Preference of the Herbivorous Marine Teleost Siganus canaliculatus for Different Macroalgae
YOU Cuihong, ZENG Fangui, WANG Shuqi, and LI Yuanyou
Marine Biology Institute of Shantou University, Guangdong Provincial Key Laboratory of Marine Biotechnology, Shantou 515063, P. R. China
Preference of the Herbivorous Marine Teleost Siganus canaliculatus for Different Macroalgae
YOU Cuihong, ZENG Fangui, WANG Shuqi, and LI Yuanyou*
Marine Biology Institute of Shantou University, Guangdong Provincial Key Laboratory of Marine Biotechnology, Shantou 515063, P. R. China
The decomposition of a large amount of unexploited macroalgal resource along the coast of China often results in heavy environmental pollution. In order to pave a way of using macroalgae as the dietary ingredient of rabbitfishSiganus canaliculatus, one of a few farmed herbivorous marine teleosts in China, its preference (feeding selectivity) for different macroalgae was determined in this study. Seven seaweed species abundantly inhabiting the coast of east Guangdong Province were exposed simultaneously to rabbitfish juveniles in laboratory (multiple-choice feeding) with their content and absolute intake assayed. It was found that the most preferred algae wereUlva prolifera,Gracilaria lemaneiformisandChaetomorpha linum, less preferred algae wereU. pertusaandPorphyra haitanensis, and least preferred ones wereSargassum fusiformeandCorallinasessilis. Such an order did not change when one to four relatively preferred seaweeds were removed. The preferred seaweeds were richer in protein and soluble sugar thus higher in energy than the least preferred. In addition, this fish was found to favor filamentous and flat algae rather than calcified ones. Accordingly, the richness of nutrients and morphological characteristics determined the preference ofS. canaliculatusfor tested macroalgae.
Siganus canaliculatus; feeding preference; macroalgae; nutrient
1 Introduction
Macroalgae are the main foods of herbivorous teleosts; they contain very rich carbohydrate and protein and diverse amino acids, vitamins and minerals (Tolentino-Pablicoet al., 2008). Macroalgal resources along the coast of China are abundant. Eighty four macroalgal species in class Phaeophyceae, Chlorophyceae and Florideophyceae have been documented around Nanao Island, eastern Guangdong Province (Chenet al., 2010); however, only a few of them, for example, red algaePorphyra haitanensisandGracilaria lemaneiformisand brown algaLaminaria japonicaare human consumed or industry used. The unused biomass of these algae decays, causing often serious pollution (Konget al., 2011). Rabbitfish in family Siganidae inhabit widely the coral reefs of Indo-Pacific region (Woodland, 1983), among them,Siganus canaliculatusandS. fuscescensinhabit the coast of Southeast China and are commercially important (Xuet al., 2011; Duet al., 2008; von Westernhagen, 1973). HerbivorousS. canaliculatusfeeding mainly algae and seagrasses is tender in meat and rich in polyunsaturated fatty acids (PUFA) (Liet al., 2008). As a wide practice, farmers feed this fish usually commercial feeds and some fresh macroalgae. The specific feeds are not available forS. canaliculatus; thus limits its farming scale.
Macroalgal meals have been formulated into the feeds for many fishes such as rainbow trout (Oncorhynchus mykiss) (Güroyet al., 2013), common carp (Cyprinus carpio) (Dileret al., 2007), tilapia (Oreochromis niloticus) (Stadtlanderet al., 2013) and European sea bass (Dicentrarchus labrax) (Valenteet al., 2006). HerbivorousS. canaliculatusshould feed macroalgae with a greater capacity than these carnivorous and omnivorous fish. In our previous investigation, the basic nutritional requirements ofS. canaliculatuson protein and lipid had been determined (Wanget al., 2010). It has been demonstrated early that incorporating dried seaweedGracilaria lemaneiformisinto the diets of rabbitfish is feasible (Xuet al., 2011).
In this study, we determined the feeding preference ofS. canaliculatusfor different seaweeds abundant around Nanao Island. Our findings may aid to choosing macroalgal species suitable as the ingredient in formulating feed ofS. canaliculatus, and enrich the knowledge of ingestion biology of herbivorous marine teleost.
2 Materials and Methods
2.1 Collection of Macroalgae and Fish
Actively growing and healthy thalli of 7 macroalgalspecies abundant around Nanao Island (23°23′33′′–23°29′11′N, 116°56′24′′–117°08′59′′E), eastern Guangdong Province, China, were collected with their characteristics of taxonomy shown in Table 1. The algae were carefully rinsed with filtered seawater in order to remove all epiphytes and used in feeding trials within 48 h indoor in seawater tanks at Nanao Marine Biology Station (NAMBS) of Shantou University.
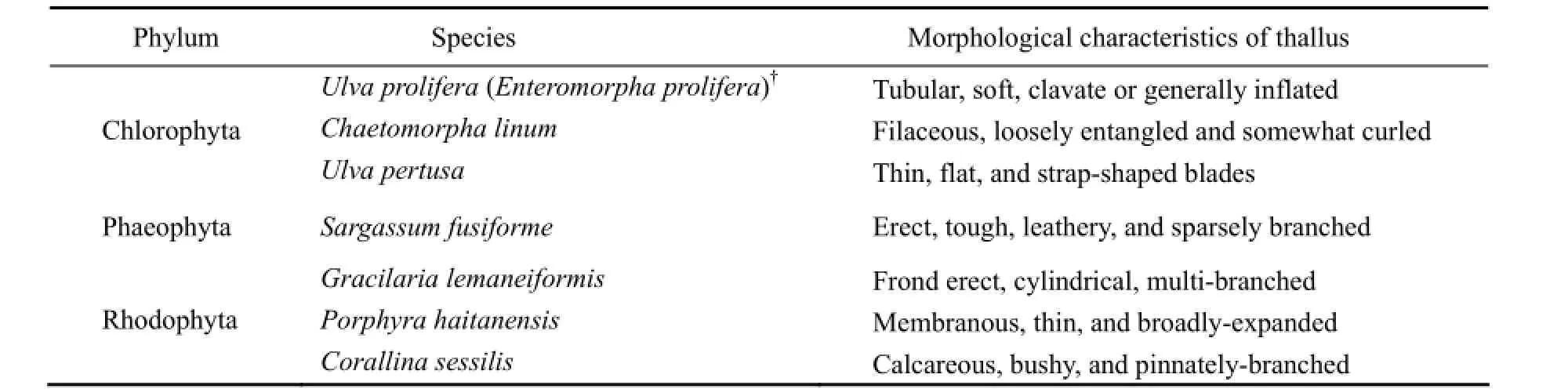
Table 1 The taxonomy and morphological characteristics of 7 seaweed species used in feeding preference assay
S. canaliculatusjuveniles (body mass around 10–50 g) were captured from the coastal area near NAMBS, and transferred to the laboratory containers with aerated seawater. The fish were kept at 22℃, 32 salinity with constant aeration and natural photoperiod, and acclimated by feedingad libituma mixture of the seven species of fresh seaweeds for two weeks. The preference assays were carried out in 200 L cylindrical tanks equipped with a continuous recirculating seawater system.
2.2 Laboratory Multiple-Choice Feeding
Two feeding strategies were taken to determine the preference of rabbitfish for macroalgae (Mantyka and Bellwood, 2007): (1) 7 macroalgae, equal in mass, fed simultaneously; and (2) 1 to 4 relatively preferred species removed (based on the findings of the first strategy).
For the first strategy, four experimental groups, the large-size fish group, 3 individuals 40.97 g in average each; the small-size fish group, 8 individuals 11.36 g in average each; and 2 controls, without fish, were set in order to determine the autogenic change of algal mass. Each group had 4 replicates. Macroalgae large enough forad libitumfeeding and in equal mass were gently blotted, weighed, randomly tied on iron wire, 65 cm in length, at approximately 8 cm intervals, and suspended 0.3 m above the bottom of tanks. Algal pieces torn off by fish were carefully collected. Remaining part of 7 seaweeds were collected, separately, blot dried and reweighed 1.5 h later to determine the mass loss each species. The trial was run for 4 consecutive days.
As algal availability changes seasonally, absence of the preferred macroalgae may force fish to adopt a different feeding pattern. For the second strategy, 1 to 4 relatively preferred seaweeds were removed to determine whether such a removing influences the preference of fish. Six groups and corresponding controls (without fish) were set up, which included 7 seaweeds exposed simultaneously (Group 1); most preferredU. proliferaandG. lemaneiformisremoved, respectively (Groups 2 and 3);U. proliferaandG. lemaneiformisremoved (Group 4);U. prolifera,G. lemaneiformisandC. linumremoved (Group 5); andP. haitanensis,S. fusiformeandC. sessilisexposed (Group 6), 8 fish, 8.19 ± 1.26 g in average each. Each group was replicated 6 times. The condition was identical to that of the first strategy. The trial was run for 6 consecutive days.
2.3 Determination of Macroalgal Biochemical Composition
Fresh thalli of each seaweed species were carefully cleaned with filtered seawater, divided into 3 portions and analyzed for biochemical composition as described previously (Xuet al., 2011; Liet al., 2008). Dry biomass was determined after air dried at 105℃ overnight. Crude protein content was expressed as 6.25 times of total nitrogen content determined with Kjeldahl method. Crude lipid content was measured with Soxhlet’s extraction. Ash content was determined by combusting in a muffle furnace at 550℃ for 16 h to constant weight. Crude fiber (insoluble dietary fiber, IDF) was determined according to Chinese Standard GB/6434-2006 (Caiet al., 2009; Taoet al., 2001). The total carbohydrate was calculated by subtracting crude protein, lipid, fiber and ash from dry biomass. Total soluble sugar content was measured with phenol-sulphuric acid colorimetry method described early (Zhouet al., 2012). Energy was measured in a digital display thermal meter (XRA-1A, Shanghai Changji Geological instruments Co, Ltd, China) as described early (Zhang, 2007).
2.4 Determination of Food Consumption and Absolute Intake of Nutrients
Food consumption of each seaweed species byS. canaliculatus(wet massin) in multiple-choice feeding experiment was calculated with the formula
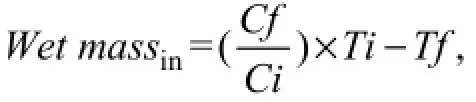
whereTiandTf, respectively, corresponded to the initial and final wet mass of seaweed during the 1.5 h feeding period, andCiandCfcorresponded, to the initial and final mass of the same species in control. The absolute intake of nutrients from seaweed by small-size fish in thefirst feeding strategy was calculated with formula
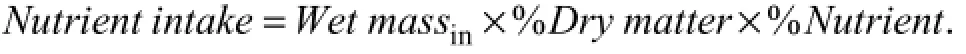
2.5 Statistical Analysis
All data were presented as mean ± SEM (n= 3, 4 or 6). One-way ANOVA in combination with Tukey’s test was used to find the difference among groups withOrigin7.0 (OriginLab, Northampton, MA). The correlation between preference of rabbitfish and biochemical composition of seaweeds, and preference of rabbitfish and nutrient intake from each seaweed was analyzed with Bivariate correlation (Pearson) implemented inSPSS19.0 (IBM, USA), and expressed inR-squared (R2). The significance was accepted whenP< 0.05.
3 Results
3.1 Feeding Preference of Rabbitfish for 7 Seaweeds Offered Simultaneously
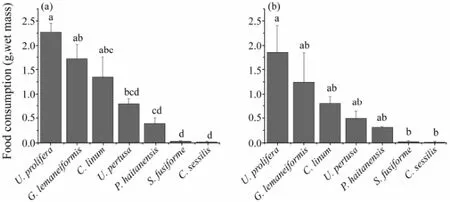
Fig.1 Feeding preference of S. canaliculatus for different macroalgae. Seven macroalgal species were offered simultaneously to rabbitfish for 1.5 h, and food consumption in (a) small-size fish and (b) large-size fish was measured for 4 consecutive days. Data are mean ± SEM (n = 4), Bars without sharing a common letter indicate a significant difference (one-way ANOVA, Tukey’s test, P < 0.05).
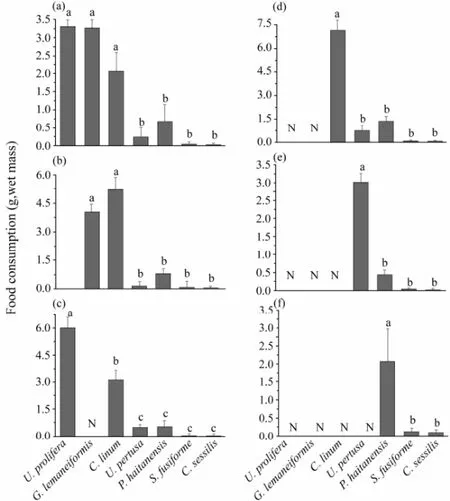
Fig.2 Feeding preference of S. canaliculatus for macroalgae when 1–4 relatively preferred algae were removed. (a) through (f) correspond to Groups 1–6. Data are mean ± SEM (n = 6). Bars without sharing a common letter indicate a significant difference (one-way ANOVA, Tukey’s test, P < 0.05).
When 7 species of seaweeds were offered simultaneously, rabbitfish exhibited constantly a highest preferenceforU. prolifera, which was followed byG. lemaneiformis,C. linum,U. pertusa,P. haitanensis,S. fusiformeandC. sessilisin order, being independent of fish size (Figs.1a, b). According to the feeding selectivity order and consumed mass for seaweeds as shown in Figs.1 and 2, we regardedU. prolifera,G. lemaneiformisandC. linumas the most preferred algae,U. pertusaandP. haitanensisas the less preferred algae,S. fusiformeandC. sessilisasas the least preferred algae.
3.2 Feeding Preference of Rabbitfish When 1–4 Relatively Preferred Seaweeds Were Removed
The preference degree of rabbitfish for seaweeds was further supported by findings in removing assays. When 7 seaweeds were offered simultaneously (Fig.2a), the fish displayed a similar preference order as shown in Fig.1 although with a certain degree of variation, which may due to the limiting number of fish. Moreover, after 1–4 relatively preferred seaweeds were removed, the preference order of fish for these seaweeds did not changed (Figs.2b–f). WhenU. prolifera,G. lemaneiformisor both were removed, rabbitfish tended to consume more of the remaining preferred algae (Figs.2b–d). However, when all the 3 most preferred algae were removed, fish had to consume a small amount (about 3 g) of less preferredU. pertusadue to hungriness (Fig.2e). Similarly, whenU. pertusawas also removed, fish was forced to consume a smaller amount ofP. haltanensls(Fig.2f). In all case, fish little consumed the least preferred algae,S. fusiformeandC. sessilisas.
3.3 Feed Preference and Nutrient Content of Macroalgae
As shown in Table 2, the nutrient content varied dramatically among seven seaweeds. The varied nutrient included crude protein, fiber and ash, carbohydrate, soluble sugar and energy. The content of crude lipid was not significantly different among species (P> 0.05). It was noteworthy that the protein content in the most preferred 3 algae (U. prolifera, G. lemaneiformis, C. linum) and less preferred 2 algae (U. pertusaandP. haitanensis) was significantly higher than that in the least preferred 2 algae (S. fusiformeandC. sessilisas) (P< 0.05). The content of soluble sugar in the most preferred 3 seaweeds was significantly higher than that in the least preferred 2 algae (P< 0.05). Moreover, the 5 most and less preferred seaweeds had a lower value of energyvsprotein proportion (E/P) than the 2 least preferred seaweeds (P < 0.05).
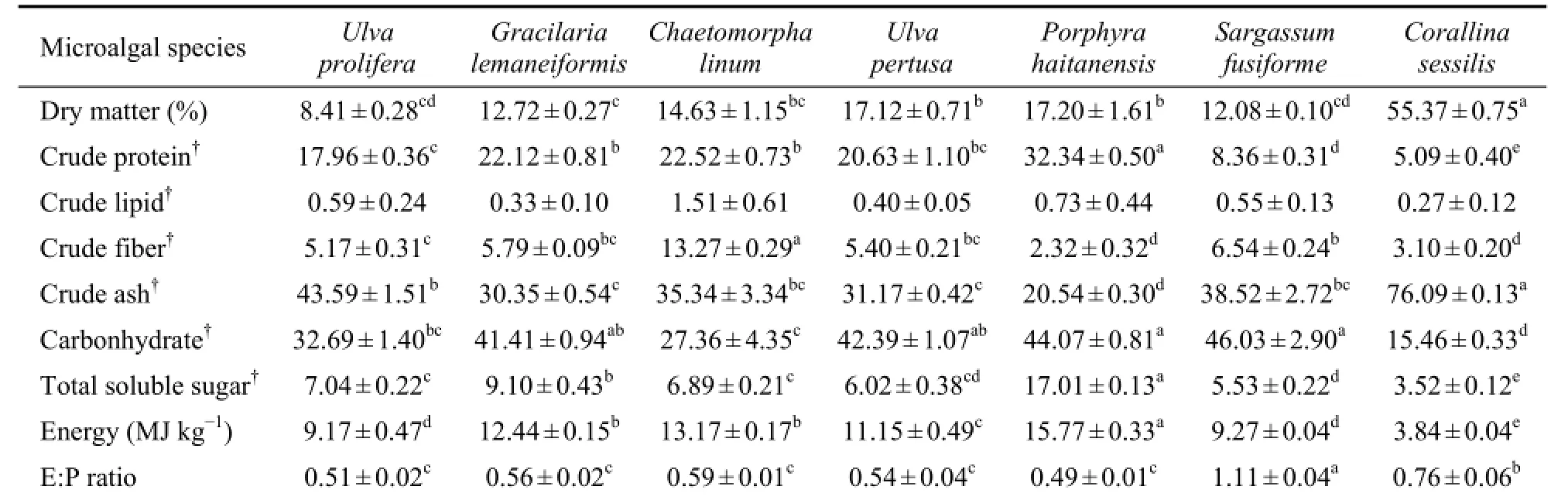
Table 2 Nutrient content of seven seaweed species used in the feeding preference trials
3.4 Feed Preference and Nutrient Intake from Macroalgae
The nutrient intake from 7 seaweeds by small-size fish was shown in Fig.3. The intake of nutrients, except crude lipid and fiber, from the 3 most preferred seaweeds was significantly higher than that from the 2 least preferred species. Such a difference was not found between the 3 most preferred seaweeds and 2 less preferred ones (P> 0.05).
A significant positive correlation was detected between food consumption and the intake of dry matter (R2= 0.922,P= 0.003), protein (R2= 0.842,P= 0.018), total carbohydrate (R2= 0.859,P= 0.013), soluble sugar (R2= 0.818,P= 0.024), ash (R2= 0.973,P= 0) and energy (R2= 0.817,P= 0.025).
4 Discussion
The preference of siganids for macroalgae has been documented early (e.g., von Westernhagen, 1973, 1974; Paulet al., 1990; Pillanset al., 2004; Lundberget al., 2004; Capperet al., 2006; Mantyka and Bellwood, 2007; Fox, 2012). It has been shown that siganids feed a wide range of macroalgae and have a distinct preference associating with algal morphological characteristics and defensive chemicals. Such a preference also associates with their specific feeding behavior, oral jaw morphology and nutrient assimilation.S. canaliculatusinhabiting different places prefers non-calcified seaweeds, for example, green algaUlvasp. (von Westernhagen, 1973) and brown algaSargassumsp. (Mantyka and Bellwood, 2007), but rejects red algaeGalaxaurasp. andAmphiroasp. (Mantyka and Bellwood, 2007); however, whether such a selection associates with the nutrient of macroalgae is less understood.
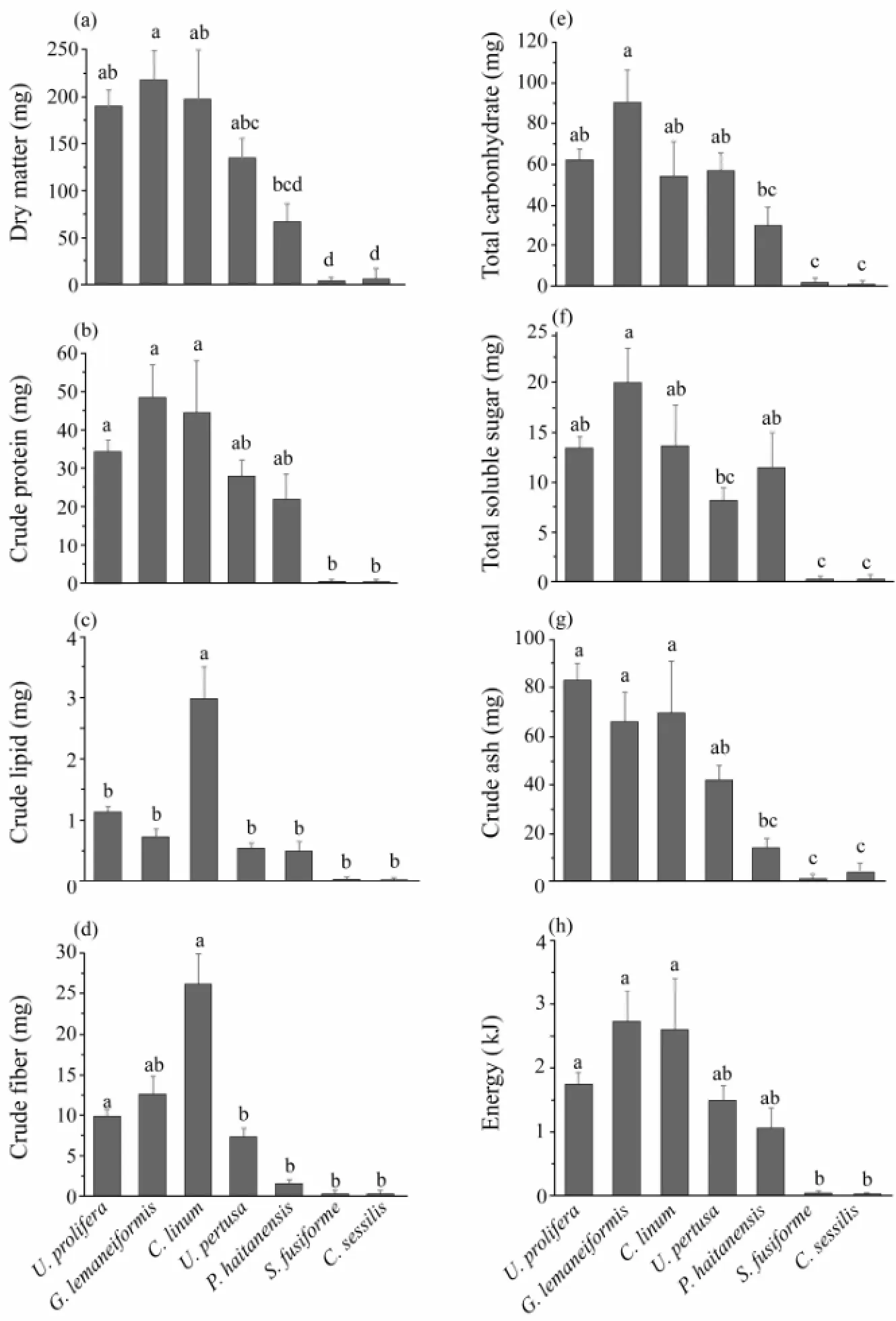
Fig.3 Absolute intake of nutrients by S. canaliculatus. Seven seaweeds were offered to small-size fish for four days. (a), dry matter; (b), crude protein; (c), crude lipid; (d), crude fiber; (e), total carbohydrate; (f), total soluble sugar; (g), crude ash; (h), energy. Data are mean ± SEM (n = 4), which without sharing a common letter above bars indicate significant differences (one-way ANOVA, Tukey’s test, P < 0.05).
In this study, we found thatS. canaliculatuspreferred green algaeU. proliferaandC. linum, and red algaG.lemaneiformismore than brown algaS. fusiformeand calcified red algaC. sessilis, which was contrary to the documented early (Mantyka and Bellwood, 2007). Such a discrepancy may be attributed to the difference in the ontogenetic stage of fish. The juveniles were used in this study; while adults were used early (Mantyka and Bellwood, 2007). The young fish we used should have not developed the hindgut fermentation ability, which is critical for digesting mannitol and laminarin rich in brown algae (Whiteet al., 2010). When 1–4 relatively preferred species were removed,S. canaliculatusswitched to feed the next preferred ones but neverS. fusiforme(Fig.2), being similar to the preference when 7 seaweeds were offered simultaneously (Fig.1). This finding indicated that the preference for specific seaweeds ofS. canaliculatusis determined genetically.
The correlation between food choice and macroalgal nutrients has seldom been demonstrated in marine herbivorous fish (Pillanset al., 2004). It is believed that herbivorous fish species prefer the macroalgae with high nutrient content than those with low nutrients (Horn and Neighbors, 1984). The content of protein, carbohydrate, fiber, soluble sugar and energy varied among 7 seaweeds used in this study. The preference ofS. canaliculatusfor macroalgae was closely but not significantly related to the content of protein and soluble sugar and E/P value of seaweeds (Table 2); however, such a preference significantly and positively correlated with the intake of drymatter, protein, soluble sugar, carbohydrate and energy from macroalgae (Fig.3), meeting the requirement of protein and energy of juvenile fish. It has been shown that nitrogen (protein) is a limiting factor of growth of many herbivores (Fenchel and Jorgensen, 1977) including some marine herbivorous fish species (Mattson, 1980; Choat and Clements, 1998; Raubenheimeret al., 2005). However, a correlation was not observed between the preference ofS. fuscescens(a close relative ofS. canaliculatus) and macroalgal nutrient including protein content (Pillanset al., 2004). The discrepancy between Pillans’s findings and ours may be attributed to the difference of seaweeds used. When energy and protein were considered together, the feeding preference was found to be negatively and weakly relative with the value of energyvsprotein proportion (E/P ratio) (Table 2), agreeing with the optimal foraging theory stating as herbivores maximize their intake of the most energetic and nutrient feeds (Hughes, 1980).
Although red algaP. haitanensispossesses the highest energy and content of protein and low E/P ratio among 7 algae tested, it was not highly selected byS. canaliculatus. In fact, many factors such as algal morphology and defensive chemicals and others play roles in determining the feeding selectivity of fish (von Westernhagen, 1973, 1974; Pillanset al., 2004; Mantyka and Bellwood, 2007). Tolentino-Pablicoet al. (2008) demonstrated that physical structure,i.e., toughness and resistance, seem to affect algal consumption by rabbitfish. The present study showed that the rabbitfishS. canaliculatusdid not like calcified (C. sessilis) seaweeds, less liked the sheet-like (U. pertusa), but most like the brittle branched (G. lemaneiformis) and the filamentous (U. proliferaandC. linum) as these seaweeds have a large surface area for digestion. The membranousP. haitanensishas a sheet texture and a relatively strong breaking tenacity, which may deter rabbitfish from grazing. Therefore, algal structural features have a direct effect on the macroalgal selectivity of fish.
In conclusions,S. canaliculatusprefersU. prolifera,G. lemaneiformisandC. linummost,U. pertusaandP. haitanensisless, andS. fusiformeandC.sessilisleast. Such a preference associates with algal nutrient and morphology. Rabbitfish prefers the macroalgae with rich protein, soluble sugar and energy and filamentous, flat and non-calcified thalli. These findings should aid to formulating rabbitfish feeds with seaweeds.
Acknowledgements
We thank Prof. Douglas R. Tocher of University of Stirling and Prof. Wei Chiju of Shantou University for revising the manuscript. This work was financially supported by grants from National Natural Science Foundation of China (No. 41276179), National Science and Technology Support Plan Project (No. 2012BAC07B05), Team Project of Natural Science Foundation of Guangdong Province (S2011030005257), Producing, Teaching and Research Cooperation Projects of Guangdong Province and Ministry of Education (No. 2011B090400039).
Cai, C., Yao, B., Shen, W. R., and He, P. M., 2009. Determination and analysis of nutrition compositions in Enteromorpha clathrata. Journal of Shanghai Ocean University, 18 (2): 155-159 (in Chinese with English abstract).
Capper, A., Tibbetts, I. R., O’Neil, J. M., and Shaw, G. R., 2006. Feeding preference and deterrence in rabbitfish Siganus fuscescens for the cyanobacterium Lyngbya majuscula in Moreton Bay, south-east Queeensland, Australia. Journal of Fish Biology, 68: 1589-1609.
Chen, W. Z., Liu, T., Du, H., Cao, H. B., Wang, L. G., and Ma, Q. T., 2010. Diversity of macrophytes of the Nanao Island of Guangdong province. In: Proceedings of the 2ndAcademic Conference on Algae Diversity and Classification in China, Taiyuan, 2.
Choat, J. H., and Clements, K. D., 1998. Vertebrate herbivores in marine and terrestrial environments: A nutritional ecology perspective. Annual Review of Ecology and Systematics, 29: 375-403.
Diler, I., Tekinay, A., Güroy, D., Güroy, B., and Soyuturk, M., 2007. Effects of Ulva rigida on the growth, feed intake and body composition of common carp, Cyprinus carpio. Journal of Biological Sciences, 7: 305-308.
Du, Y. B., Li, Y. Y., Zhen, Y. J., Hu, C. B., Liu, W. H., Chen, W. Z., and Sun, Z. W., 2008. Toxic effects in Siganus oramin by dietary exposure to 4-tertoctylphenol. Bulletin of Environmental Contamination and Toxicology, 80 (6): 534-538.
Fenchel, T. M., and Jorgenson, B. B., 1977. Detritus food chains of aquatic ecosystems: The role of bacteria. Advances in Microbial Ecology, 1: 1-58.
Fox, R. J., 2012. The trophic and spatial ecology of rabbitfishes (Perciformes, Siganidae) on coral reefs. PhD thesis, James Cook University.
Güroy, B., Ergün, S., Merrifield, D. L., and Güroy, D., 2013. Effect of autoclaved Ulva meal on growth performance, nutrient utilization and fatty acid profile of rainbow trout, Oncorhynchus mykiss. Aquaculture International, 21: 605-615.
Hayden, H. S., Blomster, J., Maggs, C. A., Silva, P. C., Stanhope, M. J., and Waaland, J. R., 2003. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology, 38: 277-294.
Horn, M. H., and Neighbors, M. A., 1984. Protein and nitrogen assimilation as a factor in predicting the seasonal macroalgal diet of the monkeyface prickleback. Transactions of the American Fisheries Society, 113: 388-396.
Hughes, R. N., 1980. Optimal foraging theory in the marine context. Oceanography and Marine Biology Annual Review, 18: 423-481.
Kong, F., Mao, Y. X., Cui, F. J., Zhang, X. K., and Gao, Z., 2011. Morphology and molecular identification of Ulva forming green tides in Qindao, China. Journal of Ocean University of China, 10 (1): 73-79.
Li, Y. Y., Hu, C. B., Zhen, Y. J., Xia, X. A., Xu, W. J., Wang, S. Q., Chen, W. Z., Sun, Z. W., and Huang, J. H., 2008. The effects of dietary fatty acids on liver fatty acid composition and Δ6-desaturase expression differ with ambient salinities in Siganus canaliculatus. Comparative Biochemistry and Physiology, 151B: 183-190.
Lundberg, B., Ogorek, R., Galil, B. S., and Goren, M., 2004. Dietary choices of siganid fish at Shiqmona reef, Israel. Israel Journal of Zoology, 50: 39-53.
Mantyka, C. S., and Bellwood, D. R., 2007. Macroalgal grazingselectivity among herbivorous coral reef fishes. Marine Ecology Progress Series, 352: 177-185.
Mattson Jr., W. J., 1980. Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics, 11: 119-161.
Paul, V. J., Nelson, S. G., and Sanger, H. R., 1990. Feeding preferences of adult and juvenile rabbitfish Siganus argenteus in relation to chemical defenses of tropical seaweeds. Marine Ecology Progress Series, 60: 23-34.
Pillans, R. D., Franklin, C. E., and Tibbetts, I. R., 2004. Food choice in Siganus fuscescens: Influence of macrophyte nutrient content and availability. Journal of Fish Biology, 64: 297-309.
Raubenheimer, D., Zemke-White, W. L., Phillips, R, J., and Clements, K. D., 2005. Algal macronutrients and food selection by the omnivorous marine fish Girella Tricuspidata. Ecology, 86 (10): 2601-2610.
Stadtlander, T., Khalil, W. K. B., Focken, U., and Becker, K., 2013. Effects of low and medium levels of red alga Nori (Porphyra yezoensis Ueda) in the diets on growth, feed utilization and metabolism in intensively fed Nile tilapia, Oreochromis niloticus (L.). Aquaculture Nutrition, 19: 64-73.
Tao, P., Xu, Q. L., Yao, J. G., and Gao, X., 2001. An analysis of nutrient components of thirteen kinds of seaweeds for food in Dalian coastline. Journal of Liaoning Normal University (Natural Science Edition), 24 (4): 406-410 (in Chinese with English abstract).
Tolentino-Pablico, G., Bailly, N., Froese, R., and Elloran, C., 2008. Seaweeds preferred by herbivorous fishes. Journal of Applied Phycology, 20: 933-938.
Valente, L. M. P., Gouveia, A., Rema, P., Matos, J., Gomes, E. F., and Pinto, I. S., 2006. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture, 252: 85-91.
von Westernhagen, H., 1973. The natural food of the rabbitfish Siganus oranim and S. striolata. Marine Biology, 22: 367-370.
von Westernhagen, H., 1974. Food preferences in cultured rabbitfishes (Siganidae). Aquaculture, 3: 109-117.
Wang, S. Q., Xu, S. D., Wu, Q. Y., Zhang, L., Zhang, T., You, C. H., Zhen, H. P., and Li, Y. Y., 2010. Optimal levels of protein and lipid in diets for rabbitfish Siganus canaliculatus juvenile. Marine Science, 34 (11): 18-22 (in Chinese with English abstract).
White, W. L., Coveny, A. H., Robertson, J., and Clements, K. D., 2010. Utilisation of mannitol by temperate marine herbivorous fishes. Journal of Experimental Marine Biology and Ecology, 391 (1-2): 50-56.
Woodland, D. J., 1983. Zoogeography of the Siganidae (Pisces): An interpretation of distribution and richness patterns. Bulletin of Marine Science, 33: 713-717.
Xu, S. D., Zhang, L., Wu, Q. Y., Liu, X. B., Wang, S. Q., You, C. H., and Li, Y. Y., 2011. Evaluation of dried seaweed Gracilaria lemaneiformis as an ingredient in diets for teleost fish Siganus canaliculatus. Aquaculture International, 19: 1007-1018.
Zhou, Z., and Zhou, N., 2012. Determination of total sugar in Ren-dong garlic by phenol-sulfuric acid method. Food Research and Development, 33 (6): 137-142 (in Chinese with English abstract).
Zhang, L. Y., 2007. Feed Analysis and Quality Inspection Technology. China Agricultural University Press, Beijing, 435pp.
(Edited by Qiu Yantao)
(Received December 1, 2013; revised December 27, 2013; accepted January 18, 2014)
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Correspondending author. Tel/Fax: 0086-754-86503157
E-mail: yyli@stu.edu.cn
 Journal of Ocean University of China2014年3期
Journal of Ocean University of China2014年3期
- Journal of Ocean University of China的其它文章
- Effects of Sulfate Chitosan Derivatives on Nonalcoholic Fatty Liver Disease
- Variation of Bioaccumulation Ability of 2,2’,4,4’-Tetrabromodiphenyl Ether by Marine Diatom Skeletonema costatum Under Different N:P Ratios
- Effect of Temperature on Gene Expression in the Pearl Oyster Pinctada fucata
- Nitrogen and Phosphorus Budget of a Polyculture System of Sea Cucumber (Apostichopus japonicus), Jellyfish (Rhopilema esculenta) and Shrimp (Fenneropenaeus chinensis)
- Structure of Mitochondrial DNA Control Region of Pholis fangi and Its Phylogenetic Implication
- Fishery Biology of Jumbo Flying Squid Dosidicus gigas off Costa Rica Dome
