Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
Mahipal S. Shekhawat, M. Manokari
Biotechnology Laboratory, Department of Plant Science, M.G.G.A.C., Mahe, Pondicherry-673311, India
Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
Mahipal S. Shekhawat*, M. Manokari
Biotechnology Laboratory, Department of Plant Science, M.G.G.A.C., Mahe, Pondicherry-673311, India
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Hybanthus enneaspermus
Somatic embryogenesis
In vitro fl owering
Conservation
Objective: Present study reports the various factors af fecting somatic embryogenesis and in vitro fl owering in Hybanthus enneaspermus (L.) F. Muell. Methods: The effect of the salts strength of Murashige and Skoog’s (MS) medium, concentration of sucrose and plant growth regulators were analyzed for the induction of direct somatic embryogenesis using nodal segments as explants. Results: High frequency of somatic embryogenesis was reported on full strength MS medium (with 3% sucrose) and additives supplemented with 0.5 mg/L 6-benzylaminopurine (BAP) and 0.25 mg/L indole-3-acetic acid (IAA). Maximum somatic embryos (207.0±4.2) were germinated on 1/2 strength MS medium augmented with 0.5 mg/L BAP. Microscopic studies revealed the typical developmental patterns in somatic embryogenesis from globular to heart-shaped and followed by bipolar torpedo-shaped somatic embryos from nodal explants. The plantlets raised from the somatic embryos resulted in fl owering on full strength MS medium augmented with 1.0 mg/L each of BAP and Kinetin (Kin) + 0.5 mg/L IAA at 50 μmol/(m2·s) SFPD for 13 h/d photoperiod. About 92% plantlets were successfully acclimatized in the greenhouse. Field transferred plants exhibit normal f lowering and fruit setting. Conclusions: The study could be exploited for large scale propagation of true to type plants as conservation strategies of this rare and endemic medicinal plant.
1. Introduction
Hybanthus enneaspermus (L.) F. Muell. (family Violaceae) is an ancient Indian medicinal plant traditionally valued for its aphrodisiac and stimulant activity[1], popularly known as Rathanapurush (Sanskrit). This plant has been disappeared from the Western Ghats[2] and considered as rare and endemic species of Deccan Peninsular India[3,4]. Recently this plant has attracted much attention due to its multipotent bioactivities as antirheumatic, antiinfertility[5], antioxidant[6] and antidiabetic[7]. Coumarins, alkaloids, fl avonoids, saponins, tannins, glycosides and triterpenoids are major bioactive compounds of H. enneaspermus[8].
Over harvesting for medicinal use, sporadic distribution, poor seed viability and germination are the major threats to this plant[9]. The conventional breeding methods are unable to improve the sustainability of H. enneaspermus. Plant tissue culture techniques represent a useful tool for mass propagation as well as an attractive alternative to conventional breeding[10]. In vitro culture of plants is increasingly used in conservation of biodiversity, especially for rare and endemic species, and considered as an important component of plant genetic resource management[11].
Somatic embryogenesis represents an important in vitro tool for large scale propagation of elite genotypes and it is one of the important prerequisites for genetic interventions. Somatic embryogenesis and plantlet regeneration have been achieved in Leucojum vernum[12], Acacia Senegal[13], Curcuma longa[14], and Sugarcane[15] for successful propagation. Somatic embryogenesis has added advantages over organogenesis because it leads to the formation of bipolar structures possessing both root and shoot meristems[16]. Somatic embryo resembles the genotype of parent cells, assumed to be originating from single cell and will result in the formation of number of embryos per cell mass volume[17].
Somatic embryogenesis involves a process of development ofembryogenic mass derived from the somatic explant in vitro and their subsequent development to regenerate plants directly. This potential mechanism aids to understand a change from vegetative to reproductive phase[18]. In vitro flowering can be obtained repeatedly in the shoots raised from somatic embryos. According to Simpson et al. the competent bud meristems are responsive to environmental or autonomous signals that eventually lead to fl ower formation[19]. Somatic embryogenesis and in vitro fl owering has been achieved in Bamboo species[20], Chamomilla recutita[21] and Boerhaavia diffusa[22].
So far, there are no reports on direct somatic embyogenesis and in vitro flowering in H. enneaspermus. Earlier reports are available on indirect somatic embryogenesis through callus regeneration from leaf and stem explants[23-25]. The absence of direct somatic embryogenesis protocol is probably the reason that there is no report of stable genetic transformation and disease free stock production protocols in this plant. The present study introduced a rare species Hybanthus enneaspermus to direct somatic embryogenesis and fl owering in vitro which can be used as strategy of conservation of this rare plant species.
2. Materials and methods
2.1. Plant material and surface sterilization
Hybanthus enneaspermus was selected from Coromandel Coast (Kanchipuram, Villipuram, Puducherry, Cudalore, Nagapattinam and Karaikal districts) of India for the present study. Young emerging slender stems were used as the source of explants from a 2 months old mature plant. The nodal segments (approximately 3.0 cm in length) were harvested from the 2 months old fi eld grown plant. The explants were sterilized with the systemic fungicide (0.1% Bavistin; BASF India Ltd., India) and then with 0.1% HgCl2 (w/v) for 4–5 min. The sterilized explants were washed with autoclaved double distilled water for 5–6 times to remove the adhered traces of HgCl2.
2.2. Medium and culture conditions
The sterilized explants were cultured on Murashige and Skoog (MS) medium[26] containing 3% sucrose, additives (50 mg/L ascorbic acid, 25 mg/L each of citric acid, L-arginin and adenine sulphate)[13] and diff erent concentrations of indole-3-acetic acid (IAA), α-naphthalene acetic acid (NAA) and 6-benzylaminopurine (BAP) for the induction of somatic embryogenesis. The medium was solidifi ed with 0.8% (w/v) agar, and the pH was brought to 5.8 with 0.1 mol/L NaOH or HCl and autoclaved at 121 °C for 15 min. All the chemicals used in the present study were procured from Himedia, Mumbai, India. The cultures were maintained in growth/ culture room at (25±2) °C under a photoperiod of 12 h/d with a light intensity of 30–40 μmol/(m2·s). Spectral fl ux photon density (SFPD) was maintained using cool white fl uorescent lamps (Philips Kolkata, India).
2.3. Microscopic studies of somatic embryogenesis
In order to understand the development of somatic embryogenesis, microscopic analysis of in vitro regenerated tissues was performed. The tissue samples were fi xed in Formalin, acetic acid, ethyl alcohol, FAA (1:1:3) and the thin sections (12 μm) were stained with 1.0% (w/v) safranin and observed under the microscope ((Labomed iVu 3100, USA) for histological studies.
2.4. Effects of growth hormones, strength of MS salts and sucrose on induction of somatic embryogenesis
Juvenile green nodal explants were cultured on MS medium incorporated either alone or in combination of BAP (0–2.0 mg/L) and IAA/ NAA (0–1.0 mg/L). To evaluate the eff ect of strength of MS salts on the induction of somatic embryogenesis, explants were cultured on optimized concentration of growth hormones (0.5 mg/L BAP and 0.25 mg/L IAA + additives) supplemented in full, 1/2 and 1/4 strength of MS salts and 3.0%, 1.50% and 0.75% sucrose. The explants were inoculated horizontally on growth medium to study the induction of somatic embryos.
2.5. Germination of somatic embryos and shoot elongation
In order to germinate the somatic embryos, the meristemoid portion with mother explants were excised and cultured on diff erent strength (full, 1/2 and 1/4) of MS medium supplemented with different concentrations of BAP ranging from 0.25, 0.50, 0.75 and 1.0 mg/L. The embryogenic callus was transferred to fresh medium for the maturation of embryos in vitro. Initially cultures were maintained in dark for a week. After induction of embryogenic potential by the explants on MS medium, the proembryo masses have been transferred to auxin free medium for elongation of adventitious shoots.
2.6. In vitro flowering
The fully elongated shoots (3–5 cm in length) of 30 d old shoot clumps from proliferated cultures were used for in vitro fl owering. These were cultured on MS medium supplemented with diff erent concentration of BAP and Kin along with IAA. To evaluate the optimum in vitro environment of light and temperature, these cultures were maintained at (25±2) °C to (28±2) °C under a photoperiod of 12–15 h/d with the light intensity of 50–70 μmol/(m2·s) SFPD.
2.7. Hardening and field transfer
Fully developed plantlets were carefully separated from culture vessels and the traces of medium removed. The rooted plantlets were transferred to the paper cups containing soilrite?(a combination of perlite with peat moss and exfoliated vermiculite procured from KelPerlite, Bangalore, India), moistened with 1/4 MS salts and kept in the greenhouse. After 5 weeks the plantlets were shifted to nursery bags containing garden soil, soilrite?, manure and vermi compost (1:1:1:1) and fi nally transplanted to the natural fi elds.
2.8. Experimental design, data collection and statistical analysis
The experiments conducted with 20 explants for each treatmentand each experiment was repeated thrice. The frequency of embryogenesis was calculated as the percentage of cultures showing somatic embryos. The results were expressed as mean ± SD of triplicates. The data were statistically analyzed using SPSS ver. 16 (SPSS Inc., Chicago, USA) and the significance of differences among mean values was carried out using Duncan’s multiple range test (DMRT) at P < 0.05.
3. Results
3.1. Induction of somatic embryogenesis
The nodal shoot segments were inoculated on the MS medium with plant growth regulators to induce somatic embryos from the surface of the explants. The resulted bipolar structures were closely resembles the zygotic embryo morphologically (Figure 1A). The maximum development of meristemoids (92%) was observed when the explants were cultured on full strength MS medium supplemented with 3.0% sucrose, 0.50 mg/L BAP and 0.25 mg/L IAA for 4 weeks (Table 1). The frequency and intensity of somatic embryogenesis were considerably high when the explants cultured on combination of BAP and IAA. BAP alone with the 1/2 and 1/4 strength MS medium fortified 1.50% and 0.75% sucrose did not support the formation of somatic embryoids (Table 2). The transition of somatic embryos from globular, heart-shaped and bipolar (torpedo) embryo were the critical steps in somatic embryogenesis (Figure 1B to 1D).
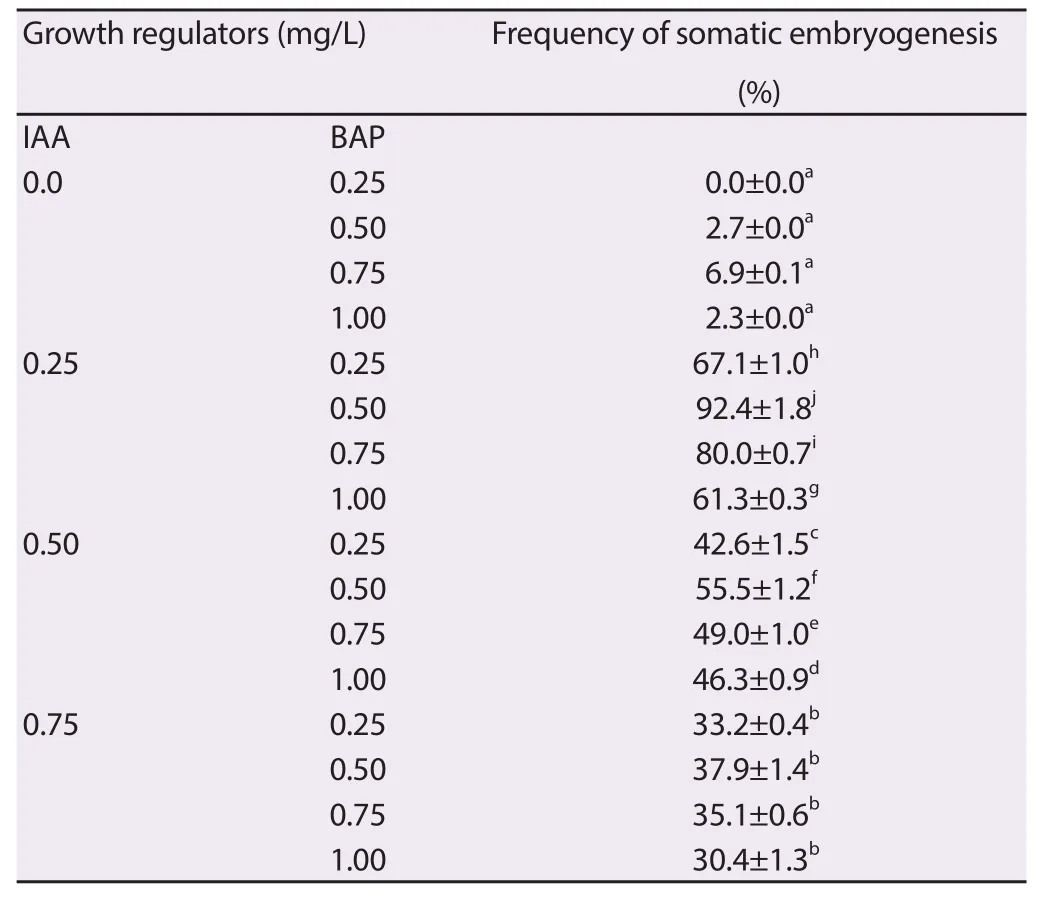
Table 1 Eff ect of BAP and IAA in induction of somatic embryogenesis.
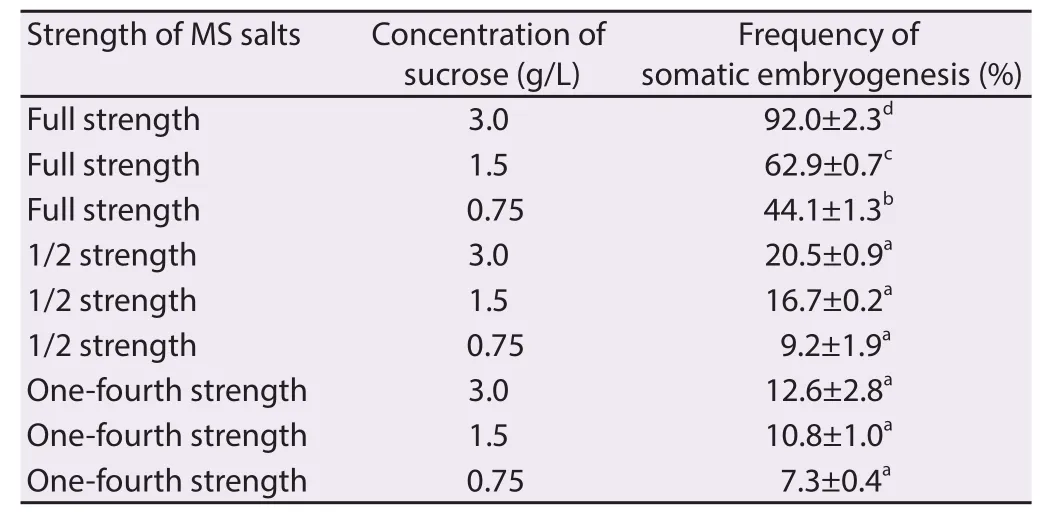
Table 2 Eff ect of strength of MS salts and concentration of sucrose on induction of somatic embryogenesis.
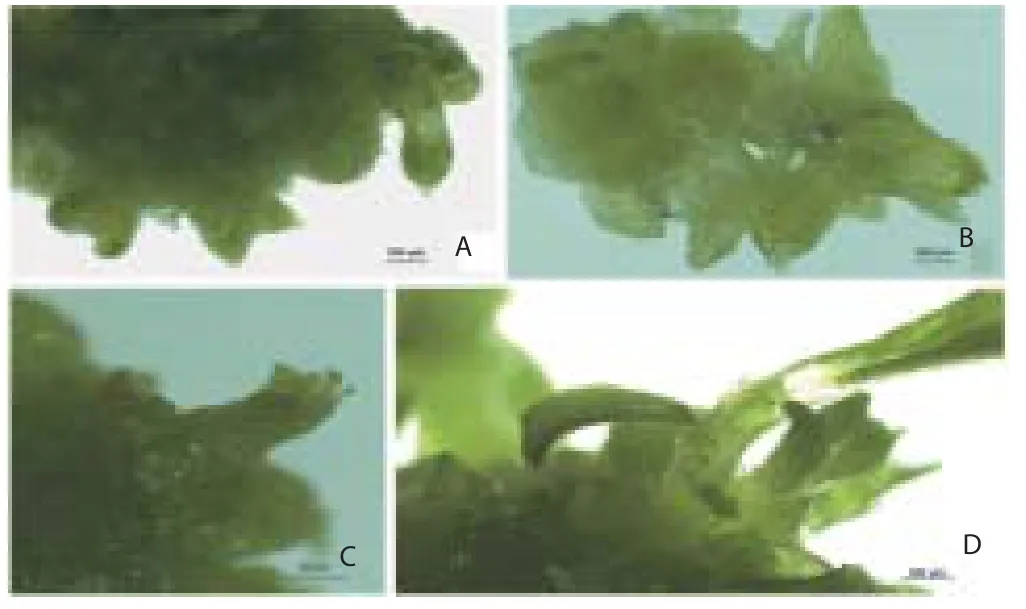
Figure 1. Diff erent stages in the development of somatic embryos. (1A and 1B) Globular and bipolar embryos from the surface of explants. (1C and 1D) Advanced stages in somatic embryogenesis.
3.2. Microscopic analysis of somatic embryos
Development of globular somatic embryos was observed after 10 d of inoculation. The globular embryos proceed further in the formation of heart, torpedo and cotyledonary stage in another 30 d (Figure 2A to 2C). Asynchronous development of somatic embryos with diff erent stages in the development of embryos was observed on the same explant. Initiation of new meristemoids was observed throughout the culture period (meristemoid, primordium, bud and shoots) as developmental stages. Microscopic studies of the intact embryogenic cell masses observed similar in appearance, size but diff erent in shapes. It has been reported that the somatic embryos were originated from single epidermal or subepidermal cells (Figure 3A to 3H).
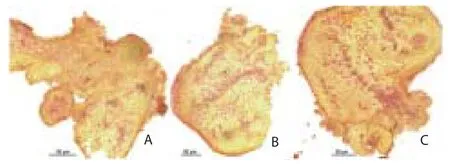
Figure 2. Microscopic study of the somatic embryogenesis from the explants. (2A and 2B) Globular and torpedo shaped embryos. (2C) Germination of embryos and formation of shoot primordia.
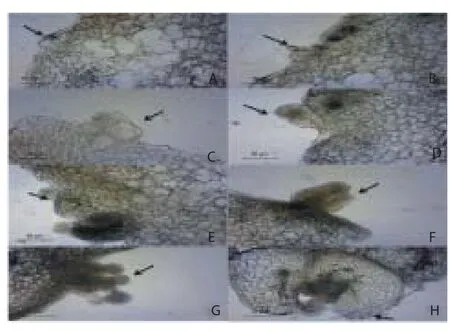
Figure 3. Developmental stages in the formation of somatic embryos.
3.3. Germination of somatic embryos and elongation of shoots
Low concentration of cytokinin (BAP) with 1/2 strength MS medium was used for germination of somatic embryos into plantlets. Somatic embryos were converted into well developed plantlets (shoots and roots) within 4 weeks when the meristemoids transferred onto 1/2 strength MS medium containing 1.50% sucrose and additives supplemented with BAP 0.50 mg/L (Figure 4A and 4C). Full and 1/4 strength MS medium were reported comparatively less eff ective in plantlet regeneration from the somatic embryos. The regular subculture of these cultures on fresh medium with the same medium combinations was able to yielded 207 plantlets per culture vessel in this study (Figure 4B and Table 3).

Figure 4. Diff erent stages of somatic embryogenesis and hardening of the plantlets.
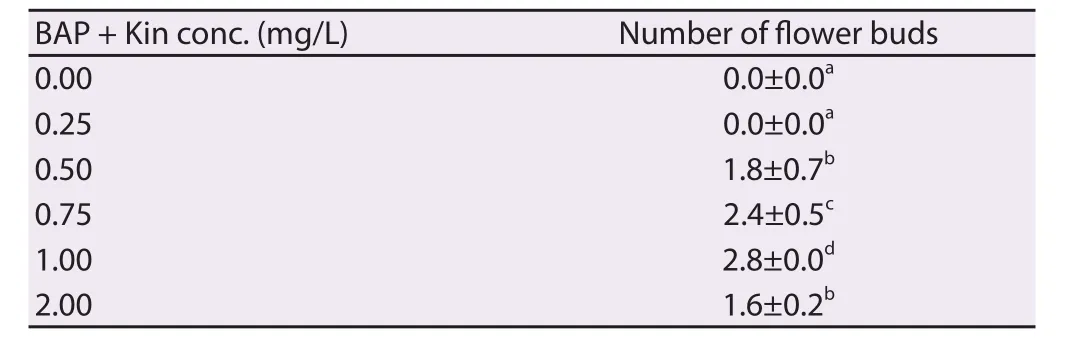
Table 3 Eff ect of BAP on germination of somatic embryos.
3.4. In vitro flowering in H. enneaspermus
In vitro induction of fl ower buds was achieved by the subculture of elongated shoots into diff erent culture environment and growth regulators. Among the plant growth regulators examined, full strength MS medium fortifi ed with additives and 1.0 mg/L each of BAP and Kin + 0.5 mg/L IAA was reported suitable for fl ower bud induction from the shoots (Table 4). The present study reveals that the lengthy shoots (4–5 cm long) only responded for photoperiod for in vitro fl owering.
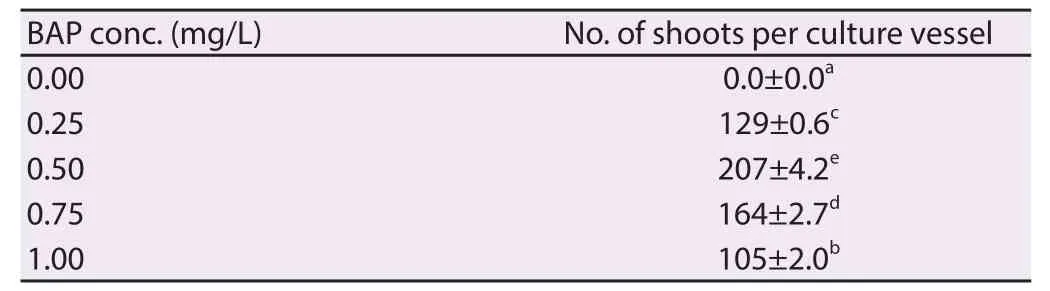
Table 4 Eff ect of cytokinins in fl ower bud induction on MS medium containing 0.5 mg/L IAA.
induction, full strength MS medium was responded positively. There was no fl ower bud observed on 1/2 and 1/4 strength MS medium with the same hormone concentrations even after 8 weeks of incubation on various SFPD. The maximum number of fl ower buds (2.8) formed at 50 μmol/(m2·s) SFPD for 13 h/d photoperiod (Figure 4D).
3.5. Hardening and field transfer
The well rooted plantlets were separated from culture vessels and transferred to the soilrite?containing cups, moistened with aqueous 1/4 MS salts solution and maintained in the greenhouse for hardening and then to the nursery poly-bags containing garden soil, soilrite?, manure and vermi compost in 1:1:1:1 ratio after 5 weeks (Figure 4E). The plantlets were hardened in the greenhouse for about 2 months. Normal fl owering and fruiting has been observed while hardening in the greenhouse (Figure 4F). About 92% of the plants were survived in the greenhouse and the hardened plants were fi nally shifted to the natural fi eld.
4. Discussion
Somatic embryos were induced by the series of morphological and biochemical changes in the somatic tissues under specifi c culture conditions. Direct somatic embryogenesis from the explants is subjected to induce embryogenic competence by culture conditions and plant growth regulators in the medium. The maximum development of meristemoids was observed when explants cultured on BAP and IAA in this study and the bipolar structures formed were morphologically similar to the zygotic embryos. The combined eff ect of BAP and IAA in direct somatic embryogenesis was also reported in Nicotiana species[27] and Solanum lycopersicum[28]. About 60% meristemoids were observed with 0.50 mg/L BAP and 0.25 mg/L NAA. These fi ndings are in contrast with the results of Ramaswamy et al.[29] in Solanum surratense and Kintzios and Michaelakis[21] in Chamomilla recutita, where NAA with BAP played signifi cant role in formation of somatic embryos.
BAP alone in the 1/2 and 1/4 strength MS medium with 1.50% and 0.75% sucrose did not support the formation of somatic embryoids in present study. Auxins, especially 2,4-D (2,4-dichlorophenoxy acetic acid) alone or in combination with other auxins play a key role in the induction of somatic embryogenesis. Even combination of auxin and cytokinin can produce suffi cient number of somatic embryos[30]. Altered response to diff erent combination of growth hormones may be due to diff erences in genetic make-up among different plant species[31]. Juvenile explants on full strength MS medium supplemented with 0.50 mg/L BAP and 0.25 mg/L IAA induced the expression of cellular totipotency in H. enneaspermus plant cells.
Normal development of somatic embryos required a fine temporal and spatial regulation of cell division, elongation and diff erentiation[32]. The growth medium and culture conditions promoted the plant cell to undergo a series of complex metabolic and morphological coordinated steps to produce a complete and normal sporophyte[30,33].
The direct somatic embryogenesis was further confi rmed through microscopic observations. It was reported from the embryogenic cell masses that the somatic embryos were originated from single epidermal or subepidermal cells. Maturation of somatic embryos is an essential phase between embryo development and germination. The accumulated storage proteins in embryos assist to develop into normal plants[34,35]. The full strength MS salts medium played a significant role in maturation of somatic embryos due to the synthesis of certain metabolites and storage proteins.
Low concentration of BAP in 1/2 strength MS medium was reported suitable for germination of somatic embryos. Since rooting was also achieved on the same medium combination from bipolar somatic embryos, further rooting experimentation was exempted. The results revealed that an optimum level of BAP concentration was needed for germination of somatic embryos and multiplication of shoots in vitro. Use of full and 1/4 strength MS medium resulted in less number of shoots with compact mass of roots caused diffi culty in separation of the plantlets.
The role of plant growth regulators, carbon, nitrogen source and minerals in development of fl oral organs has been discussed widely in number of plants[36-38]. In vitro flower buds formation was achieved by the subculture of elongated shoots into full strength MS medium at 50 μmol/(m2·s) SFPD for 13 h/d photoperiod. In vitro fl owering through somatic embryogenesis has been achieved in Brassica nigra[18] and Dendrocalamus hamiltonii[39].
The selection of explants from the mother plant determined the ability of the tissue to regenerate fl ower buds directly via somatic embryogenesis. The regeneration of fl oral organs probably achieved through transport of the fl owering stimuli and due to the consecutive subculturing. In vitro flowering of the plantlets regenerated via somatic embryogenesis from nodal explants of H. enneaspermus was demonstrated for the fi rst time. It could provide better understanding of nature of various factors infl uence the in vitro fl owering in this plant. The plantlets were hardened in the greenhouse and transferred to the natural fi eld conditions after 6 weeks. Plantlets regenerated through direct somatic embryogenesis and direct shoots induction have been reported to be genetically uniform[40-42] so that the genetic stability of in vitro propagated plants could be true to type.
In conclusion, the development of somatic embryos from sporophytic cells of H. enneaspermus has off ered a great potential for the production of plantlets and its biotechnological manipulation, this could be exploited for the mass propagation of H. enneaspermus. The somatic embryos were germinated efficiently and in vitro fl owering was observed. Direct somatic embryogenesis and in vitro fl owering in H. enneaspermus were reported without intervention of callus phase. In vitro fl owering off ers better understanding of culture environments required by the plant for transition from vegetative to reproductive phase. The study could be used for mass scale production of this multipotent plant species.
Declare of interest statement
The authors disclose that they have no confl ict of interest in this publication.
Acknowledgments
Authors are grateful to the University Grants Commission, New Delhi, India and the DST&E, Government of Puducherry for providing funds to our laboratory.
[1] Awobajo FO, Olatunji-Bello II, Adegoke OA, Odugbemi TO. Phytochemical and antimicrobial Screening of Hybanthus enneaspermus and Paquentina nigricense. Recent Res Sci Technol 2009; 1: 159-160.
[2] Joseph, TS, Skaria BP, Sajithakumari. Disappearing medicinal plant resources of Kottayam district of Kerala State. Indian J Arecanut Spices Medicinal Plants 2000; 2: 79-81.
[3] Amuthapriya D, Ranganayaki S, Suganya DP. Phytochemical screening and antioxidant potential of Hybanthus enneaspermus: A rare ethnobotanical herb. J Pharm Res 2011; 4: 1497-1502.
[4] Sudeesh S. Ethnomedicinal plants used by Malyaraya tribes onVannapuram village in Idukki, Kerala, India. Indian J Sci Technol 2011; 1: 7-11.
[5] Nathiya S, Selvi SR. Anti-infertility eff ect of Hybanthus enneaspermus on endosulfan induced toxicity in male rats. Int J Med Biosci 2013; 2: 28-32.
[6] Satheeshkumar D, Kottai MA, Manavalan R. Antioxidant potential of various extracts from whole plant of Ionidium suffruticosum (Ging). Res J Pharma Biol Chem Sci 2011; 2: 286-293.
[7] Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (L.) F. Muell. in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 2011; 1: 316-322.
[8] Kumar SB, Kumar JV, Selvaraj R. Aphrodisiac activity of Cycas circinalis and Ionidium suffruticosum Ging. on male Wister albino rat. Asian J Pharma Clinical Res 2013; 6: 215-217.
[9] Arunkumar BS, Jayaraj M. Rapid in vitro callogenesis and phytochemical screening of leaf and leaf callus of Ionidium suffruticosum, Ging.- A seasonal multipotent medicinal herb. World J Agri Sci 2011; 7: 55-61.
[10] Deo PC, Taylor M, Harding RM, Tyagi AP, Becker DK. Initiation of embryogenic cell suspensions of taro (Colocasia esculenta var. esculenta) and plant regeneration. Plant Cell Tiss Org Cult 2010; 100: 283-291.
[11] Pence VC. In vitro methods and the challenge of exceptional species for Target 8 of the Global Strategy for Plant Conservation. Ann Missouri Bot Gard 2013; 99: 214-220.
[12] Ptak A. Somatic embryogenesis in in vitro culture of Leucojum vernum L. Methods Mol Biol 2010; 589: 223-233.
[13] Rathore JS, Rai MK, Shekhawat NS. Induction of somatic embryogenesis in gum Arabic tree (Acacia Senegal (L.) Willd.). Physiol Mol Biol Plants 2012; 18: 387-392.
[14] Raju CS, Aslam A, Shajahan A. High-efficiency direct somatic embryogenesis and plant regeneration from leaf base explants of turmeric (Curcuma longa L.). Plant Cell Tiss Organ Cult 2015; 122: 79-87.
[15] Kaur R, Kapoor M. Plant regeneration through somatic embryogenesis in Sugarcane. Sugar Tech 2015; 1-7.
[16] Rai MK, Akhtar N, Jaiswal VS. Somatic embryogenesis and plant regeneration in Psidium guajava L. cv. Banarasi local. Sci Hort 2007; 113: 129-133.
[17] Litz RE, Gray DJ. Organogenesis and somatic embryogenesis. In: Hammerschlag FA, Litz RE, editors. Biotechnology of Perennial Fruit Crops. Wallingford: CAB International; 1992: 3-34.
[18] Mehta UJ, Hazra S, Mascarenhas AF. Somatic embryogenesis and in vitro fl owering in Brassica nigra. In vitro Cell Dev Biol 1993; 29: 1-4.
[19] Simpson GG, Gendall AR, Dean C. When to switch to fl owering. Annu Rev Cell Dev Biol 1999; 99: 519-550.
[20] Rout GR, Das P. Somatic embryogenesis and in vitro flowering of 3 species of bamboo. Plant Cell Rep 1994; 13: 683-686.
[21] Kintzios S, Michaelakis A. Induction of somatic embryogenesis and in vitro fl owering from infl orescence of chamomile (Chamomilla reticulatta L.). Plant Cell Rep 1999; 18: 684-690.
[22] Sudarshana MS, Nianjan MH, Girisha ST. In vitro flowering, somatic embryogenesis and regeneration in Boerhaavia diffusa Linn.- A medicinal plant. Global J Biotech Biochem 2008; 3: 83-86.
[23] Arunkumar BS, Jayaraj M, Bagadekar AN, Bhandarkar V. In vitro propagation of Ionidium suffruticosum Ging. - A seasonal multipotent medicinal herb. Plant Tiss Cult Biotech 2009; 19: 143-150.
[24] Velayutham P, Karthi C, Nalini P, Jahirhussain G. In vitro regeneration and mass propagation of Hybanthus enneaspermus (L.) F. Muell. from the stem explants through callus culture. J Agri Techol 2012; 8: 1119-1128.
[25] Premkumar G, Arumugam N, Muthuramkumar S, Varatharaju G, Rajarathinam K. Improved micropropagation in Hybanthus enneaspermus L. Muell. Amer J Plant Sci 2013; 4: 1169-1172.
[26] Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 1962; 15: 473-497.
[27] Pathi KM, Tula S, Tuteja N. High frequency regeneration via direct somatic embryogenesis and effi cient Agrobacterium-mediated genetic transformation o tobacco. Plant Signal Behav 2013; 8: 1-8.
[28] Godishala V, Mangamoori L, Nanna R. Plant regeneration via somatic embryogenesis in cultivated tomato (Solanum lycopersicum L.). J Cell Tiss Res 2011; 11: 2521-2528.
[29] Ramaswamy N, Ugandhar T, Praveen M, Venkataiah P, Rambabu M, Upender M, et al. Somatic embryogenesis and plantlet regeneration from cotyledon and leaf explants of Solanum surattense. Indian J Biotech 2005; 4: 414-418.
[30] Jimenez VM. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 2005; 47: 91-110 [31] Xie DY, Hong Y. Regeneration of Acacia mangium through somatic embryogenesis. Plant Cell Rep 2001; 20: 34-40
[32] Ammirato PV. Organizational events during somatic embryogenesis. In: Green CE, Somers DA, Hackett WP, Biesboer DD, editors. Plant Tissue and Cell culture. New York: AR Liss; 1987: 57-81.
[33] Rao KS. Embryogenesis in flowering plants: recent approaches and prospects. J Biosciences 1996; 21: 827-841.
[34] Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L, Developmental pathways of somatic embryogenesis. Plant Cell Tiss Org Cult 2002; 69: 233-249.
[35] Rai MK, Jaiswal VS, Jaiswal U. Effect of ABA and sucrose on germination of encapsulated somatic embryos of guava (Psidium guajava L.). Sci Hort 2008; 117: 302-305.
[36] Dielen V, Lecouvet V, Dupont S, Kinet JM. In vitro control of floral transition in tomato (Lyco?persicon esculentum Mill.), the model for autono?mously fl owering plants, using the late fl owering uniflora mutant. J Exp Bot 2001; 52: 715-723.
[37] Naor V, Kigel J, Ziv M. Hormonal control of infl orescence development in plantlets of Calla lily (Zantedeschia spp) grown in vitro. Plant Growth Regul 2004; 42: 7-14.
[38] Xu HY, Li XG, Li QZ, Bai SN, Lu WL, Shang XS. Characterization of HoMADS1 and its induction by plant hormones during in vitro ovule development in Hyacinthus orientalis L. Plant Mol Biol 2004; 55: 209-220.
[39] Kaur D, Thapa P, Sharma M, Bhattacharya A, Sood A. In vitro fl owering– A system for tracking floral organ development in Dendrocalamus hamiltonii Nees et Arn. Ex Munro. Indian J Exp Biol 2014; 52: 825-834.
[40] Rani V, Raina SN. Genetic fidelity of organized meristem derived micropropagated plants: a critical reappraisal. In vitro Cell Dev Biol Plant 2000; 36: 319-330.
[41] Shekhawat MS, Shekhawat NS. Micropropagation of Arnebia hispidissima (Lehm). DC. and production of alkannin from callus and cell suspension culture. Acta Physiol Plant 2011; 33: 1445-1450.
[42] Shekhawat, MS, Kannan N, Manokari M, Ravindran CP. Enhanced micropropagation protocol of Morinda citrifolia L. through nodal explants. J Appl Res Med Aromat Plant 2015; 2(4): 174-181.
ent heading
10.1016/j.apjr.2016.04.005
*Corresponding author: Mahipal S. Shekhawat, Biotechnology Laboratory, Department of Plant Science, M.G.G.A.C., Mahe, Pondicherry-673311, India.
Tel: +918943157187
E-mail: smahipal3@gmail.com
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
- Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
- Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
- Changes in sperm characteristics of the three main breeds of sheep in Algeria after dietary supplementation
