 2010; 376: 23-32.
[6] Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2010; 7: CD007872.
[7] Fazel MR, Mansoure-Samimi, Esmaeil-Fakharian. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East J Anesthesiol 2013; 22(1): 41-46.
[8] Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med 2013; 26(1):1705-1709.
[9] Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak 2013; 23(7): 459-462.
[10] Gungorduk K, Y?ld?r?m G, As?c?o?lu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol 2011; 28(3): 233-240.
[11] Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet 2013; 287(3): 463-468.
[12] Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarean section: a multicenter, randomized trial. Eur J Obstet Gynecol Reprod Biol 2004; 112: 154-157.
[13] Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet 2013; 287(4): 641-645.
[14] Yang H, Zheng S, Shi C. Clinical study on the efficacy of tranexamic acid in reducing post-partum blood loss: a randomized, comparative, multicenter trial. Zhonghua Fu Chan Ke Za Zhi 2001; 36: 590-592. [Article in Chinese].
ment heading
10.1016/S2305-0500(14)60002-6
*Corresponding author: Amr H. Yehia, MD, MRCOG, Lecturer of Obstetrics and Gynaecology, Ain Shams University, Maternity Hospital, Abassyia, Cairo, Eygpt.
Tel: (+2) 0122-7900014
E-mail: am_helmy77@hotmail.com
Blood loss
Cesarean section
 Asian Pacific Journal of Reproduction2014年1期
Asian Pacific Journal of Reproduction2014年1期
- Asian Pacific Journal of Reproduction的其它文章
- Tuberculous orchitis mimicking a testicular tumor: A diagnostic dilemma
- Klinefelter syndrome and its association with male infertility
- Current insights into gonadotropic pituitary function in the polycystic ovary syndrome
- Investigation on leukocyte profile of periparturient cows with or without postpartum reproductive disease
- Maternal outcome in multiple versus singleton pregnancies in Northern Tanzania: A registry-based case control study
- Soluble fms-like tyrosine kinase-1 and vascular endothelial growth factor: Novel markers for unexplained early recurrent pregnancy loss
