Effects of Sulfate Chitosan Derivatives on Nonalcoholic Fatty Liver Disease
YU Mingming, WANG Yuanhong, JIANG Tingfu, and LV Zhihua
Key Laboratory of Marine Drugs, Ministry of Education, Shandong Provincial Key Laboratory of Glycoscience Glycotechnolygy, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
Effects of Sulfate Chitosan Derivatives on Nonalcoholic Fatty Liver Disease
YU Mingming, WANG Yuanhong, JIANG Tingfu, and LV Zhihua*
Key Laboratory of Marine Drugs, Ministry of Education, Shandong Provincial Key Laboratory of Glycoscience Glycotechnolygy, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
Sulfate chitosan derivatives have good solubility and therapeutic effect on the cell model of NAFLD. The aim of this study was to examine the therapeutic effect of sulfate chitosan derivatives on NAFLD. The male Wistar rats were orally fed high fat emulsion and received sulfate chitosan derivatives for 5 weeks to determine the pre-treatment effect of sulfate chitosan derivatives on NAFLD. To evaluate the therapeutic effect of sulfate chitosan derivatives on NAFLD, the rats were orally fed with high concentration emulsion for 5 weeks, followed by sulfate chitosan derivatives for 3 weeks. Histological analysis and biomedical assays showed that sulfate chitosan derivatives can dramatically prevent the development of hepatic steatosis in hepatocyte cells. In animal studies, pre-treatment and treatment with sulfate chitosan derivatives significantly protected against hepatic steatohepatitis induced by high fat diet according to histological analysis. Furthermore, increased TC, ALT, MDA, and LEP in NAFLD were significantly ameliorated by pre-treatment and treatment with sulfate chitosan derivatives. Furthermore, increased TG, AST, and TNF-α in NAFLD were significantly ameliorated by treatment with sulfate chitosan derivatives. Sulfate chitosan derivatives have good pre-treatment and therapeutic effect on NAFLD.
NAFLD; sulfate chitosan derivatives; histological analysis; hepatocyte cells; rats
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of abnormal liver dysfunction in the clinical setting. NAFLD involves a wide spectrum of liver disease, ranging from fatty liver alone to steatohepatitis, steatonecrosis, and nonalcoholic steatohepatitis (NASH) (Matteoniet al., 1999; Maet al., 2007). The overall prevalence of NAFLD is estimated to be 20%-30% in the general population (D Kimet al., 2012), and it has become the most common cause of chronic liver disease and liver transplantation in western countries (Nakaoet al., 2006).
The metabolism involved in the induction and progression of NAFLD is very complex. Although obesity, insulin resistance, diabetes, and hypertriglyceridemia are often associated with NAFLD, the pathogenesis of NAFLD and its progression to fibrosis are still unclear (Mastertonet al., 2010; Pettaet al., 2009). The treatments for NAFLD currently focuses on decreasing metabolic risk factors, with therapy mainly targeting lifestyle adaptations such as gradual weight loss by diet and exercise (Mattaret al., 2005; Rafiq and Younossi, 2008). However, there is no single intervention that has convincingly improved liver histology.
Chitin is the second-most abundant biopolymer in nature, widely distributed in the shell of crustaceans, the cuticles of insects, and the cell walls of fungi. Chitosan is a non-toxic, biocompatible polymer that has found a number of applications in drug delivery including that of an absorption enhancer of hydrophilic macromolecular drugs (Kimet al., 2008; Kumar, 2000). Chitosan derivatives have been developed to overcome chitosan’s limited solubility and effectiveness as an absorption enhancer at neutral pH values such as those found in the intestinal tract (Khor and Lim, 2003). Chitin can be synthesized to get sulfate chitosan derivatives (β-(1, 4) polyglycosamine-3-O-sulfate-6-O-sulfate-6′-O-carboxyl-methyl ether sodium, Fig.1) by molecular modification. Sulfate chitosan derivatives have been synthesized by our lab as anti-atherosclerotic drug candidate in clinic study in China. Previous studies showed that sulfate chitosan derivatives have good effect on alcoholic fatty liver (YUet al., 2010). However, the potential therapeutic effect of sulfate chitosan derivatives on NAFLD has not been studied.
The aim of this study is to examine the therapeutic effect of the sulfate chitosan derivatives synthesized by our lab and to investigate the possible mechanisms of sulfate chitosan derivatives in the prevention of steatohepatitis.
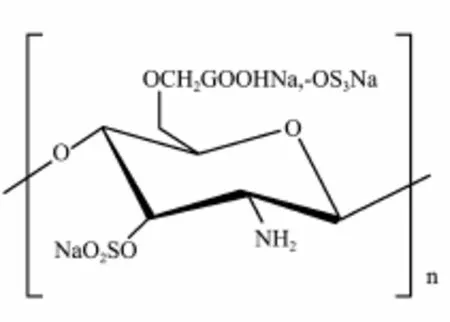
Fig.1 Chemical structure of sulfate chitosan derivatives.
2 Materials and Methods
2.1 Cell Culture and Fat-Overloading Induction of L-02
Human hepatocytes (L-02) (China Cell Culture Center, Shanghai, China) were grown in RPMI 1640 culture medium containing 10% fetal bovine serum in a humidified atmosphere of 5% CO2at 37℃ until the cells reached 90% confluence (Wanget al., 2010). Before each experiment, human hepatocytes were cultured in RPMI culture medium for cell cycle synchronization (Jinet al., 2009). After cell cycle synchronization, the human hepatocytes (L-02) were divided into six groups, which were the control group, model group, positive drug group, and treatment groups (low-dose group, middle-dose group, and high-dose group) (n=6), and each group had 2×105cells. The control group was cultured in RPMI culture medium containing 10% fetal bovine serum, and the others were cultured in RPMI culture medium containing 50% fetal bovine serum for 24 h to induce fat-overloading of cells. In the next 24 h, all groups were cultured in RPMI culture medium containing 10% fetal bovine serum, the positive drug group received silymarin (20 μg mL-1) (Madaus AG), and the treatment groups were treated with sulfate chitosan derivatives (5, 25, and 100 μg mL-1) (Key Laboratory of Marine Drugs, Ocean University of China). The cells from each group were stained with Oil Red O for microscopic photography. For the triglyceride (TG) assay, cultured cells were scraped in PBS, and then centrifuged at 3000 r min-1for 10 min (Taylor and Harker, 2006). Supernatants were subsequently collected for TG content assay.
2.2 Rat Model of NAFLD
Male Wistar rats (180 - 220 g, from Lukang Pharmaceutical Group Co. Ltd. of Shandong, China) were maintained under controlled temperature conditions of 20℃with a 12-hour light/dark cycle and free access to normal chow and water. Our studies were carried out following the guidelines of the Qingdao Committee for care and use of laboratory animals. The rats were orally fed 10mLkg-1high fat emulsion once per day for 5 weeks to induce NAFLD (Zouet al., 2006). The high fat emulsion composition contains lard 25%, cholesterol 10%, sodium cholate 3%, propylthiouracide 0.4%, propanediol 20%, polysorbate 80 25%, distilled water 16.6% (Denget al. 2009; G?beleet al., 2011; Unoet al., 2008).
2.3 Pre-Treatment and Treatments
Rats were randomly divided into four groups (10 rats per group). Rats in the control group were fed normal chow; rats in the model group received physiological saline (2 mL kg-1) with high fat emulsion; rats in the positive drug group received silymarin (20 mL kg-1) with high fat emulsion; rats in the pretreatment group received sulfate chitosan derivatives (100 mg kg-1) with fed high fat emulsion for five weeks.
Rats were randomly divided into six groups (10 rats per group). Rats in five groups were orally fed high fat emulsion for five weeks to induce NAFLD. Over the same period, the rats in the control group were fed normal chow. After five weeks, rats in all groups were fed normal chow for three weeks. Over the same period, rats in model group received physiological saline (2 mL kg-1); rats in positive drug group received silymarin (20 mg kg-1); rats in the treatment groups (low-dose, middle-dose, and high-dose group) received sulfate chitosan derivatives (25, 50, and 100 mg kg-1) by intragastric administration for three weeks.
After experiment, the rats were sacrificed by decapitation. Serum samples were collected for chemical assay and the livers were fixed in 10% formalin for histological analysis.
2.4 Histology Analysis
The formalin-fixed livers from rats were processed according to the routine techniques, and 5 μm thick paraffin sections were stained with hematoxylin and eosin for histological analysis (Tevaret al., 2011; Rosselliet al., 2009).
2.5 Samples Analysis
Serum total cholesterol (TC), triglyceride (TG), highdensity lipoprotein (HDL-C), low-density lipoprotein (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and liver homogenate malondialdehyde (MDA) were assayed by commercial analysis kits (Jiancheng Institute of Biotechnology, Nanjing, China). Serum leptin (LEP) and tumor necrosis factor alpha (TNF-α) were determined by enzyme-linked immunosorbent assay (R&D Systems, United States) (Ohet al., 2011; Mancoet al., 2007).
2.6 Statistical Analysis
All measured values were shown as means or means ± standard error of the mean (SEM). Data was analyzed by one-way analysis of variance andP< 0.05 was considered statistically significant.
3 Results
3.1 Effects of Sulfate Chitosan Derivatives on Fatoverloading in Hepatocytes

Fig.2 Effect of treatment with sulfate chitosan derivatives on fat-overloading hepatocytes. Oil Red O stained cells: Control group shows few droplets; model group shows significant amount of fat droplets (red in figure) compared to control group; Positive drug group shows less fat droplets than model group; Low-dose group (5 μg mL-1sulfate chitosan derivatives) shows less fat droplets than model group; Middle-dose group (25 μg mL-1sulfate chitosan derivatives) shows less fat droplets than low-dose group; High-dose group (100 μg mL-1sulfate chitosan derivatives) shows similar amount of fat droplets to control group (original magnification, ×100).

Fig.3 Comparison of triglyceride (TG) level in hepatocytes treated with saline and different doses of sulfate chitosan derivatives. Positive drug group received silymarin (20 mg kg-1); low-dose, middle-dose, and high-dose groups received sulfate chitosan derivatives 25, 50, and 100 mg kg-1(n=10).
The fat-overloading model that can be well-represented by the cell characteristic of NAFLD was successfully established by treating hepatocytes with 50% fetal bovine serum in 24 h. As shown from the Oil Red O staining, there was a significant increase in neutral fat droplets within hepatocytes in the 50% fetal bovine serum-treated hepatocytes (model group) compared to the cells cultured with 10% fetal bovine serum (control group); and there was a marked decrease in neutral fat droplets within the sulfate chitosan derivative treated hepatocytes (treatment group) compared to the cells in the model group (Fig.2). The amount of triglycerides (TG) increased significantly in model group compared with control group (P< 0.05), while the level of TG dropped significantly in three-dose treatment groups (5, 25, and 100 μgmL-1sulfate chitosan derivatives) compared with model group indicating dose dependency (Fig.3).
3.2 Effects of Pre-Treatment Effects of Sulfate Chitosan Derivatives on NAFLD Induced by High Fat Emulsion in Rats
3.2.1 Liver index
Liver index is the ratio between liver weight and body weight. The liver index of rats in model increased significantly compared with rats in control group, and there was a significantly decrease in liver index in sulfate chitosan derivatives treated (treatment group) compared with model group (Table 1).

Table 1 Values of liver index in groups (n =10)
3.2.2 Histology of liver samples
Steatosis affected numerous hepatocytes causing diffuse ballooning and inflammatory cell infiltration in model group. The presence of diffuse ballooning and inflammatory infiltrate were significantly reduced by sulfate chitosan derivatives compared to model group (Fig.3). Rats that received sulfate chitosan derivatives have less fat droplets than those that received silymarin (positive drug) (Fig.4).
3.2.3 Serum biochemical parameters
The levels of serum TC, ALT, MDA, and LEP were significantly raised in the rats fed with HFD emulsion (treatment group) compared to the control group (Fig.5). The levels of serum TC, ALT, MDA, and LEP were significantly decreased in the pre-treatment group that received sulfate chitosan derivatives compared to the model group (Fig.5).
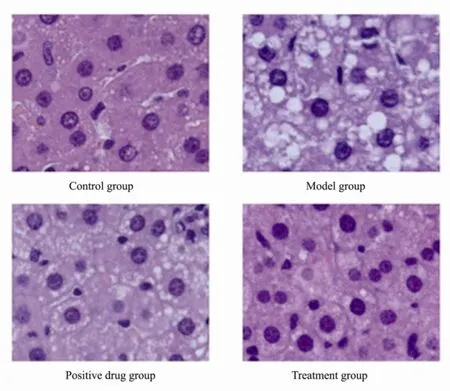
Fig.4 Hematoxylin and eosin-stained liver sections from control group, model group, positive drug group, and treatment group (Original magnification × 400).
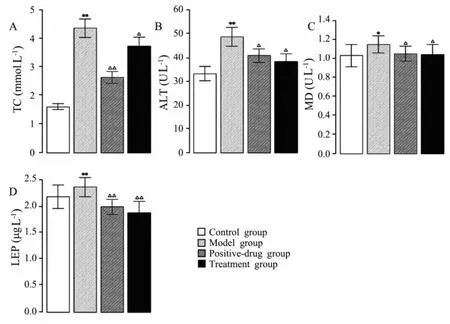
Fig.5 Results of TC (A), ALT (B), MDA (C) and LEP (D) detection from rats fed normal chow (control group), rats fed high fat emulsion (model group), rats fed high fat emulsion and received silymarin (positive-drug group), and rats fed high fat emulsion and received sulfate chitosan derivatives (treatment group, 100 mg kg-1) (n=10). Compared with the control group, *P < 0.05; **P < 0.01. Compared with model group, △P< 0.05; △△P < 0.01.
3.3 Therapeutic Effects of Sulfate Chitosan Derivatives on NAFLD Induced by High Fat Emulsion in Rats
3.3.1 Histology of liver samples
Steatosis affected hepatocytes, with diffuse ballooning and inflammatory cell infiltration in model group. Larger number of hepatocytes were affected by steatosis with diffuse ballooning and inflammoatory cell infiltration in the model group compared to the control group. The presence of diffuse ballooning and inflammatory infiltrate was markedly reduced by sulfate chitosan derivatives. There were notably fewer fat-droplets in rats that received high-dose sulfate chitosan derivatives (Fig.6).
3.3.2 Serum biochemical parameters
As shown in Fig.7, the levels of serum TC, TG, LDL, AST, ALT, and MDA were significantly raised, while that of HDL was significantly reduced in model group compared to control group. The levels of serum TC, TG, AST, and MDA were significantly lowered in treatment groups that received sulfate chitosan derivatives, and those of LDL and ALT were significantly lowered in both middle-dose and high-dose groups compared to the model group. The level of HDL was significantly raised in high-dose group compared to the model group.
The levels of serum LEP and TNF-α were significantly raised in model group (Fig.8). The levels of serum LEPand TNF-α were significantly lowered in the treatment groups that received sulfate chitosan derivatives.
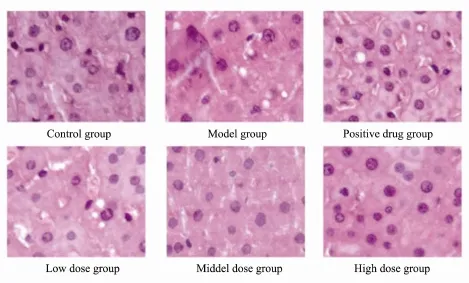
Fig.6 Hematoxylin and eosin-stained liver sections from control group, model group, positive drug group, and treatment groups (Low-dose, middle-dose, and high-dose group) (Original magnification × 400).
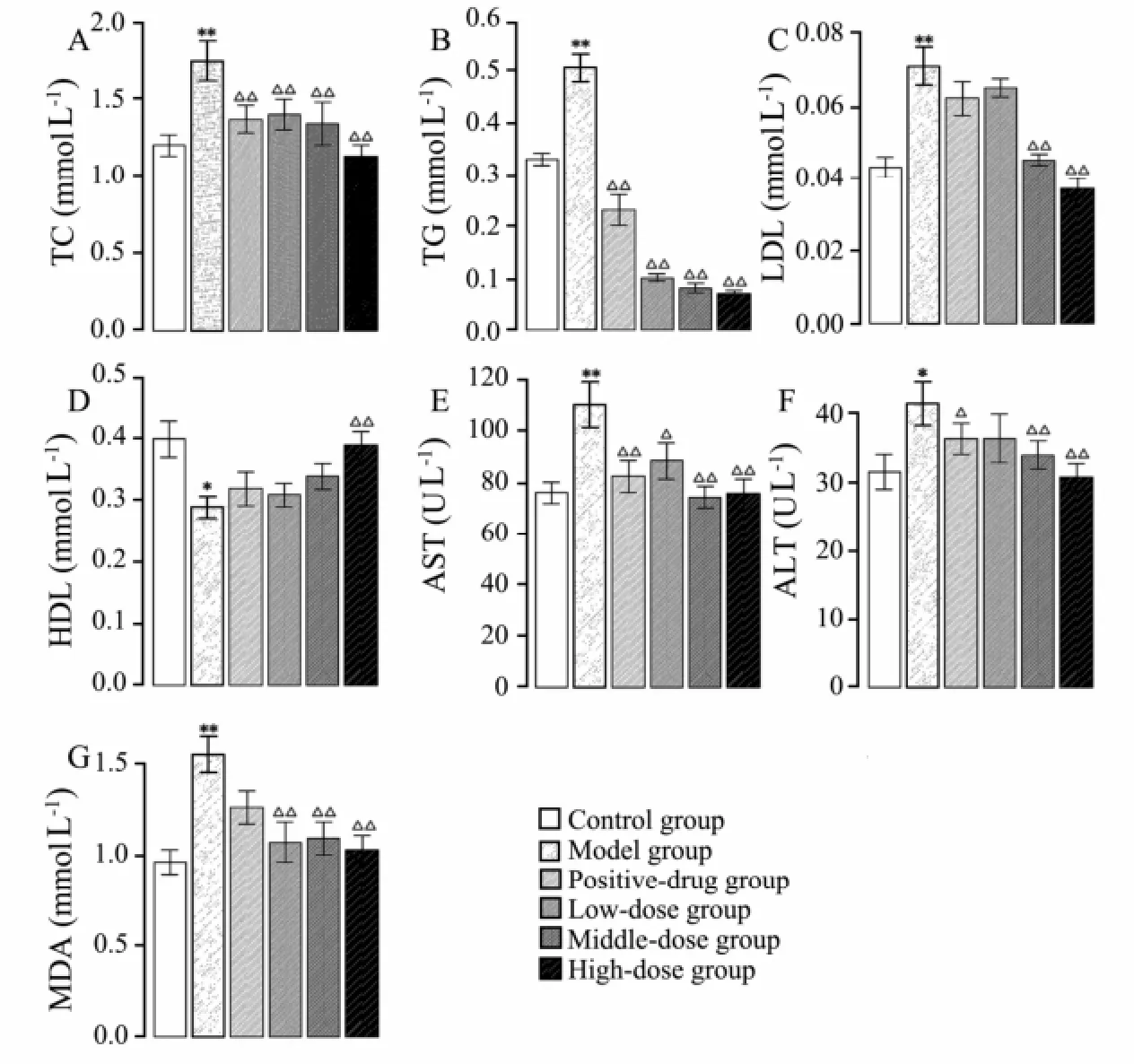
Fig.7 Levels of TC (A), TG (B), LDL (C), HDL (D), AST (E), ALT (F), and MDA (G) detected from rats in control group, model group, and positive-drug group that received silymarin; low-dose, middle-dose, and high-dose groups received sulfate chitosan derivatives (n=10). *P < 0.05; **P < 0.01. Compared with model group, △P< 0.05; △△P < 0.01.
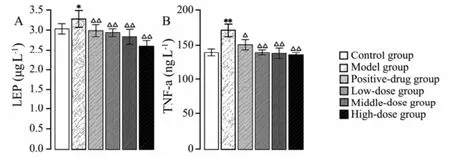
Fig.8 Levels of LEP (A) and TNF-α (B) detected from rats in control group, model group, positive-drug group treated with silymarin; low-dose, middle-dose, and high dose groups treated with sulfate chitosan derivatives (n=10). Compared to the control group, *P < 0.05; **P < 0.01. Compared with model group, △P< 0.05; △△P < 0.01.
4 Discussion
In this study, a new rat model of NAFLD was successfully established by feeding rats with high-fat emulsion (Denget al., 2009; G?beleet al., 2011; Unoet al., 2008). NAFLD model reproduced several typical hepatic lesion of NAFLD, such as steatosis, hyperlipidemia, and inflammation. In this model, the changes in ALT, AST, MDA, LEP, and TNF-α showed similar patterns in patients with NAFLD (Mancoet al., 2007). The rat model of NAFLD was used to explore the preventive effect of sulfate chitosan derivatives therapy on NAFLD. The results of liver histology and serum parameter assay demonstrated that pre-treatment and treatment with sulfate chitosan derivatives markedly improved hepatic steatosis, hepatocyte ballooning, and hyperinsulinemia. Some studies show that increased triglycerides (TG) is associated with NAFLD (Fabbriniet al., 2010). Our results indicate that sulfate chitosan derivatives can significantly lower the level of TG in L02 cells with fat-overloading and rats with NAFLD.
The alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are makers of hepatic steatosis or hepatic dysfunction related to liver fat accumulation(Ohet al., 2011; Clarket al., 2003). The proinf l ammatory and prof i brogenic effects of the aldehyde end products of lipid peroxidation (MDA and 4-hydroxynonenol) can account for all of the typical histologic features observed in NAFLD (Browning and Horton, 2004). Our findings also reveal that treatment with sulfate chitosan derivatives significantly improves liver function as demonstrated by the observed reduction in ALT, AST and MDA in rats with NAFLD.
Leptin (LEP) and TNF-α as adipokines have a strong association with histologic features of NAFLD, and TNF-α overexpression will induce inflammation and fibrosis (Zouet al., 2006; Mancoet al., 2007). Leptin known as a long-term energy store factor plays a key role in the progression of fibrogenesis and carcinogenesis in NASH (Kitadeet al., 2006). Our results show that sulfate chitosan derivatives can significantly reduce the levels of serum TNF-α and leptin to improve liver function.
In conclusion, the rat model of NAFLD has been successfully established in this study. By using this model, we demonstrate that sulfate chitosan derivatives have a therapeutic effect on NAFLD. The results also suggest that the therapeutic effect might be activated by exerting the influence on ALT, MDA, or adipokines. The sulfate chitosan derivatives have good therapeutic effect on NAFLD rats and have low toxicity, which can make it a drug candidate in further studies.
Acknowledgements
This work is supported by the National High Technology Research and Development Program of China (863 Program 2006AA090401).
Browning, J. D., and Horton, J. D., 2004. Molecular mediators of hepatic steatosis and liver injury. Journal of Clinical Investigation, 114 (2): 147-152.
Clark, J. M., Brancati, F. L., and Diehl, A. M., 2003. The prevalence and etiology of elevated aminotransferase levels in the United States. The American Journal of Gastroenterology, 98 (5): 960-967.
Deng, Z. B., Liu, Y., Liu, C., Xiang, X., Wang, J., Cheng, Z., Shah, S. V., Zhang, S., Zhang, L., Zhuang, X., Michalek, S., Grizzle, W. E., and Zhang, H. G., 2009. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology, 50 (5): 1412-1420.
Fabbrini, E., Mohammed, B. S., Korenblat, K. M., Magkos, F., McCrea, J., Patterson, B. W., and Klein, S., 2010. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. The Journal of Clinical Endocrinology and Metabolism, 95 (6): 2727-2735.
G?bele, E., Dostert, K., Dorn, C., Patsenker, E., Stickel, F., and Hellerbrand, C., 2011. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcoholism, Clinical and Experimental Research, 35 (7): 1361-1367.
Jin, X., Yang, Y., Chen, K., Lv, Z., Zheng, L., Liu, Y., Chen, S., Yu, C., Jiang, X., Zhang, C., and Li, Y., 2009. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H2O2 levels: A novel mechanism for NAFLD. Journal of Hepatology, 50 (5): 1019-1028.
Khor, E., and Lim, L. Y., 2003. Implantable applications of chitin and chitosan. Biomaterials, 24 (13): 2339-2349.
Kim, D., Choi, S. Y., Park, E. H., Lee, W., Kang, J. H., Kim, W.,
Kim, Y. J., Yoon, J. H., Jeong, S. H., Lee, D. H., Lee, H., Larson, J., Therneau, T. M., and Kim, W. R., 2012. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology, 56 (2): 605-613.
Kim, I., Seo, S. J., Moon, H. S., Yoo, M. K., Park, I. Y., Kim, B. C., and Cho, C. S., 2008. Chitosan and its derivatives for tissue engineering applications. Biotechnology Advances, 26 (1): 1-21.
Kitade, M., Yoshiji, H., Kojima, H., Ikenaka, Y., Noguchi, R., Kaji, K., Yoshii, J., Yanase, K., Namisaki, T., Asada, K., Yamazaki, M., Tsujimoto, T., Akahane, T., Uemura, M., and Fukui, H., 2006. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology, 44 (4): 983-991.
Kumar, R., 2000. A review of chitin and chitosan applications. Reactive and Functional Polymers, 46 (1): 1-27.
Ma, X., Hua, J., Mohamood, A. R., Hamad, A. R. A., Ravi, R., and Li, Z., 2007. A high-fat diet and regulatory t cells influence susceptibility to endotoxin-induced liver injury. Hepatology, 46 (5): 1519-1529.
Manco, M., Marcellini, M., Giannone, G., and Nobili, V., 2007. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. American Journal of Clinical Pathology, 127 (6): 954-960.
Masterton, G. S., Plevris, J. N., and Hayes, P. C., 2010. Review article: Omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics, 31 (7): 679-692.
Mattar, S. G., Velcu, L. M., Rabinovitz, M., Demetris, A. J., Krasinskas, A. M., Barinas-Mitchell, E., Eid, G. M., Ramanathan, R., Taylor, D. S., and Schauer, P. R., 2005. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Transactions of the Meeting of the American Surgical Association, 123: 304-314.
Matteoni, C., Younossi, Z., Gramlich, T., Boparai, N., Liu, Y., and Mccullough, A., 1999. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology, 116 (6): 1413-1419.
Nakao, Y., Yoshida, S., Matsunaga, S., Shindoh, N., Terada, Y., Nagai, K., Yamashita, J. K., Ganesan, A., van Soest, R. W. M., and Fusetani, N., 2006. Azumamides A–E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge mycale izuensis. Angewandte Chemie, 118 (45): 7715-7719.
Oh, E., Kim, T. H., Sohn, Y. W., Kim, Y. S., Oh, Y. R., Cho, E. Y., Shim, S. Y., Shin, S. R., Han, A. L., Yoon, S. J., and Kim, H. C., 2011. Association of serum alanine aminotransferase and Γ-glutamyltransferase levels within the reference range with metabolic syndrome and nonalcoholic fatty liver disease. The Korean Journal of Hepatology, 17 (1): 27-36.
Petta, S., Muratore, C., and Craxì, A., 2009. Non-alcoholic fatty liver disease pathogenesis: The present and the future. Digestive and Liver Disease, 41 (9): 615-625.
Rafiq, N., and Younossi, Z. M., 2008. Effects of weight loss on nonalcoholic fatty liver disease. Seminars in Liver Disease, 28 (4): 427-433.
Rosselli, M. S., Burgue?o, A. L., Carabelli, J., Schuman, M., Pirola, C. J., and Sookoian, S., 2009. Losartan reduces liver expression of plasminogen activator inhibitor-1 (PAI-1) in a high fat-induced rat nonalcoholic fatty liver disease model. Atherosclerosis, 206 (1): 119-126.
Taylor, S., and Harker, A., 2006. Modification of the ultrafiltration technique to overcome solubility and non-specific binding challenges associated with the measurement of plasma protein binding of corticosteroids. Journal of Pharmaceutical and Biomedical Analysis, 41 (1): 299-303.
Tevar, A. D., Clarke, C. N., Schuster, R., Wang, J., Edwards, M., J., and Lentsch, A. B., 2011. The effect of hepatic ischemia reperfusion injury in a murine model of nonalcoholic steatohepatitis. The Journal of Surgical Research, 169 (1): e7- 14.
Uno, M., Kurita, S., Misu, H., Ando, H., Ota, T., Matsuzawa-Nagata, N., Kita, Y., Nabemoto, S., Akahori, H., Zen, Y., Nakanuma, Y., Kaneko, S., and Takamura, T., 2008. Tranilast, an antifibrogenic agent, ameliorates a dietary rat model of nonalcoholic steatohepatitis. Hepatology, 48 (1): 109-118.
Wang, H., Chan, P. K., Pan, S. Y., Kwon, K. H., Ye, Y., Chu, J. H., Fong, W. F., Tsui, W. M. S., and Yu, Z. L., 2010. ERp57 is up-regulated in free fatty acids-induced steatotic L-02 cells and human nonalcoholic fatty livers. Journal of Cellular Biochemistry, 110 (6): 1447-1456.
Yu, C., Lv, Z., Wang, Y., and Jiang, T., 2010. Study on the therapeutic effect of sulfated chitosan derivative on experimental fatty liver in rats. Periodical of Ocean University of China, 40 (5): 27-32.
Zou, Y., Li, J., Lu, C., Wang, J., Ge, J., Huang, Y., Zhang, L., and Wang, Y., 2006. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sciences, 79 (11): 1100-1107.
(Edited by Ji Dechun)
(Received October 8, 2013; revised March 25, 2014; accepted April 7, 2014)
? Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-82032064
E-mail: lvzhihua@ouc.edu.cn
 Journal of Ocean University of China2014年3期
Journal of Ocean University of China2014年3期
- Journal of Ocean University of China的其它文章
- Variation of Bioaccumulation Ability of 2,2’,4,4’-Tetrabromodiphenyl Ether by Marine Diatom Skeletonema costatum Under Different N:P Ratios
- Preference of the Herbivorous Marine Teleost Siganus canaliculatus for Different Macroalgae
- Effect of Temperature on Gene Expression in the Pearl Oyster Pinctada fucata
- Nitrogen and Phosphorus Budget of a Polyculture System of Sea Cucumber (Apostichopus japonicus), Jellyfish (Rhopilema esculenta) and Shrimp (Fenneropenaeus chinensis)
- Structure of Mitochondrial DNA Control Region of Pholis fangi and Its Phylogenetic Implication
- Fishery Biology of Jumbo Flying Squid Dosidicus gigas off Costa Rica Dome
