Variation of Bioaccumulation Ability of 2,2’,4,4’-Tetrabromodiphenyl Ether by Marine Diatom Skeletonema costatum Under Different N:P Ratios
CHAI Chao, GE Wei, and YIN Xundong
1) College of Resources and Environment, Qingdao Agricultural University, Qingdao 266109, P. R. China
2) College of Life Sciences, Qingdao Agricultural University, Qingdao 266109, P. R. China
Variation of Bioaccumulation Ability of 2,2’,4,4’-Tetrabromodiphenyl Ether by Marine Diatom Skeletonema costatum Under Different N:P Ratios
CHAI Chao1),*, GE Wei2), and YIN Xundong1)
1) College of Resources and Environment, Qingdao Agricultural University, Qingdao 266109, P. R. China
2) College of Life Sciences, Qingdao Agricultural University, Qingdao 266109, P. R. China
The growth, biochemical content and bioaccumulation quantity of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) inSkeletonema costatumwere studied under different N:P ratios (1, 4, 16, 64 and 128). All cellular biochemical contents ofS.costatumpresented decreasing trend over cultivation time. At early stage of cultivation, the cellular protein, carbohydrate and lipid inS.costatumpresented higher values in treatments of N:P=4 and 16. However, they were lower in these treatments at the late stage, but higher in treatments N:P=1 and 128. Similarly, BDE-47 levels per cell ofS.costatumwere higher in treatments of N:P=4 and 16 at early stage of cultivation, which were 3.8 and 3.7 ng (106cells)-1, respectively. At the middle stage of cultivation, the BDE-47 level perS.costatumcell lowered; and it further reduced in the treatments of N:P=4 and 16 at the late stage with the values 0.6 and 0.5 ng (106cells)-1, respectively. However, it rose in N:P=128, reaching up to 2.3 ng (106cells)-1. Compared with BDE-47 per cell, BDE-47 per algal volume under different N:P ratios did not present obvious difference. The quantity BDE-47 accumulated per cell ofS.costatumwas positively correlated with protein, carbohydrate and lipid per cell; meanwhile, the BDE-47 per volume had a positive correlation with biochemical content per volume. The variation of bioaccumulation ability of BDE-47 inS.costatumcan be explained by biochemical changes due to N:P ratios.
N:P ratio; bioaccumulation; polybrominated diphenyl ethers; microalgae; nutrient
1 Introduction
Polybrominated diphenyl ethers (PBDEs) are halogenated flame-retardant chemicals, widely used in a number of consumer products including textiles, plastics, wire insulation, and automobiles (Rahmanet al., 2001). PBDEs were detected in environments and organisms, and their contents increased exponentially in the past 30 years (Hites, 2004; Suzukiet al., 2006; Lawet al., 2006; Borghesiet al., 2008; Luoet al., 2008). Due to toxicity, persistence and bioaccumulation, PBDEs are of global concern.
PBDEs consist of 209 congeners with 1-10 bromine atoms. Compared with higher brominated PBDEs, lower brominated PBDEs (1-5 bromine atoms per molecule) are more dangerous because they more efficiently bioaccumulate (De Witet al., 2006; Wanget al., 2007). It has been reported that the 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) is the predominant lower brominated congener in Chinese coastal waters (Guanet al., 2009) and has a relatively high abundance in seafood (Guoet al., 2007; Menget al., 2008).
Due to their rich lipid and small size, microalgae can bioaccumulate hydrophobic organic compounds (HOCs) which are then transferred to higher trophic levels. The bioaccumulation of HOCs by microalgae depends on environmental factors, such as nutrient status (Carlson and Swackhamer, 2006; Huet al., 2008; Liet al., 2010). Halling-Sùrensenet al. (2000) reported that nitrogen limitation affected the bioconcentration of polychlorinated biphenyl (PCBs) bySelenastrum capricornutum. Lynnet al. (2007) found that silica deficiency induces the increase of bioaccumulation factor of PCBs byStephanodiscus minutulus. These studies were aiming at bioaccumulation of PCBs by freshwater algae under nutrient-deficient condition. However, few studies focus on the effect of nutrient ratios on the bioaccumulation of PBDEs by marine microalgae.
Skeletonema costatum, a diatom, is a dominant species in Chinese coastal water. Zhaoet al. (2009) found that biochemical content ofS.costatumvaries in different nitrogen and phosphorus levels. The objective of this study focuses on whether the bioaccumulation of BDE-47 byS.costatumwill change with N:P ratios in different cultivation periods. The effects of N:P ratios on cellular biochemical contents and bioaccumulation ability ofBDE-47 byS.costatumin different cultivation periods are also investigated.
2 Materials and Methods
2.1 Algal Growth
The stock culture ofS.costatumwas provided by Institute of Oceanology, Chinese Academy of Sciences, and maintained in sterilized seawater with F/2 medium. The stock and experimental cultures were kept with an illumination of 45 μmol (m2s)-1(light:dark cycle, 12 h: 12 h) at 20 ± 1℃.
2.2 Experimental Design
Before inoculation,S.costatumwas grown for 48 h in N-free and P-free seawater. After starvation, algal cultures ofS.costatumin log-phase were inoculated into the 5 L flasks with sterilized artificial seawater. N:P ratios were designed as follows: (1)N:P=1 (N, P, 16 μmol L-1); (2)N:P=4 (N, 64μmol L-1; P, 16μmol L-1); (3)N:P=16 (N, 256μmol L-1; P, 16μmol L-1); (4)N:P=64 (N, 256μmol L-1; P, 4 μmol L-1); (5)N:P=128 (N, 256 μmol L-1; P, 2 μmol L-1). The N was added as NaNO3and P as NaH2PO4. The silicate and other nutrients corresponded to the F/2 medium. Each treatment was carried out in triplicate.
Studies found that the microalgae could accumulate HOCs rapidly. For example, the maximum bioaccumulation amount of PCBs, phthalic acid esters (PAEs) and phenol by microalgae were detected in 0.5 h-24 h (Halling-S?rensenet al., 2000; Chiet al., 2005; Siet al., 2000). Furthermore, the degradation of HOCs by microalgae increased with time, which may result in the decline of HOCs in microalgae. Therefore, according to Lynnet al. (2007), the exposure time ofS.costatumto BDE-47 was set for 24 h. On the first, third and sixth day of cultivation, 600 mL of algal culture was separated from the flask and inoculated with 0.6 mL BDE-47 working solution (0.2 mg L-1, AccuStandard Inc).S.costatumwas exposed to BDE-47 for 24 h. On Day 2, 4 and 7 of cultivation, the subsamples of BDE-47 were obtained.
2.3 Nutrients, Algal Density and Biochemical Content
About 100 mL algal culture was filtered through a 0.45 μm micro-pore filter membrane every 24 h to measure nitrate and phosphate. Nitrate was measured with the cadmium-copper reduction method (Grasshoff, 1976) and phosphate with phosphomolybdenum blue (Strickland and Parsons, 1972). Another 20 mL algal culture was fixed with Lugol’s solution to determine the algal density.
After the 24 h exposure, 150 mL algal culture was collected onto fiberglass Whatman GF/F filters (pre-combusted at 450℃ for 7 h) to determine biochemical contents including protein, carbohydrate and lipid. They were extracted according to Rausch (1981), Bligh and Dyer (1959). The protein, carbohydrate and lipid were quantified using the Coomassie Brilliant Blue dye-binding method (Bradford, 1976), phenol-sulfuric acid method (Kochert, 1978) and method of Pandeet al. (1963), respectively.
2.4 BDE-47 Analysis
250 mL algal culture was filtered through pre-combusted Whatman GF/F filters. Filters were Soxhlet extracted for 48 h in hexane (chromatographically pure grade) and 50 ng 2,2’,4,4’,5,5’-hexabromodiphenyl ether was added as surrogate standards. Interferences were removed according to Chaiet al. (2013). 0.01 mL of PCB-103 (2,2’,4,5’,6-pentachlorobiphenyl, 1 mg L-1) was added as an internal standard in the final extracts. Instrumental analysis was conducted on a gas chromatograph (GC, Agilent 6890N) equipped with an electron-capture detector and an HP-5 column (30m × 0.25mm i.d., 0.25 μm film thickness). The temperature program and injector temperature were set according to Chaiet al. (2013). The GC was operated in the splitless injection mode.
The recoveries of surrogate standards were 84% ± 8% and BDE-47 data were reported with no surrogate recovery correction.
2.5 Statistical Analysis
The specific growth rate (μ, d-1) was estimated according to Landry and Hassett (1982). The differences were analyzed by ANOVA (LSD test) in whichP< 0.05 was considered to separate significant differences. Pearson’s correlation (α = 0.05) analysis was used to detect correlation. Statistical analyses were carried out with software SPSS 16.0.
3 Results
3.1 Nutrients
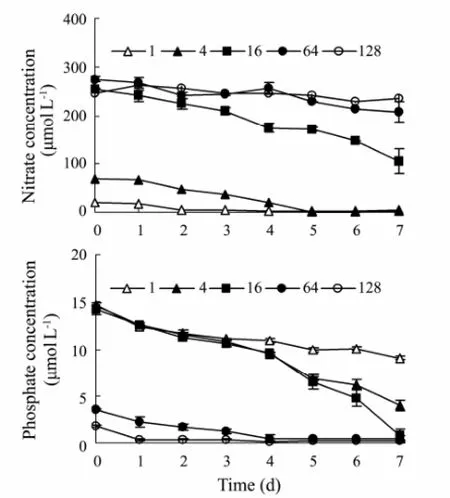
Fig.1 The change of nitrate (a) and phosphate (b) under different N:P ratios during the S. costatum cultivation.
Theoretically, Redfield ratio (N:P=16) is optimal forthe growth of microalgae (Redfieldet al., 1963). If N:P ratio is less than 16, algal growth will tend to be N limited; on the contrary, algal growth tends to be P limited. Besides N:P ratio, Justicet al. (1995) proposed that thresholds for dissolved inorganic nitrogen (DIN) and phosphate of microalgae growth are 1 μmol L-1and 0.1 μmol L-1, respectively.
The concentrations of nitrate or phosphate in all treatments before Day 7 were higher than thresholds, and so the algal cultures were not limited by N or P (Fig.1). On Day 7, nitrate in treatment of N:P=1 was 0.9 μmol L-1while phosphate in N:P=128 was 0.09 μmol L-1, and so the algal cultures in N:P=1 and 128 were limited by nitrogen and phosphorus, respectively. The algal cultures in other treatments on Day 7 were not limited by nutrients.
3.2 Algal Growth
During the early cultivation period (Day 1 - 2), no significant difference in the cell density was found among all treatments (Fig.2a). On Day 3 - 4,S.costatumstayed in the period of fast growth. The specific growth rate was high, ranging from 0.3 d-1to 0.6 d-1on Day 3. At the late stage of cultivation (Day 6 - 7), the cell density in treatments of N:P=4 and 16 was significantly higher than those in other treatments. On Day 7, the specific growth rate was nearly 0.01 d-1in the treatment of N:P=128, but it was in the range of 0.11 - 0.19 d-1in other treatments.
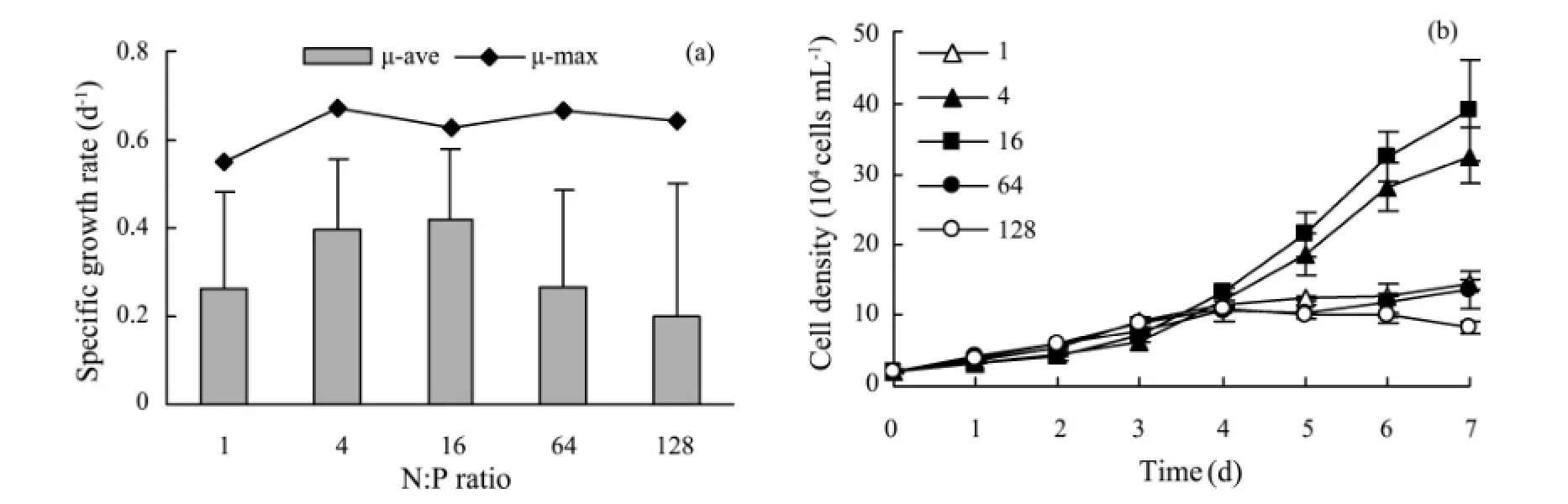
Fig.2 The cell density (a) and specific growth rate (b) of S. costatum under different N:P ratios.
There was no obvious difference in the maximum specific growth rate (μ-max) in all treatments, which was in the range of 0.55 - 0.67 d-1(Fig.2b). However, the average specific growth rate (μ-ave) presented obvious difference, which was largest in the treatment of N:P=16 (0.42 d-1) and least in N:P=128 (0.20 d-1).
3.3 Biochemical Content
On Day 2, the protein content per cell in the treatment of N:P=16 was 0.30 mg (106cells)-1, significantly higher than in other treatments (P<0.05) (Fig.3a). As time was prolonged, the protein content per cell decreased in all treatments. On Day 4, culture in N:P=1 presented the lowest protein level, which was 0.009 mg (106cells)-1. On Day 7, there was not significant difference among all treatments. The protein level per algal volume increased over time (Fig.3b). On Day 7, protein level per volume in N:P=16 was the highest, reaching up to 0.0044 mg mL-1.
The carbohydrate level per cell in N:P=16 on Day 2 (0.02 mg (106cells)-1) was significantly higher than in other ratios (P<0.05) (Fig.4a). The carbohydrate level per cell in all treatments lowered on Day 4, and there was no significant difference among all treatments (P>0.05). On Day 7, the carbohydrate level per cell in N:P=128 rose compared with that on Day 4, and was significantly higher than those in N:P=4 and 16 (P<0.05). Different from carbohydrate level per cell, the carbohydrate level per algal volume rose over time (Fig.4b). On Day 7, the level in N:P=16 reached the highest value.

Fig.3 The protein contents per S. costatum cell (a) and per algal volume of S. costatum (b) under different N:P ratios.
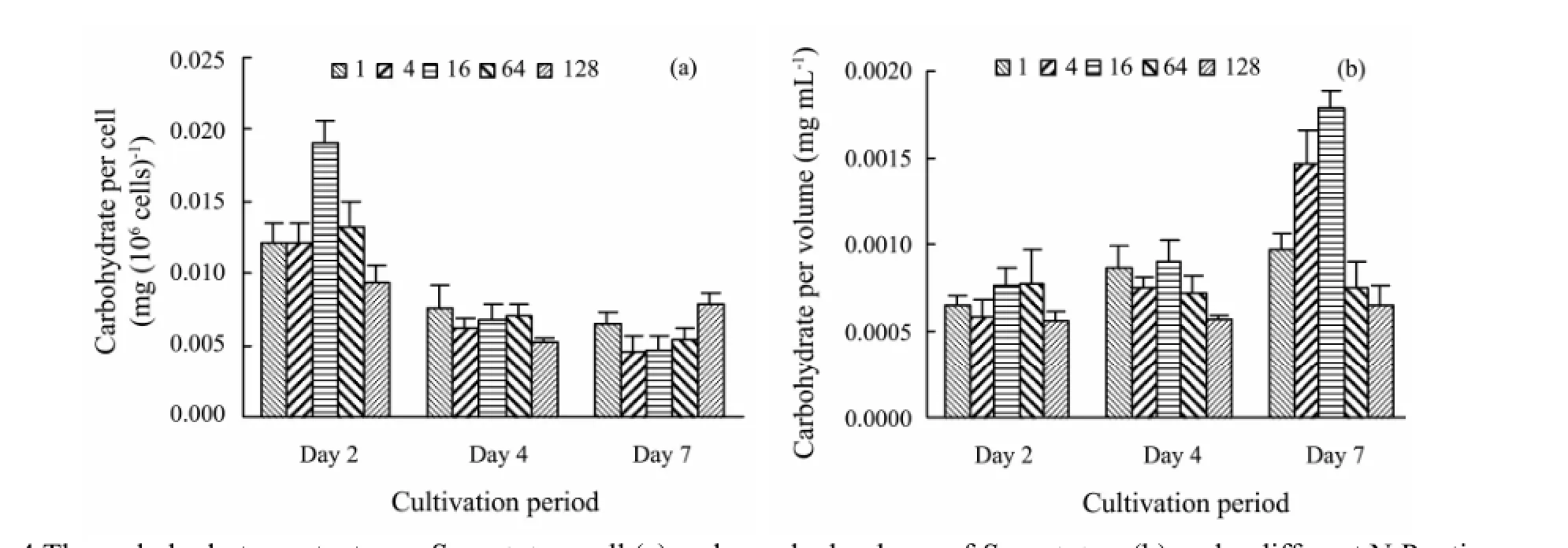
Fig.4 The carbohydrate contents per S. costatum cell (a) and per algal volume of S. costatum (b) under different N:P ratios.
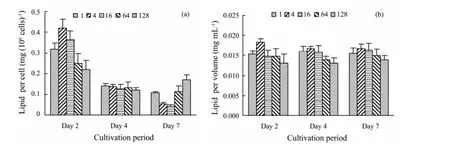
Fig.5 The lipid contents per S. costatum cell (a) and per algal volume of S. costatum (b) under different N:P ratios.
Lipid level per cell presented a similar trend with the carbohydrate (Fig.5a). On Day 2, the treatments of N:P=4 and 16 reached up to 0.42 and 0.36 mg (106cells)-1, respectively, significantly higher than in other treatments (P<0.05). It lowered on Day 4 and no difference existed among all treatments. On Day 7, the lipid levels per cell in N:P=4 (0.05 mg (106cells)-1) and 16 (0.04 mg (106cells)-1) were significantly lower than in other treatments (P<0.05); by comparison, lipid in N:P=128 presented highest value on Day 7. However, no obvious difference in lipid level per algal volume among treatments existed (Fig.5b).
3.4 Bioaccumulation of BDE-47
BDE-47 level per cell inS.costatumvaried with N:P ratios. It was higher in treatments of N:P=4 and 16 on Day 2 with the values 3.8 and 3.7 ng (106cells)-1, respectively; and it was lowest in N:P=128 (Fig.6a). On Day 4, the BDE-47 per cell was in the range of 1.4 - 1.8 ng (106cells)-1, and there was no significant difference among all treatments. On Day 7, the BDE-47 level per cell further reduced in the treatments of N:P=1, 4 and 16 with the values 1.2, 0.6, 0.5 ng (106cells)-1, respectively. However, compared with the level on Day 4, it rose in N:P=128 on Day 7, reaching up to 2.3 ng (106cells)-1. The BDE-47 levels per cell in N:P=4 and 16 on Day 7 were significantly lower than those in other treatments (P<0.05).
The difference in BDE-47 per algal volume among N:P ratios was not obvious (Fig.6b). BDE-47 per volume in the treatments of N:P=128 on Day 2 (0.15 ng mL-1) and on Day 4 (0.16 ng mL-1) was a little lower than those in other treatments. The BDE-47 levels per volume in N:P=4, 16 and 128 presented a slightly increasing trend over time.

Fig.6 The BDE-47 level per S. costatum cell (a) and per algal volume (b) under different N:P ratios.
4 Discussion
Biochemical content of microalgae varies as a function of N:P ratio. High N:P ratio raises protein level (Heraudet al., 2005). Cellular protein content inAlexandrium tamarenseincreases nearly by 30% with the increasing of N:P ratio from 8 to 48 (Murataet al., 2006) and inProrocentrum limait doubles when N:P ratio rises from 1.2 to 24 (Vanucciet al., 2010). Contrary to protein, content of cellular carbohydrate and lipid in microalgae may be reduced by low N:P ratio (Shifrin and Chisholm, 1981; Sigeeet al., 2007). It has been found that content of carbohydrate and lipid inProrocentrum donghaiensein N:P=1.8 is about twice as much as those for N:P=16 (Zhaoet al., 2009). Similarly, the high N:P ratio also results in the increase of carbohydrate and lipid in some algae (Liet al., 2005; Laiet al., 2011). However, responses of biochemical content in microalgae to nutrient ratio are different among species. Lipid increases inBacillariophyceaeandPrymnesiophyceaewith the elevating extent of phosphorus limitation (Reitanet al., 1994), but inN.atomusandTetraselmissp it decreases (Reitanet al., 1994). In this study, all biochemical contents perS.costatumcell presented decreasing trend over cultivation time (Figs.3a, 4a, 5a), which is consistent with the results of Zhaoet al. (2009). However, differences in biochemical contents ofS.costatumfor different nutrient ratios were found at each cultivation period. At early stage of cultivation (Day 2), the cellular biochemical contents inS.costatumpresented higher values in treatments of N:P=4 and 16, but it presented lower values for low or high N:P ratios (Figs.3a, 4a, 5a). Compared with the early stage, the contents of carbohydrate and lipid per cell at the late stage (Day 7) were lower in treatments of N:P=4 and 16, but having higher values in N:P=1 and 128. The variation of biochemical contents may result from the nutrient status which affected the growth ofS.costatum. The average specific growth rates ofS.costatumwere lower in treatments of N:P=1 and 128 (Fig.2b) and growth ofS.costatumwas limited by N or P at the late stage (Fig.1). Due to nutrient limitation resulting from low or high N:P ratio, growth ofS.costatumwas inhibited, which led to the storage of cellular carbohydrate and lipid as energy (Beardallet al., 2001; Zhaoet al., 2009; Laiet al., 2011).
Bioaccumulation of organic compounds in organisms is dependent on the biochemical quantity (Meadoret al., 1995; Arnot and Gobas, 2004; Magnussonet al., 2007). It has been found that the variation of organic compounds accumulated in microalgae can be explained by algal lipid content which is affected by nutrient status (Swackhamer, 1985; Halling-S?rensenet al., 2000; Lynnet al., 2000). Kilham (1998) found a difference in the bioaccumulation of tritiated polychlorinated dibenzofurans (PCDFs) byNitzschiasp. between nutrient treatments. Lynnet al. (2007) reported that uptake of PCB byStephanodiscus minutulusin Si and P limiting treatments is higher than non-limiting or N limiting treatments, due to higher cellular lipid content. In this study, BDE-47 level perS.costatumcell on Day 2 was higher in treatments of N:P=4 and 16. However, the level in treatments of N:P=4 and 16 on Day 7 was lower, but in N:P=1 and 128 it was higher (Fig.6a). This change resulted from the nutrient status and biochemical content. Over the time of cultivation, N and P presented decreasing trend (Fig.1) and their concentrations in the treatments of N:P=1 and 128 on Day 7 were lower than threshold values for growth of microalgae, resulting in the elevating of cellular carbohydrate and lipid (Figs.4a, 5a). Furthermore, BDE-47 quantity accumulated inS.costatumcell increased with the increase of lipid (Fig.7). Correlation analysis indicated that the BDE-47 per cell was positively correlated with protein (r= 0.800,P< 0.01), carbohydrate (r= 0.903,P< 0.01) and lipid (r= 0.976,P< 0.01) per cell (Table 1).
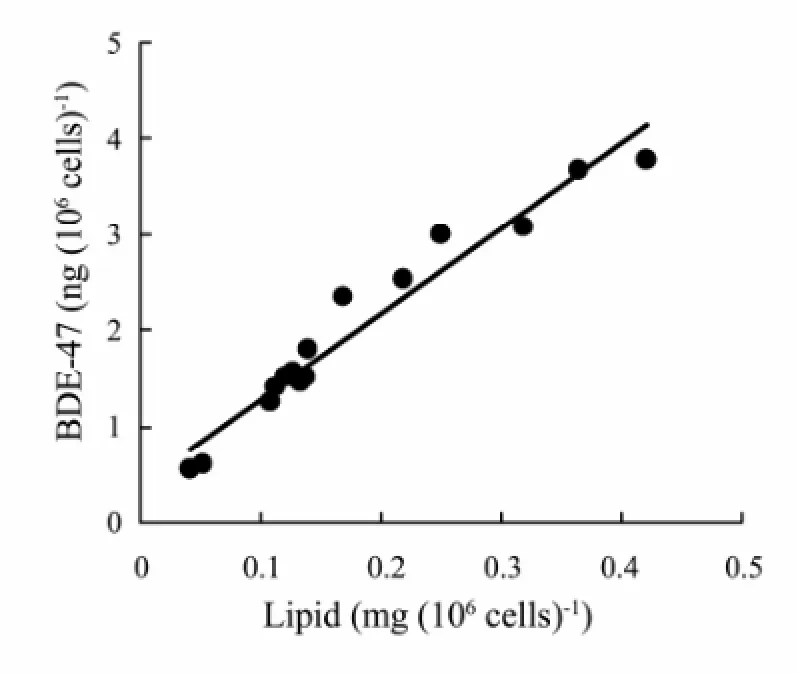
Fig.7 The relationship between BDE-47 quantity and lipid in per S. costatum cell.
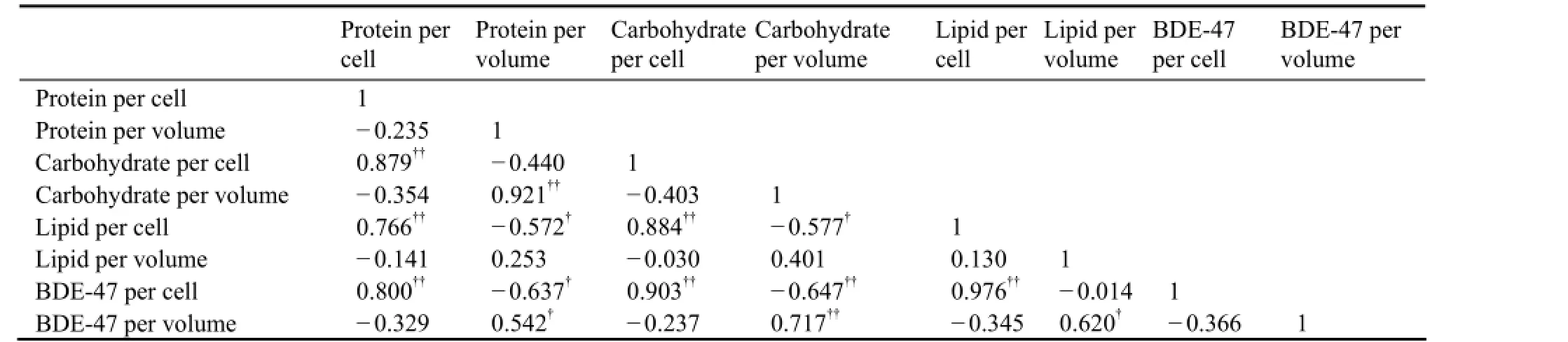
Table 1 Pearson correlations among BDE-47 and biochemical contents in Skeletonema costatum
As for BDE-47 levels per algal volume, there was no obvious difference among N:P ratios (Fig.6b). This result is similar with PCB uptake byStephanodiscus minutulusin various nutrient regimes (Lynnet al., 2007). This may result from the difference of cell density in the cultures. For example, on Day 7, cell density in treatment of N:P = 128 was lower (Fig.2a) but the accumulated BDE-47 per cell was higher due to higher lipid (Fig.5a). Therefore, this balance did result in obvious difference in the uptake of BDE-47 in algal volume among N:P ratios. This pattern was also found in the variation of lipid between calculated results per cell and per volume (Fig.5a, b). Pearson correlation indicated that there was significantly positive correlation between lipid and BDE-47 per volume ofS.costatum(Table 1). Therefore, it can be concluded that N:P ratios bring about the biochemical variation which induces the change of bioaccumulation of PBDEs inS.costatum.
Besides the lipid content, the variation of lipid composition may result in the change of bioaccumulation of organic compounds by algae. Lipid in algae includes neutral lipid, glycolipid and phospholipid (Denget al., 2011). It was reported that the BCFs of PCBs normalized in total lipid, neutral lipid or polar lipid inSelenastrum capricornutumare different (Halling-S?rensenet al., 2000). The changes of lipid composition may also be explained by nutrient status. N limitation has been found to elevate neutral lipid in Chlorella (Illmanet al., 2000; Merzlyaket al., 2007). P limitation induces the rising of neutral lipid of eustigmatophyte, but decline of phospholipid (Khozin-Goldberg and Cohen, 2006). On the contrary, the decrease of neutral lipid has been found inNannochloris atomusandTetraselmissp in P limiting media (Reitanet al., 1994). In this study, the BDE-47 per weight of lipid inS.costatumpresented a slight difference among N:P ratios (Fig.8). It was a little higher in the treatment of N:P=128, and lower in N:P=4. The bioaccumulation of PBDEs byS.costatummay be affected by its lipid composition. However, few researches have been about the lipid composition ofS.costatumunder different nutrient ratios, and further research is needed.
S.costatumis a dominant red tide alga in Chinese coastal waters and nearly 10 times of algal blooms have been caused byS.costatumannually in recent years (SOA, 2008, 2009, 2010). Besides, high N:P ratio has been found in the coastal area of China, resulting in microalgae growth being generally limited by phosphorus (Chaiet al., 2009). In this study, it was found that the bioaccumulation amount of PBDEs byS.costatumcell increased under high N:P ratio. Therefore, the bloom ofS.costatummay be helpful for the removal of PBDEs in China’s marine waters. This study provides the valuable information of variation of PBDEs behavior due to microalgae under different nutrient conditions.

Fig.8 The BDE-47 content per weight of lipid in S. costatum under different N:P ratios.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 40906061), the Science and Technology Plan Projects of Qingdao (No. 12-1-3-64-nsh), the ‘Two Districts’ Foundation of Shandong Province, China (No. 2011-Yellow-19) and the Talent Foundation of Qingdao Agricultural University (No. 630642).
Arnot, J. A., and Gobas, F. A. P. C., 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environmental Toxicology and Chemistry, 23 (10): 2343-2355.
Beardall, J., Young, E., and Roberts, S., 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences, 63 (1): 44-69.
Bligh, E. G., and Dyer, W. J., 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37: 911-917.
Borghesi, N., Corsolini, S., and Focardi, S., 2008. Levels of polybrominated diphenyl ethers (PBDEs) and organochlorine pollutants in two species of Antarctic fish (Chionodraco hamatus and Trematomus bernacchii). Chemosphere, 73 (2): 155-160.
Bradford, M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Analytical Biochemistry, 72: 248-254.
Carlson, D. L., and Swackhamer, D. L., 2006. Results from the US Great Lakes fish monitoring program and effects of lake processes on bioaccumulative contaminant concentrations. Journal of Great Lakes Research, 32 (2): 370-385.
Chai, C., Yin, X. D., Ge, W., and Wang, J. Y., 2013. Effects of nitrogen and phosphorus concentrations on the bioaccumulation of polybrominated diphenyl ethers by Prorocentrum donghaiense. Journal of Environmental Sciences, 25 (2): 376-385.
Chai, C., Yu, Z. Y., Shen, Z. L., Song, X. X., Cao, X. H., and Yao, Y., 2009. Nutrient characteristics in the Yangtze River Estuary and the adjacent East China Sea before and after impoundment of the Three Gorges Dam. Science of the Total Environment, 407: 4687-4695.
Chi, J., Li, J. J., and Liu, H., 2005. Kinetics of accumulation and biodegradation of di(2-Ethylhexyl) phthalate in Chlorella Vulgaris. Journal of Tianjin University, 38 (5): 422-425 (in Chinese with English abstract).
De Wit, C. A., Alaee, M., and Muir, D. C. G., 2006. Levels and trends of brominated flame retardants in the Arctic. Chemosphere, 64 (2): 209-233.
Deng, X. D., Fei, X. W., and Li, Y. J., 2011. The effects of nutritional restriction on neutral lipid accumulation in Chlamy-domonas and Chlorella. African Journal of Microbiology Research, 5 (3): 260-270.
Fontana, A. R., Silva, M. F., Martinez, L. D., Wuilloud, R. G., and Altamirano, J. C., 2009. Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography-mass spectrometry. Journal of Chromatography A, 1216 (20): 4339-4346.
Grasshoff, K., 1976. Methods of Seawater Analysis. Verlag Chemie, Weinheim and New York, 12-34.
Guan, Y. F., Samuel Sojinu, O. S., Li, S. M., and Zeng, E. Y., 2009. Fate of polybrominated diphenyl ethers in the environment of the Pearl River Estuary, South China. Environmental Pollution, 157: 2166-2172.
Guo, J. Y., Wu, F. C., Mai, B. X., Luo, X. J., and Zeng, E.Y., 2007. Polybrominated diphenyl ethers in seafood products of South China. Journal of Agricultural and Food Chemistry, 55: 9152-9158.
Halling-S?rensen, B., Nyholm, N., Kusk, K. O., and Jacobsson, E., 2000. Infuence of nitrogen status on the bioconcentration of hydrophobic organic compounds to Selenastrum capricornutum. Ecotoxicology and Environmental Safety, 45 (1): 33-42.
Heraud, P., Wood, B. R., Tobin, M. J., Beardall, J., and McNaughton, D., 2005. Mapping of nutrient-induced biochemical changes in living algal cells using synchrotron infrared microspectroscopy. FEMS Microbiology Letters, 249: 219-225.
Hites, R. A., 2004. Polybrominated diphenylethers in the environment and in people: A meta-analysis of concerntrations. Environmental Science and Technology, 38: 945-956.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., and Darzins, A., 2008. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant Journal, 54 (4): 621-639.
Illman, A. M., Scragg, A. H., and Shales, S. W., 2000. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology, 27 (8): 631- 635.
Justic, D., Rabalais, N. N., Turner, R. E., and Dortch, Q., 1995. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuarine, Coastal and Shelf Science, 40 (3): 339-356.
Khozin-Goldberg, I., and Cohen, Z., 2006. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry, 67 (7): 696-701.
Kilham, S. S., 1998. Effects of physiological state on the bioaccumulation of toxic chemicals in algae and their transfer to zooplankton. Verhandlungen-Internationale Vereinigung für Theoretische und Angewandte Limnologie, 26 (4): 1734-1736.
Kochert, G., 1978. Carbohydrate determination by phenol-sulfuric acid method. In: Handbook of Physiological and Biochemical Methods. Hellebust, J. A., and Craige, J. S., eds., Cambridge University Press, London, 512pp.
Lai, J. X., Yu, Z. M., Song, X. X., Cao, X. H., and Han, X. T., 2011. Responses of the growth and biochemical composition of Prorocentrum donghaiense to different nitrogen and phosphorus concentrations. Journal of Experimental Marine Biology and Ecology, 405: 6-17.
Landry, M. R., and Hassett, R. P., 1982. Estimating the grazing impact of marine microzooplankton. Marine Biology, 67 (3): 283-288.
Law, R. J., Allchin, C. R., de Boer, J., Covaci, A., Herzke, D., Lepom, P., Morris, S., Tronczynski, J., and De Wit, C. A., 2006. Levels and trends of brominated flame retardants in the European environment. Chemosphere, 64 (2): 187-208.
Li, M., Gong, R., Rao, X., Liu, Z. H., and Wang, X., 2005. Effects of nitrate concentration on growth and fatty acid composition of the marine microalgae Pavlova viridis (Prymnesiophyceae). Annals of Microbiology, 55: 51-55.
Li, X., Hu, H. Y., Gan, K., and Sun, Y. X., 2010. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresource Technology, 101 (14): 5494-5500.
Luo, X. J., Yu, M., Mai, B. X., and Chen, S. J., 2008. Distribution and partition of polybrominated diphenyl ethers (PBDEs) in water of the Zhujiang River Estuary. Chinese Science Bulletin, 53 (4): 493-500.
Lynn, S. G., Kilham, S. S., Kreeger, D. A., and Interlandi, S. J., 2000. Effect of nutrient availability on the biochemical and elemental stoichiometry in the freshwater diatom Stephanodiscus minutulus (Bacillariophyceae). Journal of Phycology, 36: 510-522.
Lynn, S. G., Price, D. J., Birge, W. J., and Kilham, S. S., 2007. Effect of nutrient availability on the uptake of PCB congener 2,2’,6,6’-tetrachlorobiphenyl by a diatom (Stephanodiscus minutulus) and transfer to a zooplankton (Daphnia pulicaria). Aquatic Toxicology, 83 (1): 24-32.
Magnusson, K., Magnusson, M., ?stberg, P., Granberg, M., and Tiselius, P., 2007. Bioaccumulation of 14C-PCB 101 and 14C-PBDE 99 in the marine planktonic copepod Calanus finmarchicus under different food regimes. Marine Environmental Research, 63 (1): 67-81.
Meador, J. P., Stein, J. E., Reichert, W. L., and Varanasi, U., 1995. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Reviews of Environmental Contamination and Toxicology, 143: 79-165.
Meng, X. Z., Yu, L. P., Guo, Y., Mai, B. X., and Zeng, E. Y., 2008. Congener-specific distribution of polybrominated diohenyl ethers in fish of China: Implication for input sources. Environmental Toxicology and Chemistry, 27: 67-72.
Merzlyak, M. N., Chivkunova, O. B., Gorelova, O. A., Reshetnikova, I. V., Solovchenko, A. E., Khozin-Goldberg, I., and Cohen, Z., 2007. Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). Journal of Phycology, 43 (4): 833-843.
Murata, A. I., Leong, S. C. Y., Nagashima, Y., and Taguchi, S., 2006. Nitrogen: Phosphorus supply ratio may control the protein and total toxin of dinoflagellate Alexandrium tamarense. Toxicon, 48: 683-689.
Pande, S. V., Parvin, R., and Venkitasubramanian, T. A., 1963. Microdetermination of lipids and serum total fatty acids. Analytical Biochemistry, 6: 415-425.
Rahman, F., Langford, K. H., Scrimshaw, M. D., and Lester, J. N., 2001. Polybrominated diphenyl ether (PBDE) flame retardants. Science of the Total Environment, 275 (1-3): 1-17.
Rausch, T., 1981. The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia, 78: 237-251.
Redfield, A. C., Ketchum, B. H., and Richards, F. A., 1963. The influence of organism on the composition of seawater. In: The Sea. Hill, M. N., ed., John Wiley, New York, 26-77.
Reitan, K. I., Rainuzzo, J. R., and Olsen, Y., 1994. Effect ofnutrient limitation on fatty acid and lipid content of marine microalgae. Journal of Phycology, 30 (6): 972-979.
Shifrin, N. S., and Chisholm, S. W., 1981. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. Journal of Phycology, 17: 374-384.
Si, Y. B., Yue, Y. D., Wu, Z. P., Wang, R. Y., and Deng, D. P., 2000. Bioaccumulation and biodegration of phenol by the algae Microcystis aeruginosa kutz. Journal of Anhui Agricultural University, 27 (3): 269-271 (in Chinese with English abstract).
Sigee, D. C., Bahrami, F., Estrada, B., Webster, R. E., and Dean, A. P., 2007. The influence of phosphorus availability on carbon allocation and P quota in Scenedesmus subspicatus: A synchrotron-based FTIR study. Phycologia, 46: 583-592.
State Ocean Administration (SOA), 2008, 2009, 2010. Bulletin of Marine Environmental Quality of China, 1-56 (in Chinese).
Strickland, J. D. H., and Parsons, T. R., 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada Bulletin, 167: 311.
Suzuki, G., Nose, K., Takigami, H., Takahashi, S., and Sakai, S. I., 2006. PBDEs and PBDD/Fs in house and office dust from Japan. Organohalogen Compounds, 68: 1843-1846.
Swackhamer, D. L., 1985. The role of water particle partitioning and sedimentation in controlling the fate and transport of PCBs in lakes. PhD thesis, University of Wisconsin, Madison, WI, 259pp.
Vanucci, S., Guerrini, F., Milandri, A., and Pistocchi, R., 2010. Effects of different levels of N- and P-deficiency on cell yield, okadaic acid, DTX-1, protein and carbohydrate dynamics in the benthic dinoflagellate Prorocentrum lima. Harmful Algae, 9 (6): 590-599.
Wang, Y. W., Jiang, G. B., Lam, P. K. S, and Li, A., 2007. Polybrominated diphenyl ether in the East Asian environment: A critical review. Environment International, 33 (7): 963-973.
Zhao, Y. F., Yu, Z. M., Song, X. X., and Cao, X. H., 2009. Biochemical compositions of two dominant bloom-forming species isolated from the Yangtze River Estuary in response to different nutrient conditions. Journal of Experimental Marine Biology and Ecology, 368 (1): 30-36.
(Edited by Ji Dechun)
(Received January 4, 2013; revised October 5, 2013; accepted November 11, 2013)
? Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-88030461
E-mail: chaichao1999@126.com
 Journal of Ocean University of China2014年3期
Journal of Ocean University of China2014年3期
- Journal of Ocean University of China的其它文章
- Effects of Sulfate Chitosan Derivatives on Nonalcoholic Fatty Liver Disease
- Preference of the Herbivorous Marine Teleost Siganus canaliculatus for Different Macroalgae
- Effect of Temperature on Gene Expression in the Pearl Oyster Pinctada fucata
- Nitrogen and Phosphorus Budget of a Polyculture System of Sea Cucumber (Apostichopus japonicus), Jellyfish (Rhopilema esculenta) and Shrimp (Fenneropenaeus chinensis)
- Structure of Mitochondrial DNA Control Region of Pholis fangi and Its Phylogenetic Implication
- Fishery Biology of Jumbo Flying Squid Dosidicus gigas off Costa Rica Dome
