Effect of Bacillus subtilis and Pseudomonas fluorescens on Growth of Greenhouse Tomato and Rhizosphere Microbial Community
Ge Xiao-ying, He Chun-e Li Tao, and Ouyang Zhu*
1Key Laboratory of Ecosystem Network Observation and Modeling, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (CAS), Beijing 100101, China
2University of Chinese Academy of Sciences, Beijing 100049, China
Effect of Bacillus subtilis and Pseudomonas fluorescens on Growth of Greenhouse Tomato and Rhizosphere Microbial Community
Ge Xiao-ying1,2, He Chun-e1, Li Tao1,2, and Ouyang Zhu1*
1Key Laboratory of Ecosystem Network Observation and Modeling, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (CAS), Beijing 100101, China
2University of Chinese Academy of Sciences, Beijing 100049, China
Bacillus subtilis (B. subtilis) and Pseudomonas fluorescens (P. fluorescens) are two of the most important plant growth promoting rhizobacteria (PGPR) in agriculture. An in situ trial was conducted on greenhouse tomato (Lycopersicum esculentum Mill.) to examine the effect of two bacterial strains, Bacillus subtilis (CGMCC 1.3343) and Pseudomonas fluorescens (CGMCC 1.1802), on tomato growth, gray mold disease control, catabolic and genetic microbial features of indigenous rhizosphere bacteria under lownitrogen conditions. A commercial inoculant (ETS) was also tested as a comparison. Both B. subtilis and P. fluorescens promoted growth and biomass of seedlings, while only B. subtilis was efficient in reducing gray mold incidence in greenhouse tomato. The two bacterial strains could colonization in tomato rhizosphere soil at the end of experiment (10 days after the last inoculation). Different AWCD trends and DGGE patterns were got in different bacterial treatments; however, analyses of microbial diversities showed that indigenous soil microbes did not seem to have significant differences at either the catabolic or genetic level among treatments. ETS, as a commercial microbial agent, promoted plant growth and gave a higher microbial diversity in rhizosphere soil.
greenhouse tomato, Bacillus subtilis, Pseudomonas fluorescens, qPCR, biolog, PCR-DGGE
Introduction
Tomato (Lycopersicum esculentum Mill.) is one of the most important greenhouse vegetables in China. The profitability of planting tomato has encouraged the use of large amounts of chemical fertilizers, insecticides, and pesticides, which has in turn led to water pollution, soil acidification, soil salinity accumulation, and increased frequency of soilborne disease (Guo et al., 2010; Singh et al., 2011).
Gray mold disease caused by Botrytis cinerea is the most common and widely distributed global disease of vegetables, fruits, and even field crops (Barka, 2000). It causes death on flower parts, leaves, shoots, seedlings, and fruits of the tomato plant, and is one of the most hazardous soil-borne diseases of greenhouse tomato.
Increasing both yield and quality of vegetables to satisfy consumers while avoiding deleterious effects on the environment is a big challenge in agriculture. Bacillus subtilis and Pseudomonas fluorescens are two of the most predominant plant growth promotion bacteria (PGPR) recognized (Ahmad et al., 2008). A number of Bacillus sp. and Pseudomonas sp. strains inoculated in several kinds of cereals and vegetablesare known to promote plant growth, increase yield (Gravel et al., 2007; Guo et al., 2004; Jetiyanon et al., 2003; Jiang et al., 2006), and reduce plant disease (Esitken et al., 2006; Herman et al., 2008; Jetiyanon et al., 2003; Recep et al., 2009; Zehnder et al., 2000). However, not all B. subtilis and P. fluorescens strains are PGPR bacteria. Two bacterial strains, B. subtilis (CGMCC 1.3343) and P. fluorescens (CGMCC 1.1802), originally separated from agriculture soil, were picked up to examine their characteristics of PGPR in long-term greenhouse tomato field in the present study.
The biodiversity of soil microbial communities is among the most important indices to assesse soil health (Jackson et al., 2007). Many studies focused on farming practices (i.e., fertilizer application and continuous cropping system) and how these affected the size, biodiversity, and activity of microbial populations (Moeskops et al., 2010; Peacock et al., 2001; Perucci, 1990). However, there was few information about how PGPR bacteria inoculated in rhizosphere soil affected microbial community, especially in a long-term continuous cropping tomato soil.
The hypothesis of the present study was the two bacterial strains of B. subtilis and P. fluorescens, we picked up were PGPR bacteria. The main objectives of this study were to: (1) evaluate the effect of single and co-inoculated the two bacterial strains on tomato seedling biomass; (2) evaluate the colonization of these two bacterial strains in tomato rhizosphere soil; (3) estimate the effect of single and co-inoculated the two bacterial strains on microbial community of rhizosphere soil. Additionally, a commercial microbial agent (ETS) was used as a comparison.
Materials and Methods
Experiment design and sample collection
An in situ culture experiment was conducted in a typical 12-year commercial greenhouse (84×8.5 m) located in Nanbei village, one of the most intensive greenhouse tomato growing areas in Yucheng County, Shandong Province, China. Experiments were conducted twice, between April 2013 and June 2013 and from August 2013 to October 2013. The greenhouse operated an annual winter-spring tomato/ autumn-winter tomato continuous cropping system. Separated in situ plots of 60 cm (width)×80 cm (length) were used in the experiment, and the border of each plot was surrounded by a sheet frame (without base, 60 cm (width)×80 cm (length)×40 cm (high), 30 cm under-ground and 10 cm above-ground). Two plants were placed in each plot. The physical and chemical properties of the upper (top 20 cm) soil were summarized as: 22.55 g · kg-1organic matter, 1.59 g · kg-1total N, 218.08 mg · kg-1available P, 509.23 mg · kg-1available K, 0.77 ms · cm-1EC, pH 8.22.
Five treatments were as follows: (1) RN: Reduced dose of nitrogen treatment as control. The conventional practice of N side-dressing rate in this region was 600 kg · N · hm-2. In order to evaluate the effect of PGPR on plant growth, we reduced the N side-dressing rate to 200 kg · N · hm-2; (2) RNB: RN+B. subtilis; (3) RNP: RN+P. fluorescens; (4) RNBP: RN+B. subtilis+P. fluorescens; (5) ETS: RN+ETS (a commercial complex microbial agent); B. subtilis, P. fluorescens, and ETS were inoculated at planting, 20 days and 40 days after planting, respectively.
All the treatments were set up in a randomized block design with three replicates. Plants were irrigated as needed and fertilized weekly. At the end of the experiment (50 days after planting), each plant was collected from the plot and measured biomass. Rhizosphere soil (shaked off the bulk soil from root, then scraped the soil adhesive to root and collected them in a sterilized plastic bag) was collected. Rhizosphere soil samples were separated into two parts, one part was subjected to biolog analysis immediately and another part was stored in sterilized plastic bags at -20℃ until DNA extraction.
Bacterial strains and culture conditions
The two bacterial strains, B. subtilis (CGMCC1.3343) and P. fluorescens (CGMCC 1.1802), originally separated from agriculture soil, were supplied by China General Microbiological Culture Collection Center (CGMCC, China). B. subtilis and P. fluorescens were grown for 24 h in beef-extractpeptone medium without agar at 35℃ and 30℃, respectively. Each bacterial suspension was finally adjusted to 1×108CFU · mL-1for soil drench. For RN treatment, 15-mL of beef-extract-peptone medium was added to the soil around each plant. For RNB and RNP treatments, 15-mL of a respective bacteria suspension was added to the soil around each plant. A 15-mL mixture containing 7.5 mL B. subtilis suspension and 7.5 mL P. fluorescens suspension was applied in RNBP treatment, and 15 mL ETS microbial agent was added in ETS treatment.
Plant growth and disease severity
Plant height, stem width, fresh and dry weight of shoot (leaves+stems), and fresh and dry weight of roots were measured after 50 days of transplant. The shoot (leaves+stems) and root dry weight of two plants were determined after drying at 75℃ for 48 h to constant weight. The measurements of two plants were considered one replicate, and there were three replications per treatment (n=3).
The two bacterial strains inoculated single or together were evaluated under greenhouse conditions for their ability of controlling tomato gray mold disease. The disease incidence was determined 50 days after transplanting. Each plant was scored for the occurrence of disease symptoms according to parameters set by Hariprasad et al (2011). The biological control efficacy was calculated using the following formula: Disease severity=[(A×1)+(B×2)+(C×3)+(D× 4)+(E×5)+(F×6)]/ Number of plant (Hariprasad et al., 2011).
qPCR and analysis
qPCR method was used to assess the colonization of B. subtilis and P. fluorescens in rhizosphere of tomato. Escherichia coli (BH5α), B. subtilis (CGMCC 1.3343), and P. fluorescens (CGMCC 1.1802) were used as reference strains for constructing standard curves to quantify total bacteria, Bacillus sp., and Pseudomonas sp. in soil, respectively. All reference strains were grown on nutrient agar (peptone 10.0 g, beef extract 3.0 g, NaCl 5.0 g, agar 15.0 g, and distilled water 1.0 L, pH 7.0) at 35℃ for 24 h.
The specific primer pairs for quantifying total bacteria, Bacillus sp., and Pseudomonas sp. content by qPCR are shown in Table 1.
A band of approximately 500 bp was obtained after PCR amplification by using DNA extracted from B. subtilis with the forward and reverse primers pair for Bacillus sp. (Bac F and Bac R), while lanes with water as control and DNA extracted from P. fluorescens showed no detectable product (data not shown). Similar result were obtained when testing the specificity of the primers for total bacteria and Pseudomonas sp. Thus, the primers were specific for Bacillus sp. and Pseudomonas sp., respectively, and could be used in this study.
Soil genomic DNA was extracted using the Power Soil DNA Isolation Kit (MOBIO Laboratories, lnc. CA, USA). DNA quality was assessed by the ratio of A260/280 and A260/230 using an ultraviolet spectrophotometer.
qPCR procedure (SYBR Green qPCR Kit with ROX, Invitrogen Co., CA., USA) and cycle threshold (Ct) values were used to calculate the cell numbers. qPCR reactions were performed using annealing temperatures of 52℃ (total bacteria), 60℃ (Bacillus sp.), and 50℃ (Pseudomonas sp.). Each reaction was performed in triplicate. Data analysis was performed using ABI Prism 7500 SDS software V1.3.1 (Applied Biosystems Co., CA., USA).
Standard curves were made using a 10-fold dilution series (ranging from 1.0×100to 1.0×10-7) of total bacteria, Bacillus sp., and Pseudomonas sp. plasmid DNA. Good linear relations between Ct values and copy numbers were found in all the cases (R2=0.996, 0.992, and 0.999, respectively, for total bacteria, Bacillus sp., and Pseudomonas sp.) (Supplement 1).
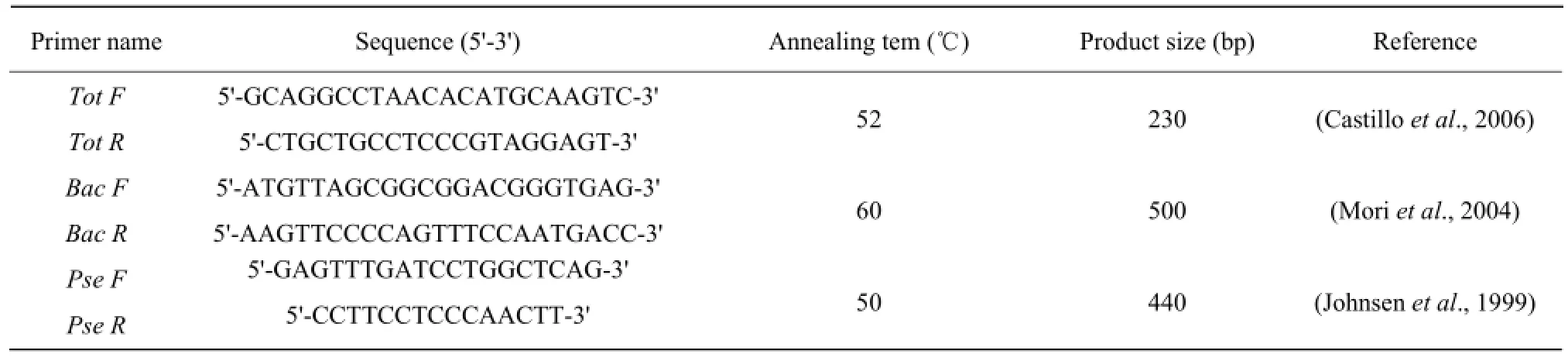
Table 1 Primer sequences used for quantifying soil total bacteria, Bacillus sp. and Pseudomonas sp.
Biolog
Biolog EcoPlate was used to assess the functional diversity of microbial community in rhizosphere soil of bacterial treatments (Biolog Inc., Hayward, USA). 10-g (wet weight) soil samples were weighed into 150-mL triangular flasks, suspended in sterile physiological saline solution (0.85% NaCl), and shaken at 200 r · min-1for 30 min. The suspensions were then further diluted by factors of 102and 103. Aliquots (150 μL) of the extracts were added to each well of a microplate, and the plates were cultured in the dark at 25℃ for 10 days. Biolog Eco-Plates were read at 0 h, then once every 12 h for 10 days, to the end of the incubation period (day 10) with a Biolog Micro Station reader (plates read at 590 nm; Biolog, lnc. CA, USA). An average well color development (AWCD) method, i.e.,

Where, C was color production within each well (optical density measurement) and R was the absorbance value of the plate's control well was used to access the metabolic activity of the soil bacterial community. Soil bacterial diversity was evaluated by Shannon's diversity index (H′) (Lupwayi et al., 2001; Zak et al., 1994):

Where, Piwas ratio of activity (i.e., optical density reading) on the ith substrate to the sum of activities on all the substrates.
PCR-DGGE
The primers used in the present study were the universal primers conserved for V3 region of the bacterial 16S rRNA gene, 338F 5'-ACTCCTACGG GAGGCAGCAG-3' and 534R 5'-CGCCCGCCGC GCGCGGCGGGCGGGGCGGGGGCACGGGGG (ATTACCGCG GCT GCTGG)-3') (Muyzer et al., 1993). PCR amplification was performed in a Master Cycler Gradient thermocycler (Eppendorf, Germany) in 50 μL reaction volumes containing 5 μL PCR buffer (200 mmol · L-1Tris-HCl pH: 8.4, 500 mmol · L-1KCl), 4 μL 2.5 mmol · L-1each dNTP mixture, 1 μL 20 μmol · L-1338F, 1 μL 20 μmol · L-1534R, 2.5 ng DNA, 0.25 μL TaKaRa rTaq (5 U · μL-1), and ddH2O (double distilled water), which was added to bring the reaction to 50 μL. Primers were synthesized by Sangon Biotech Co., Ltd., Beijing, China. The thermal cycling conditions were as the followings: initial denaturation, 94℃ for 5 min; 35 cycles of 94℃ for 1 min, annealing at 55℃ for 1 min and elongation at 72℃ for 1 min 10 s; final extension at 72℃ for 3 min 50 s. The products were examined by running 5-10 μL aliquots in 1.5% agarose gel electrophoresis.
For DGGE analysis, PCR products were separated using D-Code Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and 8% polyacrylamide gels (ratio of acrylamide andbisacrylamide 37 : 1) with a denaturing gradient of 30%-60% (100% was defined as 7 mol · L-1urea with 40% formamide). Electrophoresis was performed at 61℃ for 7 h in 0.5×TAE electrophoresis buffer under a constant voltage of 150 V. After electrophoresis, gels were stained for 20 min with SYBR Green I nucleic acid gel stain and were photographed under UV light using the Alpha Imager 2200 Imaging System (Alpha Innotech, USA). Shannon-Weaver diversity index (H′) scores were also calculated for each treatment:
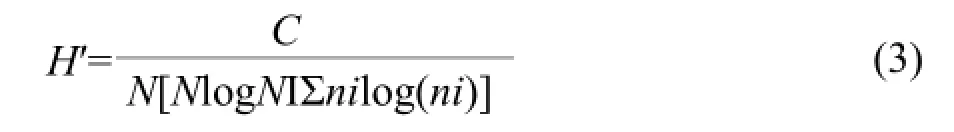
Where, C=2.3, N=the total intensity of all DNA bands and ni represented the intensity of the ith band.
Statistical analysis
Statistical analysis was performed using SPSS 12.0. Plant growth parameters and biocontrol efficacy were expressed as means±SD. Comparisons between groups were tested by one-way ANOVA analysis and LSD test. P<0.05 was considered significant.
Results
Efficacy of bacterial treatments on plant growth promotion and disease control
The average plant growth parameters are listed in Table 2. Tomato seedlings treated with bacterial suspensions did not increase plant height compared with untreated seedlings, but seedlings treated with P. fluorescens (RNP and RNBP) and ETS had significantly higher stem width. RNP and RNBP also increased both fresh weight and dry weight of shoot, compared with RN treatment. All the bacterial treatments increased the fresh weight of root, but only RNBP increased the dry weight of root.
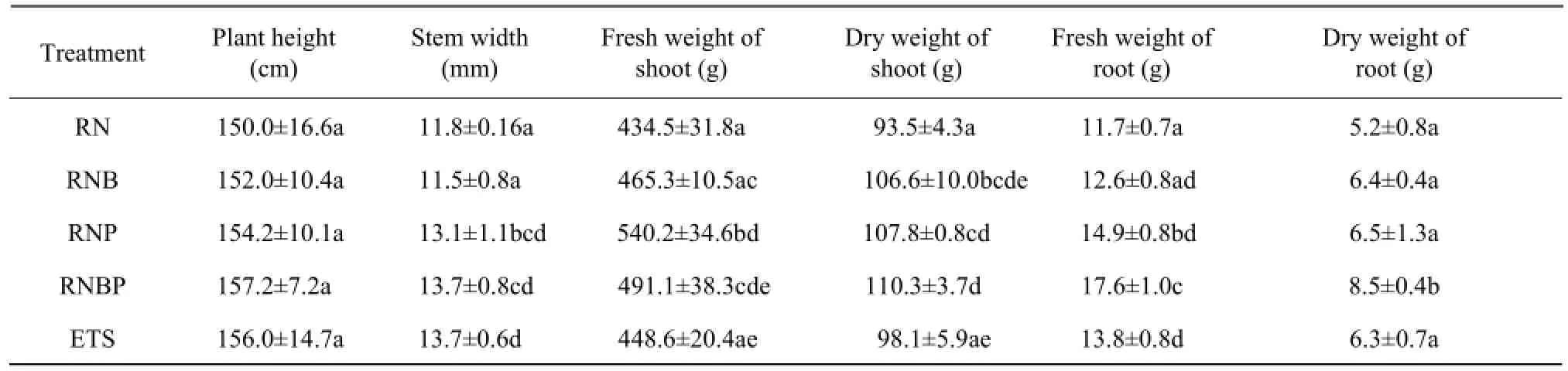
Table 2 Effect of B. subtilis, P. fluorescens and ETS on biomass of greenhouse tomato
The disease severities of inoculated different bacteria are shown in Fig. 1. Single inoculated B. subtilis treatment (RNB) and co-inoculated B. subtilis and P. fluorescens treatment (RNBP) significantly decreased the disease severity of tomato gray mold, and the disease severity of gray mold was decreased 84% by RNBP. No differences were found between treatments RNP, ETS and RN in controlling gray mold disease.
Colonization of two bacteria when single and co-inoculated in rhizoshpere soil
The colonization of the two bacteria in greenhouse tomato rhizosphere soil are shown in Fig. 2. RNP and RNBP significantly increased the copy number of Pseudomonas sp. in rhizosphere soil (P<0.05), compared with RNB and ETS treatments. RNB and RNBP significantly increased the copy number ofbacillus sp. compared with other treatments. The abundance ratio of Pseudomonas sp. and Bacillus sp. (calculated as the copy number of Pseudomonas/total bacteria, the copy number of Bacillus/total bacteria, data of the total bacteria was not shown) gave similar trends with the quantities of the two bacterial genus which are shown in Fig. 3. RNP and RNBP increased the abundance ratio of Pseudomonas sp., but not reach a significant level (P>0.05), compared with RN and RBN treatments. RBN and RNBP significantly increased the abundance ratio of Bacillus sp., compared with RN, RBP and ETS treatments.
Catabolic profiles of bacterial community
AWCD varied for different soil samples at each cultivate time (Fig. 4). At beginning 24 h, AWCD values of all treatments were low and increased rapidly thereafter until 96 h. After this point, AWCD continued to rise but at a slower rate, and reached a maximum after 216 h. In general, ETS treatment got the highest AWCD value, followed by RNBP. RN treatment resulted in the lowest AWCD value, and all other treatments returned intermediate results that were not significantly different from one another.
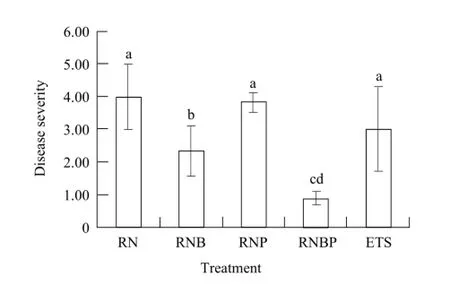
Fig. 1 Effect of B. subtilis, P. fluorescens and ETS treatment on gray mold disease severity in greenhouse tomatoa, b, c, and d are symbolized the variance used to determine significance of treatment effects, the same letter means no significant differences between different treatments (P>0.05). Statistical analysis is performed using SPSS 12.0 and one-way ANOVA procedure, LSD test analyzes the statistical significance between treatments.
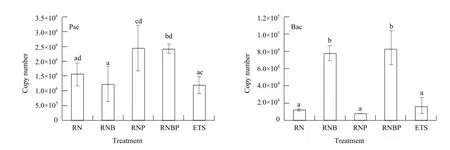
Fig. 2 Quantity of Bacillus and Pseudomonas spp. in different bacterial treatment soilsBac and Pse symbolize Bacillus sp. and Pseudomonas sp., respectively; a, b, c, and d symbolize variance used to determine the significance of treatment effects, the same letter means no significant differences between different treatments (P>0.05). Statistical analysis is performed using SPSS 12.0 and one-way ANOVA procedure, LSD test analyzes the statistical significance between treatments.
Soil microbial functional diversity influenced by different bacterial applications was calculated as the Shannon-Weaver diversity index and substrate richness, as shown in Table 3. Shannon-Weaver diversity in-dexes were not significantly different among all the treatments (P>0.05), but all the treatments with bacterial suspension got significant higher substrate richness than RN (P<0.05).
Genetic structure of bacteria community
DGGE patterns obtained from each treatment contained several distinct bands with varying intensities(Fig. 5). DNA amplification produced 12-25 detectable bands of various intensities per sample that were displayed from approximately 30%-60% denaturant. In DGGE pattern of ETS treatment, most bands were obtained in ETS treatments (25), followed by 23 bands obtained by RNBP treatment. RNB and RNP treatments got 21 and 20 bands, respectively, while RN samples contained 19 bands. In Fig. 5, some bands, including 14, 15, 18, and 20, were found in all the soil treatments, indicating that the same bacterial communities presented in the soil samples. Conversely, some bands, such as band 1 in ETS-treated samples and band 6 in RNBP-treated samples, indicated that distinct bacterial communities could be found among certain soil samples.
Based on the numbers, intensities, and positions of DGGE bands in all five treatments, Shannon-Weaver diversity indices were analyzed using Molecular Analyst software. The results showed that ETS and RNP treatments got the highest diversity index (2.61), while RNB treatment had the lowest diversity index (2.29).
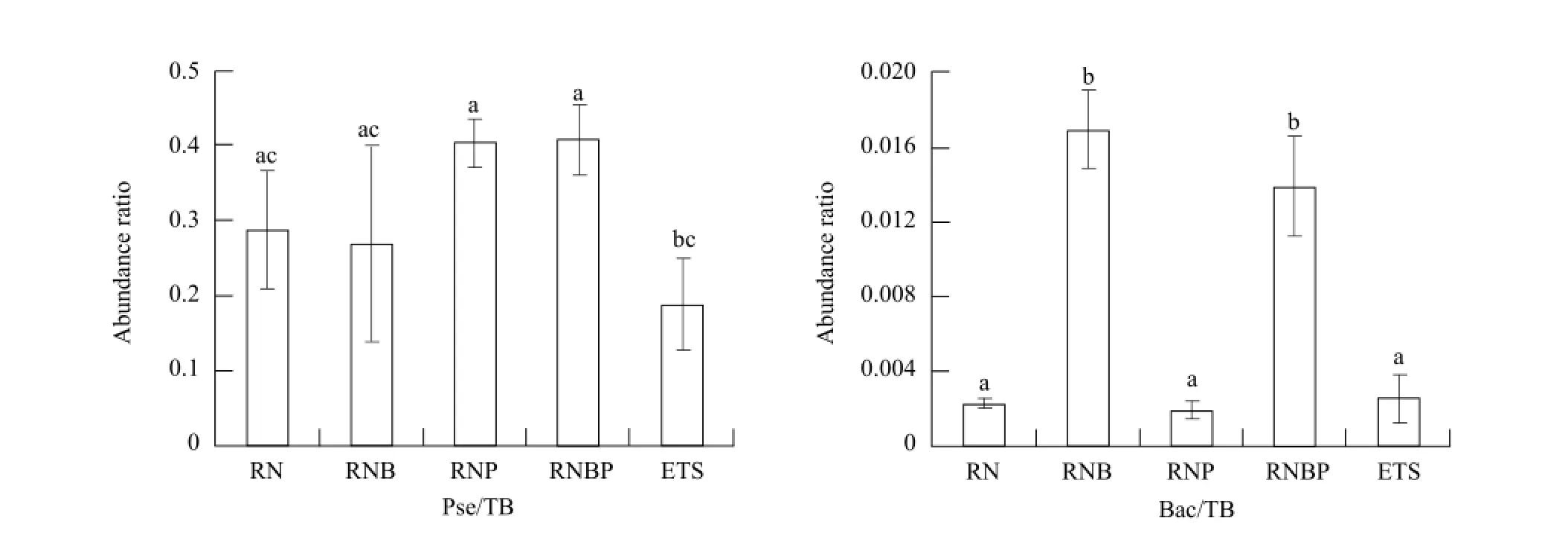
Fig. 3 Relative abundance of Pseudomonas and Bacillus spp. in different bacterial treatment soilsTB, Bac, and Pse symbolize total bacteria, Bacillus sp. and Pseudomonas sp. respectively; a, b, c, and d symbolize the variance used to determine the significance of treatment effects, the same letter means no significant differences between different treatments (P>0.05). Statistical analysis was performed using SPSS 12.0 and one-way ANOVA procedure, LSD test is used to analyze the statistical significance between treatments.
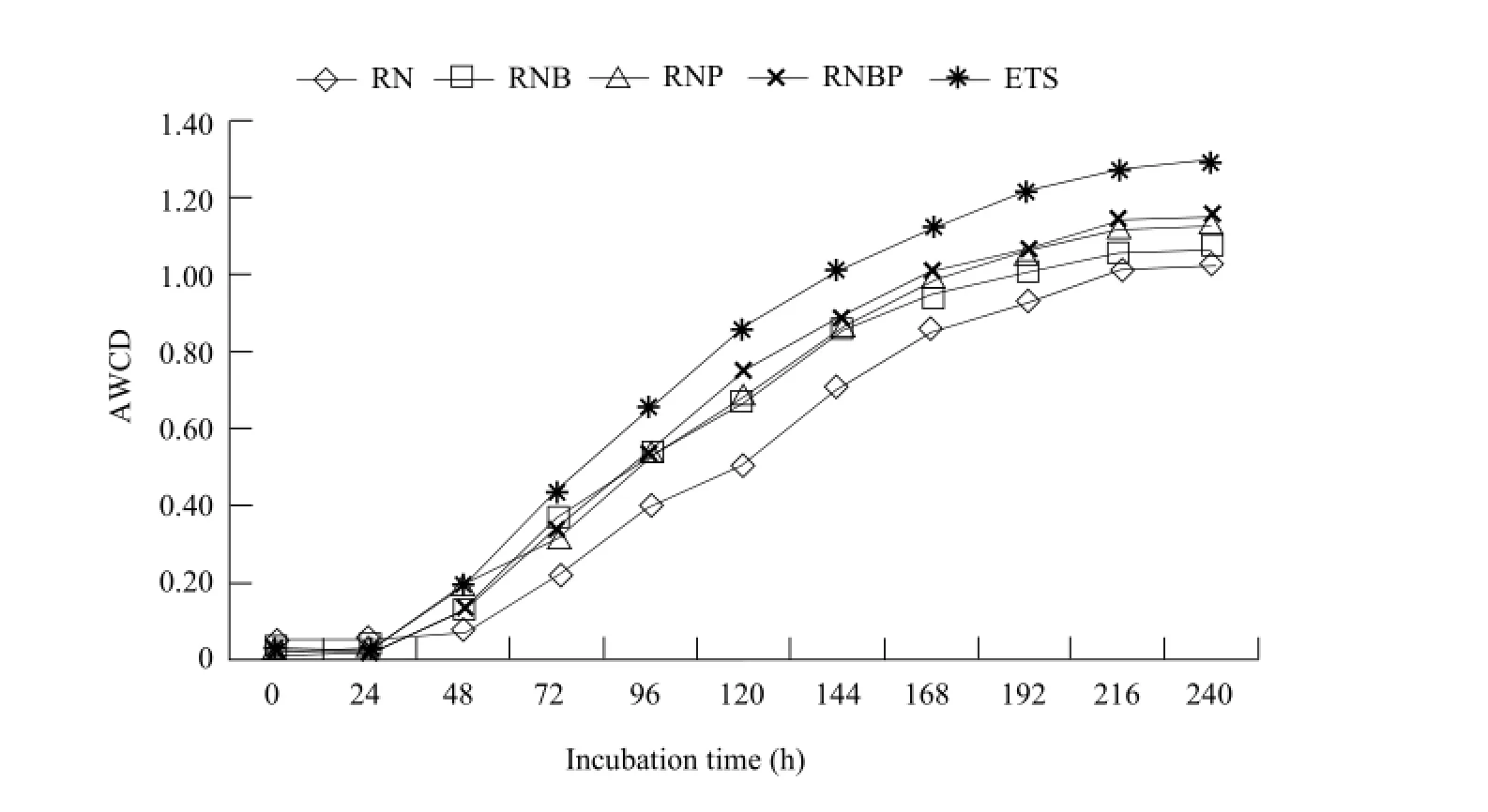
Fig. 4 Variation in average well color development (AWCD) of soil bacterial community over time for different treatments during culture timeECO Plates are incubated at 25℃ for 10 days. Plates are read at 590 nm.
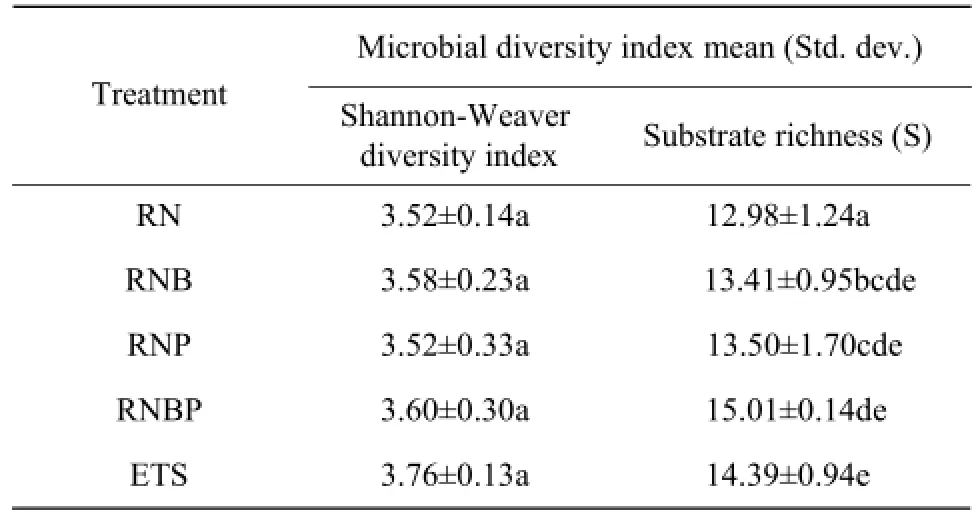
Table 3 Shannon-Weaver diversity and substrate richness of soil microbial communities following different bacterial treatments

Fig. 5 DGGE profiles of soil bacteria in different bacterial treatment soilsDGGE profiles of bacterial 16S rDNA PCR products (based) amplified against the metagenome of different bacterial treatments of greenhouse tomato soils; the denaturing gradient ranges from 30%-60%.
Discussion
Effect of single and co-inoculated B. subtilis and P. fluorescens on plant growth and controlling gray mold disease
Improvements in plant growth and disease resistance to a broad array of plant pests can be accomplished using PGPR (Kloepper et al., 2004). Our first objective was to determine whether the application of two bacterial strains (P. fluorescens and B. subtilis), ETS, and/or their combination affected tomato seedling growth. This was performed under reduced nitrogen conditions in a long-term continuous cropping greenhouse system. All the treatments with bacteria inoculated had increased biomass of seedlings indicated that the two bacteria we picked up to inoculated had a characteristics of PGPR. RNP and RNBP had increased most of seedling biomass index we had measured, but RNB only significantly increased fresh root weight only, and ETS significant increased the stem width and fresh root weight; RNB and RNBP significantly decreased disease severity of gray mold, while RNP was not, might indicate that P. fluorescens strain we tested was more efficient in promoting plant growth while B. subtilis strain in our experiment was more efficient in disease controlling.
Among various PGPR identified, P. fluorescens and B. subtilis were two of the most extensively studied rhizobacteria because of their known effects on plant growth (Kavino et al., 2010; Singh et al., 2011). In the present study, we found that P. fluorescens was a stronger promoter of plant growth, while B. subtilis was a potent inhibitor of gray mold. Similar results following inoculation with P. fluorescens or B. subtilis alone could be found in some other crops, such as maize, wheat, rice, and peanut (Dey et al., 2004; Gholami et al., 2009). Additionally, a combination of compatible PGPR could lead to better and more consistent disease suppression (Barka, 2000; Felici et al., 2008; Latha et al., 2011). In the present study, results of qPCR showed that quantities of Pseudomonas sp. and Bacillus sp. were higher in RNP, RNB and RNBP than those in RN, respectively, indicating that the two bacteria, single or co-inoculated in rhizosphere, could survive 10 days after bacteria inoculated. Also, we showed that combined treatment with B. subtilis and P. fluorescens was an effective strategy for biocontrol (Bai et al., 2002; Felici et al.,2008; Joe et al., 2009; Roberts et al., 2005; Vestberg et al., 2004).
Effect of single and co-inoculated B. subtilis and P. fluorescens on rhizosphere soil bacteria communities
AWCD results showed that soil microbial functional structure was altered by different bacterial treatments and ETS treatment got the highest AWCD through all the incubation time. AWCD values represented metabolic activity of soil bacterial community as it utilized carbon sources, suggesting that the metabolic activity of soil bacteria was increased by ETS which was a commercial complex microbial agent.
Analyses of the substrate richness also showed that all the bacterial treatments led to a higher substrate richness than RN and reached to significant level (P<0.05). ETS treatment also got the highest Shannon-Weaver diversity index but the difference was not significant, compared with other treatments. As the biolog analyzed density and activity of culturable microbes, Naiman et al. (2009) showed that the number of culturable rhizobacteria clones was significantly higher in the treatment inoculated with P. fluorescens than that in control, while Kokalis-Burelle et al. (2006) reported the opposite results. The utilization ratio of six groups of carbon resources varied according to specific treatment (data not shown), indicating that soil microbial functional structure was altered by the precise formulation of bacterial input. We could infer that the soil diversity was relatively high, and therefore the addition of one or two bacterial species did not significantly change the microbial structure.
The biolog eco-plate method we used to reflect microbial functional diversity was based on culturable bacteria which would miss most of soil bacteria; a number of more recent studies used molecular genetics profiling of soil microbial structural diversity had probed this drawback (Agnelli et al., 2004). We proposed that a combination of the two methods could give a more complete understanding of soil microbial communities. DGGE profiling revealed different characteristics among the treatments, but they did not reach significance according to the Shannon-Weaver index, indicating that the two bacteria, single or co-inoculated, did not have prominent effects on the structure of the microbial community. Similar results were reported by Correa et al. (2009) following inoculation of soybean with B. amyloliquefaciens, and by Piromyou et al. (2009) following inoculation of forage corn with Pseudomonas and Brevibacillus. The roles of tomato growth promotion by PGPR might come from other factors, such as ACC-deaminase, P-solubilization and N-fixing (Correa et al., 2009; Piromyou et al., 2011).
Conclusions
In conclusion, single or co-inoculated the two bacterial strains, B. subtilis and P. fluorescens, to greenhouse tomato seedlings under reduced chemical N conditions enhanced plant growth, improved the control efficiency of gray mold disease, and exhibited a characteristic of PGPR. qPCR results proved that 10 days after inoculation, the two bacterial strains could survive in rhizosphere soil. Biolog and PCRDGGE methods were used to evaluate the soil microbial communities. Different AWCD trends and DGGE patterns were got in different bacterial treatments; however, analyses of microbial diversities showed that indigenous soil microbes did not seem to have significant differences at either the catabolic or genetic level among treatments. ETS, as a commercial microbial agent, promoted plant growth and gave a higher microbial diversity in rhizosphere soil. The characteristics of the two bacterial strains to promote growth and control disease in greenhouse tomato might be due to other mechanisms, such as ACC-deaminase activity, P-solubilization and chitinase production.
Agnelli A, Ascher J, Corti G, et al. 2004. Distribution of microbial communities in a forest soil profile investigated by microbialbiomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol Biochem,36(5): 859-868.
Ahmad F, Ahmad I, Khan M S. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res,163(2): 173-181.
Bai Y, Pan B, Charles T C, et al. 2002. Co-inoculation dose and root zone temperature for plant growth promoting rhizobacteria on soybean [Glycine max (L.) Merr ] grown in soil-less media. Soil Biol Biochem,34: 1953-1957.
Barka E. 2000. Enhancement of in vitro growth and resistance to gray mould of vitis vinifera co-cultured with plant growth-promoting rhizobacteria. FEMS Microbiol Lett,186(1): 91-95.
Castillo M, Martin-Orue S M, Manzanilla E G, et al. 2006. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet Microbiol,114(1/2): 165-170.
Correa O S, Montecchia M S, Berti M F, et al. 2009. Bacillus amyloliquefaciens BNM122, a potential microbial biocontrol agent applied on soybean seeds, causes a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol,41(2): 185-194.
Dey R, Pal K K, Bhatt D M, et al. 2004. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res,159(4): 371-394.
Esitken A, Pirlak L, Turan M, et al. 2006. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Scientia Horticulturae,110(4): 324-327.
Felici C, Vettori L, Giraldi E, et al. 2008. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: effects on plant growth and rhizosphere microbial community. Appl Soil Ecol,40(2): 260-270.
Gholami A, Shahsavani S, Nezarat S. 2009. The effect of plant growth promoting rhizobacteria ( PGPR ) on germination: seedling growth and yield of maize. Int J Biol life Sci,5(1): 35-40.
Gravel V, Antoun H, Tweddell R J. 2007. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem,39(8): 1968-1977.
Guo J H, Qi H Y, Guo Y H, et al. 2004. Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol Control,29(1): 66-72.
Guo J H, Liu X J, Zhang Y, et al. 2010. Significant acidification in major Chinese croplands. Science (New York),327(5968): 1008-1010.
Hariprasad P, Divakara S T, Niranjana S R. 2011. Isolation and characterization of chitinolytic rhizobacteria for the management of Fusarium wilt in tomato. Crop Prot,30(12): 1606-1612.
Herman M a B, Nault B a, Smart C D. 2008. Effects of plant growthpromoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot,27(6): 996-1002.
Jackson R B, Fierer N, Schimel J P. 2007. New directions in microbial ecology. Ecology,88(6): 1343-1344.
Jetiyanon K, Fowler W D, Kloepper J W. 2003. Broad-spectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis,87(11): 1390-1394.
Jiang Z Q, Guo Y H, Li S M, et al. 2006. Evaluation of biocontrol efficiency of different Bacillus preparations and field application methods against Phytophthora blight of bell pepper. Biological Control,36(2): 216-223.
Joe M M, Jaleel C A, Sivakumar P K, et al. 2009. Co-aggregation in Azospirillum brasilensense MTCC-125 with other PGPR strains: effect of physical and chemical factors and stress endurance ability. J Taiwan Inst Chem E,40(5): 491-499.
Johnsen K, Enger O, Jacobsen C S, et al. 1999. Quantitative selective PCR of 16S ribosomal RNA correlates well with selective agar plating in describing population dynamics of indigenous Pseudomonas spp. in soil hot spots. Appl Environ Microb,65(4): 1786-1788.
Kavino M, Harish S, Kumar N, et al. 2010. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl Soil Ecol,45(2): 71-77.
Kloepper J W, Ryu C M, Zhang S A. 2004. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology,94(11): 1259-1266.
Kokalis-Burelle N, Kloepper J W, Reddy M S. 2006. Plant growthpromoting rhizobacteria as transplant amendments and their effects on indigenous rhizosphere microorganisms. Appl Soil Ecol,31(1/2): 91-100.
Latha P, Anand T, Prakasam V, et al. 2011. Combining Pseudomonas, Bacillus and Trichoderma strains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Appl Soil Ecol,49: 215-223.
Lupwayi N Z, Arshad M A, Rice W A, et al. 2001. Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Appl Soil Ecol,16(3): 251-261.
Moeskops B, Buchan D, Sleutel S, et al. 2010. Soil microbialcommunities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl Soil Ecol,45(2): 112-120.
Mori K, Iriye R, Hirata M, et al. 2004. Quantification of Bacillus species in a wastewater treatment system by the molecular analyses. Biotechnol Bioproc E,9(6): 482-489.
Muyzer G, Dewaal E C, Uitterlinden A G. 1993. Profiling of complex microbial-populations by denaturing gradient gel-electrophoresis analysis of polymerase chain reaction-amplified genes-coding for 16s ribosomal-RNA. Appl Environ Microb,59(3): 695-700.
Naiman A D, Latrónico A, García de Salamone I E. 2009. Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: impact on the production and culturable rhizosphere microflora. Eur J Soil Biol,45: 44-51.
Peacock A D, Mullen M D, Ringelberg D B, et al. 2001. Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol Biochem,33(7/8): 1011-1019.
Perucci P. 1990. Effect of the addition of municipal solid-waste compost on microbial biomass and enzyme-activities in soil. Biol Fert Soils,10(3): 221-226.
Piromyou P, Buranabanyat B, Tantasawat P, et al. 2011. Effect of plant growth promoting rhizobacteria (PGPR) inoculation on microbial community structure in rhizosphere of forage corn cultivated in Thailand. Eur J Soil Biol,47(1): 44-54.
Recep K, Fikrettin S, Erkol D, et al. 2009. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol Cont,50(2): 194-198.
Roberts D P, Lohrke S M, Meyer S L F, et al. 2005. Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Prot,24(2): 141-155.
Singh J S, Pandey V C, Singh D P. 2011. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agr Ecosyst Environ,140(3-4): 339-353.
Vestberg M, Kukkonen S, Saari K, et al. 2004. Microbial inoculation for improving the growth and health of micropropagated strawberry. Applied Soil Ecology,27(13): 243-258.
Zak J C, Willig M R, Moorhead D L, et al. 1994. Functional diversity of microbial communities - a quantitative approach. Soil Biol Biochem,26(9): 1101-1108 .
Zehnder G W, Yao C B, Murphy J F, et al. 2000. Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growthpromoting rhizobacteria. Biocontrol,45(1): 127-137.
S154.36
A
1006-8104(2015)-03-0032-11
Received 30 April 2015
Supported by the National High-tech Research and Development Program of China (2013AA102903)
Ge Xiao-ying (1984-), female, Ph. D student, engaged in the research of plant nutrient management and soil microbial ecology. E-mail: xiaoying_ge@126.com
* Corresponding author. Ouyang Zhu, professor, engaged in the research of ecological management of agriculture. E-mail: ouyz@igsnrr.ac.cn
 Journal of Northeast Agricultural University(English Edition)2015年3期
Journal of Northeast Agricultural University(English Edition)2015年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Design of Non-contact On-load Automatic Regulating Voltage Transformer
- Chinese Comprehensive Rural Reform: Institutional Vicissitude, Theoretic Framework and Content Structure
- Acceptability of Bush Meat as a Source of Animal Protein in Delta State, Nigeria: Implication for Extension Services
- Preparation of Mouse Embryonic Stem Cells and Cardiomyocyte Differentiation Induced with Retinoic Acid and Ascorbic Acid
- Contents of Trace Metal Elements in Cow Milk Impacted by Different Feedstuffs
- Predatory Efficacy of Cotton Inhabiting Spiders on Bemisiatabaci, Amrascadevastans Thripstabaci and Helicoverpa armigera in Laboratory Conditions
