Soluble fms-like tyrosine kinase-1 and vascular endothelial growth factor: Novel markers for unexplained early recurrent pregnancy loss
Mahmoud Fathy Hassan
Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Soluble fms-like tyrosine kinase-1 and vascular endothelial growth factor: Novel markers for unexplained early recurrent pregnancy loss
Mahmoud Fathy Hassan
Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
ARTICLE INFO
Article history:
Received 25 December 2013
Received in revised form 30 December 2013
Accepted 30 December 2013
Available online 20 January 2014
Recurrent miscarriage
Objective: To evaluate the role of soluble fms-like tyrosine kinase-1 (sFlt-1) and vascular endothelial growth factor (VEGF) for prediction of pregnancy loss in patients with history of unexplained early recurrent pregnancy loss (RPL). Methods: A prospective case control study was conducted in 42 women with history of unexplained early RPL, and 170 pregnant controls with history of uncomplicated pregnancy. We measured maternal serum sFlt-1 and VEGF at gestational age 6-9 weeks as predictor for pregnancy loss. Results: Mean serum levels of sFlt-1 and VEGF were significantly higher in RPL group than controls (10439.7±385.4 vs 3304.5±104.8; P<0.0001, for sFlt-1, and 1885.0±98.3 vs 709.8±24.8; P<0.0001, for VEGF). Receiver operating characteristic (ROC) curves analyses established that sFlt-1 and VEGF were able to discriminate women at risk of developing pregnancy loss with area under ROC curve 0.970 for sFlt-1 and 0.953 for VEGF. Cutoff value of 5159.5 pg/mL for sFlt-1 was predictor for early pregnancy loss with 95.2% sensitivity, 91.2% specificity, and odds ratio (OR) 206 [95%confidence interval (CI), 45.4-941]. Cutoff value of 915.5 pg/mL for VEGF was predictor for early pregnancy loss with 92.9% sensitivity, 90.6% specificity, and OR 125 [95% CI: 34.7-451]. Conclusion: These data advocate a relationship between sFlt-1, VEGF, and RPL suggesting that the high levels of sFlt-1 and VEGF might be associated with the pathogenesis of RPL.
1. Introduction
Pregnancy loss is the most common complication of pregnancy which affects up to 15% of the reproducing couples and recurs in 2% to 3% of them [1]. Recurrent pregnancy loss (RPL) is defined as two or more unsuccessful pregnancies, in which the pregnancy is defined as a clinical pregnancy documented by ultrasonography or histopathological test [2]. The pathophysiology of RPL is complex with many unknown contributing factors and mechanisms. Despite a wide range of investigations, no obvious reason can be established in more than 50% of cases[3].
The success of the implantation of an embryo depends on the formation of its own blood vessels. Therefore, the successful establishment of fetoplacental circulation is essential for the continuation of pregnancy[4]. A defective maternal hemostatic response leading to hypoxia secondary to thrombosis of the utero-placental vasculature has been hypothesized to subsequently lead to miscarriage. This may comprise impairment of trophoblast invasion, villitis, thrombosis in decidual vessels, and placental microthrombi [5]. Recent studies reported that high expression levels of the soluble fms-like tyrosine kinase-1 (sFlt-1) mRNA and the vascular endothelial growth factor
(VEGF) mRNA are found in the chorionic villus tissue of women with RPL [6]. VEGF, which has a mitogenic effect on endothelial cells and increases their migratory capacity, is secreted from many tissues, including the placenta. VEGF controls physiological and pathophysiological vascular development [7]. The biological activity of VEGF is regulated by a soluble portion of sFlt-1 receptor which is primarily secreted by the placenta. Excess circulating sFlt-1 binds to VEGF with high affinity, thereby neutralizing it [8]. The circulating sFlt-1 is an antiangiogenic factor that is secreted by the placenta [9]. Two-thirds of pregnancies that are lost to miscarriage are believed to be due to defective placentation associated with an absence of physiological changes in maternal spiral arteries [10]. A poorly vascularized (hypoxic) placenta stimulates excessive sFlt-1 and VEGF production, which leads to endothelial dysfunction. However, the mechanisms by which VEGF and sFlt-1 induce miscarriage remain unclear [11].
We conducted a prospective case control study to evaluate the hypothesis of association between elevated sFlt-1 and VEGF as markers of defective placentation and early pregnancy loss in patients with history of unexplained early RPL.
2. Materials and methods
2.1. Subjects
A prospective case control study was carried out between March, 2011 and February, 2013 at the Department of Obstetrics and Gynecology at a large Governmental Hospital, Dhahran, Saudi Arabia. The local Institutional Ethics Committee approved the study protocol and a written informed consent was obtained from all participants. All investigations were directed along with the principles stated in the Declaration of Helsinki.
The study enrolled 524 women that had experienced unexplained early recurrent pregnancy loss (RPL) prior to the current pregnancy. Only 42 patients, that subsequently miscarried, were included in this study. The study also included a matching control group including 170 randomly allocated women who had no previous history of early pregnancy loss and continued the current pregnancy with a living fetus beyond 13 weeks.
Inclusion criteria for the study were the presence of informed consent, maternal age >16 years and < 40 years, regularly menstruating women, and women with at least one live birth in the control group. Exclusion criteria were multiple gestation, history of obstetric or medical complications as intrauterine growth restriction, preeclampsia, preterm labor, diabetes, renal insufficiency, or chronic hypertension.
Early recurrent pregnancy loss was defined as 2 or more losses wherein the pregnancy was documented by an ultrasonography or a histopathological test occurring before gestational age of 13 weeks [2]. Unexplained pregnancy loss was made after other presumptive etiological causes of RPL were found to be normal i.e. glucose tolerance test, hysterosalpingography that excludes any anatomic abnormality, cervical incompetence, and intrauterine adhesions, karyotyping of parents, antiphospholipid antibody syndrome assessment, and hormonal profile.
Baseline characteristics of all women were recorded at the first visit of antenatal care. Gestational age was calculated from the menstrual dates or dates of the first trimester ultrasound. Fetal cardiac activity was confirmed by an ultrasound. All women enrolled were examined one time per week until gestational age of 13 weeks. Examinations included clinical symptoms, serumβ-human chorionic gonadotropin (β-hCG) levels and ultrasound. In all patients that miscarried, an ultrasound was done to confirm embryonic death before inclusion in the study. Meanwhile, the outcome of pregnancy was monitored until term in the control group.
Peripheral blood samples for sFlt-1 and VEGF were taken from RPL patients at gestational age 6-9 weeks. Meanwhile, samples were taken in the controls after documentation of fetal cardiac activity in the period between 6-9 weeks of gestation. Maternal plasma samples were isolated by centrifugation at 2 500 g for 10 minutes. Aliquots of plasma samples were stored at -80 ℃ until future analysis. Levels for sFlt-1 and VEGF were measured with a commercially available enzyme-linked immunosorbent assay (ELISA) (ELISA, R&D System, Minneapolis, MN, USA) according to the manufacturer’s instructions. All plasma samples were run twice on the assay plate. If more than 10% variation existed, the assay was repeated and the average was entailed. The sensitivity of the test assay was 13.3 and 9 pg/ mL for sFlt-1 and VEGF respectively. The detection limit for sFlt-1 and VEGF was 31.2 pg/mL. The interassay and intraassay coefficients of variation were 5.4% and 11.2% for sFlt-1 and VEGF. Personnel who performed the assays of analytes were blinded to the clinical information.
2.2. Statistical analysis
At the start of this study, no other published trial had evaluated the association between both early pregnancy levels of sFlt-1 and VEGF and early RPL. Thus, we conducted a pilot trial of 20 patients in the RPL group and 80 patients in the control group before the full trial. A large Cohen’s effectsize (d) was found. Thus, Priori analyses using the G*power3 program (Heinrich-Heine-Universit? t, Düsseldorf, Germany) estimated that a sample size of 42 participants from the case group and 166 participants from the control group (ratio 1:4) would provide at least 80% power with an α error of 0.05 and 2-tails to detect differences of approximately 30% between the case group and the control group.
The Statistical Package for Social Sciences, version 14.0 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism, version 6 (GraphPad Software Inc., La Jolla, CA, USA) were used for statistical analyses. Normal distribution of continuous variables was assessed by the Kolmogorov Smirnov test. The Student t test was used for continuous variables with normal distribution, and the Mann Whitney U test was used for variables that were not normally distributed. The Fisher exact test was used for analysis of categorical variables. Results are expressed as mean ± standard error of mean, median (range), and number (percentage). Association between measured variables and pregnancy loss were evaluated using receiver operating characteristics (ROC) curve. Odds ratio (OR) with 95% confidence interval (CI) was calculated at the optimum cutoff value. Validity of study variables was valuated in terms of sensitivity and specificity. A two tailed P value < 0 .05 was considered to indicate statistical significance.
3. Results
The baseline clinical characteristics of each group were presented in Table 1. There were no significant differences between the two groups in the patients’ age, BMI, gestational age at blood sampling, and number of women previously using oral contraceptive pills.
The mean sFlt-1 level was significantly higher in the RPL group compared with the control group (10439.7±385.4 pg/mL, and 3304.5±104.8 pg/mL; respectively, P<0.0001). As well, the mean VEGF level was significantly higher in the RPL group compared to the control group (1885±98.3 pg/mL, and 709.8±24.8 pg/mL; respectively, P<0.0001) (Table 2).
Table 3 displayed area under the curve from receiver operating characteristic (ROC) for sFlt-1 and VEGF. Analysis of ROC curves for sFlt-1 and VEGF demonstrated a considerable evidence for the use of these markers in identifying women at risk for developing pregnancy loss with areas under the curve equal to 0.970 and 0.953 for sFlt-1 and VEGF respectively (Figure 1, 2). Our data indicated that serum level of sFlt-1 at cutoff value 5 159.5 pg/mL was predictor of pregnancy loss with 95.2 % sensitivity, 91.2 % specificity, and an odds ratio of 206.7 [95% confidence interval (CI) ; 45.4-941.0]. Similarly, serum VEGF level at cutoff value 915.5 pg/mL would predict pregnancy loss with 92.9% sensitivity, 90.6 % specificity, and an odds ratio of 125.1 [95% CI; 34.7-451] (Table 3).
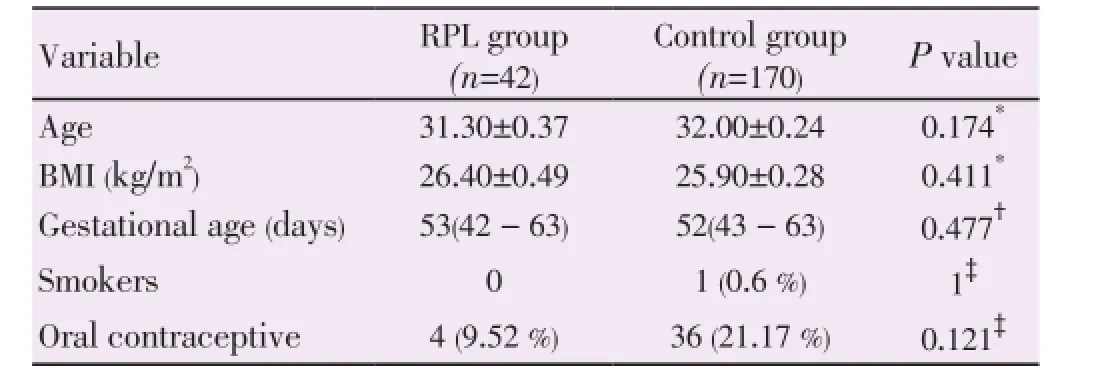
Table 1Baseline characteristics at time of blood sampling.
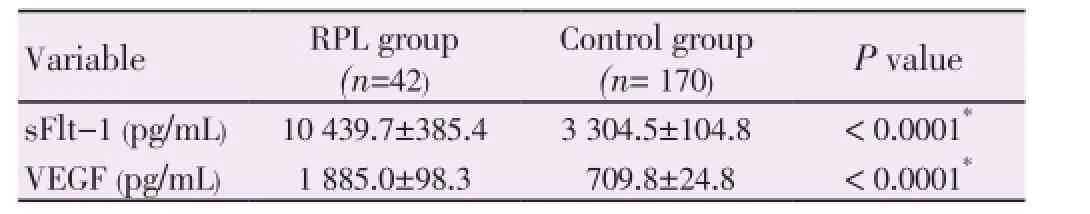
Table 2Serum concentrations of sFlt-1 and VEGF.
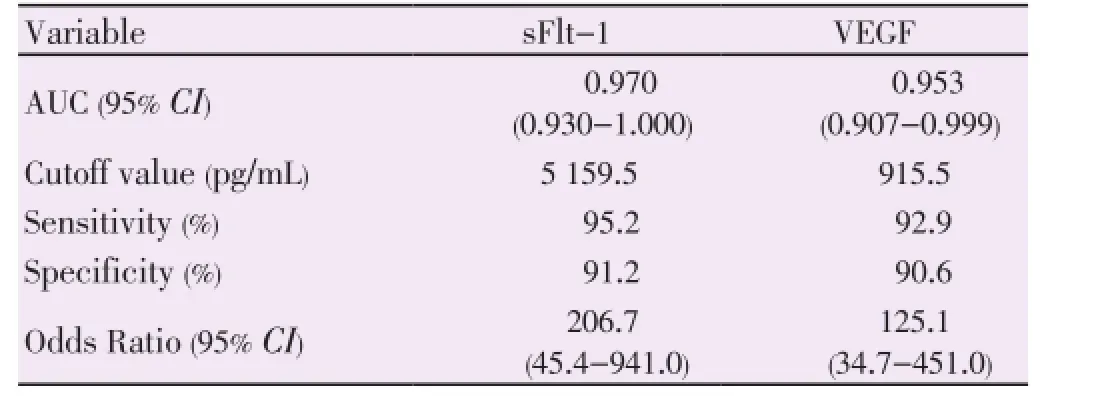
Table 3The utility of the measurements for subsequent development of pregnancy loss.
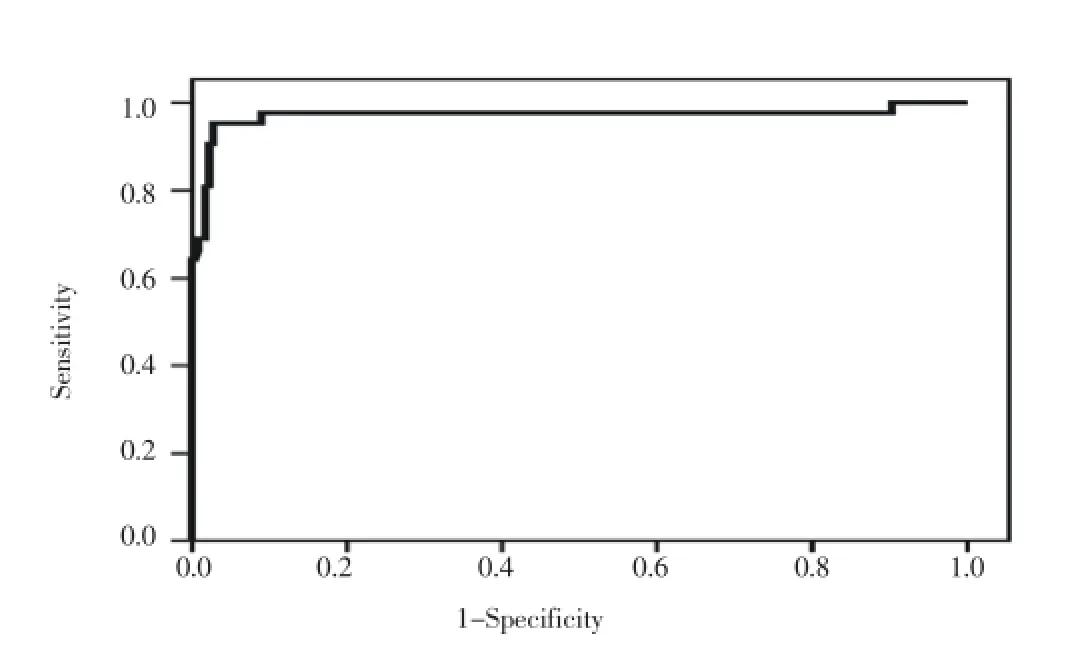
Figure 1. Receiver operating characteristic (ROC) curve of sFlt-1 in the prediction of early pregnancy loss. Area under the curve (AUC) = 0.97 (95% CI: 0.93 to 1; P<0.0001).
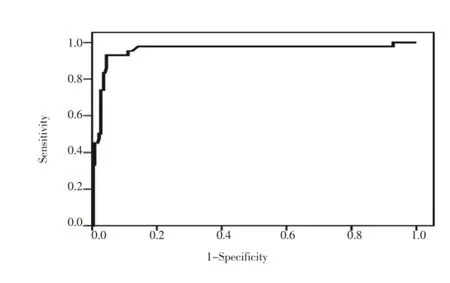
Figure 2. Receiver operating characteristic (ROC) curve of VEGF in the prediction of early pregnancy loss.Area under the curve (AUC) = 0.953 (95% CI: 0.907 to 0.999; P<0.0001).
4. Discussion
The etiology of recurrent pregnancy loss (RPL) appears to be diverse and controversial. However, in up to 50% of cases, no etiology can be identified [3]. In this study, we found a high level of expression of VEGF and sFlt-1 in the serum of unexplained early RPL patients that subsequently miscarried, when compared to controls. This supported the hypothesis that the over-expression of sFlt-1 and VEGF may be associated with the pathogenesis of RPL. Furthermore, our data revealed that the serum levels of sFlt-1 and VEGF could be used as potential predictive markers of miscarriage in women with history of RPL. The serum sFlt-1 at a cutoff value 5 159.5 pg/mL had 95.2% sensitivity and 91.2% specificity to predict pregnancy loss. Meanwhile, VEGF at a cutoff value 915.5 pg/mL had 92.9% sensitivity and 90.6% specificity.
Recent studies have indicated that placental ischemia/ hypoxia and endothelial dysfunction may contribute to cases of RPL [12, 13]. VEGF and sFlt-1 are secreted by the placenta, and they are especially important for a successful pregnancy[8]. Studies carried out on VEGF knock-out mice, showed that VEGF plays a significant role in the early stages of pregnancy during the angiogenesis process[14, 15]. VEGF is responsible for improved vascular permeability and the proliferation of endothelial cells for the successful implantation of the embryo. Any dysfunction or reduced expression of the gene and its products may lead to failure of implantation [15]. The sFlt-1, an endogenous inhibitor of VEGF, may play an important role in endothelial dysfunction. Extra circulating sFlt-1 attaches to VEGF with high affinity, thereby neutralizing it [8]. A poorly vascularized (hypoxic) placenta stimulates excessive sFlt-1 formation. The sFlt-1 is liberated to the circulation, and attached with VEGF, leading to endothelial dysfunction [7]. It has been reported that early stages of placental development occur under relatively hypoxic conditions. Expression of sFlt-1 in trophoblasts is a response to chronic hypoxia [13]. Genbacev et al. advocated that, to a certain limit, the placenta is in favor of invasive cytotrophoblast proliferation under conditions of chronic hypoxia [16]. This physiological hypoxia of the early gestational sac protects the developing fetus against the deleterious and teratogenic effects of O2free radicals [17]. However, Zhou et al. reported that increased sFlt-1 secretion was associated with reduced cytotrophoblast invasion in vitro [18]. Moreover, Germain and colleagues stated that maternal endothelial dysfunction could impair the invasion of extra-villous trophoblasts into the spiral arteries which is necessary to create the high flow and low resistance in the utero-placental vascular system that provides adequate blood supply for fetal growth. Consequently, the placentation defect and the decreased vascular smooth muscle dilatation and endometrial width may result from that increased vascular damage, and hence lead to miscarriage [19].
Many studies have reported that the utero-placental ischemia can result in an elevated sFlt-1 level, which designates a close association between the placental ischemia/hypoxia and over-expression of sFlt-1 [20, 21]. Until now, few studies have inspected the relationship between sFlt-1and VEGF and RPL. Pang and colleagues explored the relationship between early RPL and the expression of the genes encoding VEGF and sFlt-1, and demonstrated that there was a relationship between early RPL and VEGF and sFlt-1 levels, suggesting that over-expression of the sFlt-1 and VEGF may be associated with the pathogenesis of RPL [6]. In addition, ?aml? et al. displayed that One of the common polymorphisms of the VEGF gene (1154 G/A polymorphism) was found to be more frequent in women with recurrent pregnancy loss when compared to controls (23.7 vs 3.4%; respectively) indicating that disruption of VEGF function and placental angiogenesis can contribute to pregnancy loss in women with recurrent pregnancy loss [5].
On the contrary, Kaitu’u-Lino et al. indicated that sFlt-1 was unlikely to be a useful predictor of miscarriage in the first trimester clinically, since sFlt-1 had a significant overlap between cases and controls, and a poor sensitivity and specificity for predicting miscarriage in asymptomatic women [22]. Moreover, Muttukrishna and colleagues reported that serum sFlt-1 level was increased by several folds in early pregnancy and its level was markedly decreased in threatened miscarriage patients who subsequently had a miscarriage [23]. Those previously reported studies have shown that VEGF and sFlt-1 decrease after miscarriage. However, these studies included cases with threatened miscarriage, a condition with premature and excessive entry of maternal blood inside the placenta and might have two effects on the villous tissue. First, a direct mechanical effect with most of the villi is becoming progressively embedded inside large intervillous blood thrombi. Second, an indirect widespread oxygen mediated effect causing oxidative damage leading to major apoptosis and necrosis of the villous trophoblast. Overall, the consequences are placental degeneration with complete loss of syncytiotrophoblast function and detachment of the placenta from the uterine wall, resulting in decreased level of sFlt-1 prior to a complete miscarriage and even before the appearance of clinical symptoms of miscarriage [24]. In contrast, our data indicated that it is possible that a poorly vascularizedplacenta stimulates excessive sFlt-1 and VEGF production and that these factors are released into the maternal circulation shortly before miscarriage. The high sFlt-1 level, in particular, leads to endothelial dysfunction and pregnancy loss. However, the serum levels of sFlt-1 and VEGF were evaluated prior to any clinical manifestation of pregnancy loss, and hence no entry of maternal blood inside the placenta, allowing evaluation of the sFlt-1 and VEGF levels prior to placental degeneration and detachment.
In conclusion, the present study displayed evidence indicating that there is a correlation between the overexpression of serum VEGF and sFlt-1 and early pregnancy loss in patients with history of unexplained early RPL. These observations suggest that VEGF and sFlt-1 play an important role in the pathogenesis of RPL. Future research is needed to clarify the clinical relevance of elevated early pregnancy VEGF and sFlt-1 serum levels in women who experienced unexplained early RPL. Clinically, this may lead to the development of screening biomarkers for women with history of RPL and, more importantly, customized treatment strategies during pre-conceptual and prenatal care.
Cinflict of interest statement
We declare that we have no conflict of interest.
[1] Saunders RD, Nakajima ST, Rai SN, Pan J, Gercel-Taylor C, Taylor DD. Alterations in antibody subclass immune reactivity to trophoblast derived fetal fibronectin and α2 macroglobulin in women with recurrent pregnancy loss. Am J Reprod Immunol 2012; 68(5): 438-449.
[2] Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1): 63.
[3] Szekeres-Bartho J, Balasch J. Progestogen therapy for recurrent miscarriage. Hum Reprod Update 2008; 14(1): 27-35.
[4] ?aml? H, Demir BC, ?zg?z A, Atalay MA, Uncu G. Vascular endothelial growth factor gene 1154 G/A, 2578 C/A, 460 C/T, 936 C/ T polymorphisms and association with recurrent pregnancy losses. Genet Mol Res 2012; 11(4): 4739-4745.
[5] Rai R. Is miscarriage a coagulopathy? Curr Opin Obstet Gynecol 2003; 15(3): 265-268.
[6] Pang LH, Li MJ, Li MQ, Yang DM, Shi L. Vascular endothelial growth factor (VEGF) and the VEGF soluble receptor-1 (sFlt-1) in chorionic villus tissue from chinese women with early recurrent spontaneous abortion. J Int Med Res 2011; 39(3): 830-837.
[7] McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol 2004; 191(4): 1240-1246.
[8] Goldman CK, Kendall RL, Cabrera G, Soroceanu L, Heike Y, Gillespie GY, et al. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc Natl Acad Sci USA 1998; 95(15): 8795-8800.
[9] Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998; 59(6): 1540-1548.
[10] Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006; 12(6): 747-755.
[11] Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, et al. Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS One 2009; 4(4): e5155.
[12] Dib C, Araoz PA, Davies NP, Dearani JA, Ammash NM. Hypoplastic right heart syndrome presenting as multiple miscarriages. Tex Heart Inst J 2012; 39(2): 249-254.
[13] Muttukrishna S, Suri S, Groome N, Jauniaux E. Relationships between TGF beta proteins and oxygen concentrations inside the first trimester human gestational sac. PLoS One 2008; 3(6): e2302.
[14] Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380(6673): 439-442.
[15] Rowe AJ, Wulff C, Fraser HM. Localization of mRNA for vascular endothelial growth factor (VEGF), angiopoietins and their receptors during the peri-implantation period and early pregnancy in marmosets (Callithrix jacchus). Reproduction 2003; 126(2): 227-238.
[16] Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science 1997; 277(5332): 1669-1672.
[17] Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat 2009; 215(1): 27-35.
[18] Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate huma cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes and low platelets syndrome. Am J Pathol 2002; 160(4): 1405-1423.
[19] Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007; 49(1): 90-95.
[20] Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt-1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111(5): 649-658.
[21] Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 2007; 71(10): 977-984.
[22] Kaitu’u-Lino TJ, Whitehead CL, Ngian G-L, Permezel M, Tong S. Serum concentrations of soluble Flt-1 are decreased among women with a viable fetus and no symptoms of miscarriage destined for pregnancy loss. PLoS One 2012; 7(2): e32509.
[23] Muttukrishna S, Swer M, Suri S, Jamil A, Calleja-Agius J, Gangooly S, et al. Soluble Flt-1 and PlGF: new markers of early pregnancy loss? PLoS One 2011; 6(3): e18041.
[24] Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancy. Am J Pathol 2003; 162(1): 115-125.
ment heading
10.1016/S2305-0500(13)60181-5
*Corresponding author: Mahmoud Fathy Hassan, King Abdulaziz Airbase Hospital, Post Office: 570 Dhahran, Post code: 31932; Dhahran, Saudi Arabia.
Tel: 00966532887062
E-mail: mahmoudfathy74 @yahoo.com
Fax: 00966133305223
Soluble fms-like tyrosine kinase-1
Vascular endothelial growth factor
 Asian Pacific Journal of Reproduction2014年1期
Asian Pacific Journal of Reproduction2014年1期
- Asian Pacific Journal of Reproduction的其它文章
- Tuberculous orchitis mimicking a testicular tumor: A diagnostic dilemma
- Klinefelter syndrome and its association with male infertility
- Current insights into gonadotropic pituitary function in the polycystic ovary syndrome
- Investigation on leukocyte profile of periparturient cows with or without postpartum reproductive disease
- Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial
- Maternal outcome in multiple versus singleton pregnancies in Northern Tanzania: A registry-based case control study
