Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
Mark Anthony C. Mamon, Julie Andrea P. A?ano, Louise C. Abanador, Genesis Julyus T. Agcaoili, Caira B. Sagum, Ria Lorraine H. Pagliawan, Jean Mherck B. Tapere, Jessie Bob M. Agravante, John Harold G. Arevalo, Abdul Jabbar A. Minalang
Science Department, Las Pi?as National High School, Las Pi?as City, Philippines
Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
Mark Anthony C. Mamon*, Julie Andrea P. A?ano, Louise C. Abanador, Genesis Julyus T. Agcaoili, Caira B. Sagum, Ria Lorraine H. Pagliawan, Jean Mherck B. Tapere, Jessie Bob M. Agravante, John Harold G. Arevalo, Abdul Jabbar A. Minalang
Science Department, Las Pi?as National High School, Las Pi?as City, Philippines
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Perna viridis
Manila Bay
Pollution
Allometric parameters
Histological structure
Objective: To determine the eff ects of the water quality of Manila Bay on allometric parameters and histological biomarkers of selected organs of Perna viridis (P. viridis). Methods: Green mussels were collected from two coastal sites of Manila Bay, Las Pi?as–Para?aque (LPP) and Bacoor, Cavite (BC). Twenty-four green mussels from each site were used for the assessment of allometric parameters, and six green mussels from LPP and eight from BC were used for the assessment of histological structures of gonads, gut, and digestive glands. Gonad development were categorized into f ive stages, whereas gut and digestive glands were scored into four categories. Results: Allometric parameters that include shell height, weight, and total wet and dry soft tissue weight were signifi cantly diff erent between LPP and BC. It was also observed that exposure to the pollutants in Manila Bay resulted to delays in gonadal development, and detrimental changes and lesions in the histostructure of digestive gland and gut. Conclusions: Pollutants in Manila Bay have detrimental eff ects to the growth, reproductive development, and histological structure of digestive organs of P. viridis.
1. Introduction
Water pollution is one of the biggest problems that our world is facing. Water is considered polluted when certain substances or contaminants are present and makes it unfi t for specifi c purposes. These pollutants are mostly products of anthropogenic activities, specifi cally as agricultural, domestic and industrial wastes. Common pollutants include heavy metals such as lead, copper, chromium, cadmium, and mercury[1,2]. Moreover, pesticides used in protecting crops and other plants are also considered pollutants once they are introduced in the water system[3].
In the Philippines, many bodies of water are now classified as massively polluted. One of which is Manila Bay, a semienclosed marine inlet surrounded by Metro Manila and diff erent municipalities of Cavite, Bulacan, Bataan, and Pampanga[4-6]. At present, it has deteriorating water quality because of the intensifi ed disposal of human wastes[5,6]. Water samples collected from the coastal lagoon of Manila Bay have varying levels of heavy metals[6,7], polycyclic aromatic hydrocarbon (PAH)[8], benzotriazole ultraviolet stabilizers and organophosphorus fl ame retardants and plasticizers[9,10], and tribultylin (TBT). These water pollutants directly endanger the health of aquatic animals[2] resulting to a decline of marine resource production.
Perna viridis (P. viridis), commonly known as Asian green mussel or tahong, is one of the marine resources that are harvested in the coastal areas of Manila Bay. Green mussels generally grow on hard surfaces, and is said to be invasive for its wide range of tolerance. However, a recent report showed that there is a decline in the population of this aquatic species[6]. According to DENR (2004), the decline in mollusk production is attributed to the high levels of heavy metals, oil and grease, and suspended solids in Manila Bay. This problem in green mussel population tremendously aff ects the livelihood of people living in the coastal areas of the bay which rely mostly on fi sheries and aquaculture[9]. As a common and aff ordable food and rich source of iodine, green mussels are available in the local markets in the cities and municipalities around Manila Bay. Aside from the alarming state of P. viridis population, thismollusk species is considered as a bioindicator responding well to environmental changes. It was reported that green mussel is an organism suited for biomonitoring studies of coastal waters because of their widespread geographical distribution, sedentary mode of life, and fi lter feeding mechanism[11,12]. As a fi lter feeder, they have the ability to store and accumulate several organic and inorganic contaminants[13], which is used for studies in detecting chemical pollutants in the coastal waters and their possible effects to the aquatic organism. Pollution impacts to organisms can be assessed or measured through biomarkers at the cellular, histological, molecular, biochemical or physiological level[11]. The abundance of P. viridis in Manila Bay is a good criterion for its use as a bioindicator, specifi cally in determining the eff ects of chemical pollutants present in the bay on bivalves. This will clarify and explain the reports on the gradual decline of mollusk production, which vehemently results to economic losses in the aquaculture sector. At present, no other studies were conducted on determining the health and growth status of green mussels in Manila Bay.
Hence, this study was conducted to determine the effects of the water quality of Manila Bay on allometric parameters and histological biomarkers of selected organs of green mussels (Perna viridis). Specifically, this study assessed shell and soft tissue allometric parameters and histological structures of the gonads, gut and digestive glands of P. viridis collected from Manila Bay.
2. Materials and methods
2.1. Sampling of Green mussels (P. viridis)
Green mussels (P. viridis) were collected from two coastal sites of Manila Bay, Las Pi?as–Para?aque (LPP) and Bacoor, Cavite (BC). Form each site, 30 green mussels were randomly collected by the local fi sherman. The collected samples were placed in a container with water from the Bay, and were immediately transported to the Science Laboratory of Las Pi?as National High School (LPNHS) for assessment.
2.2. Taxonomic identification of P. viridis
The taxonomic identifi cation of green mussels collected from two sampling sites of Manila Bay was based on the certifi cation of the Zoology Division, National Museum of the Philippines.
2.3. Assessment of allometric parameters
Twenty-four green mussels from each site were used for the assessment of allometric parameters. Using a ruler, the following shell dimensions were measured in centimeters: shell length (SL) measured from anterior to posterior; shell width (SWI) measured in the lateral edge; and shell height (SH) measured from dorsal to ventral[14]. Shell volume (SV) was computed using this formula: Shell volume (SV) = SL x SWI x SH.
Using an electronic weighing scale, shell weight (SWE), total wet (WWE) and dry soft tissue weights (DWE) were measured in grams[14]. The wet soft tissue was weighed 10 min after it was removed from the container full of water[15]. The dry soft tissue on the other hand, was weighed after the soft tissues were dried for at least 60 °C for 48 h[16].
Consequently, condition index (CI) was computed by dividing total dry soft tissue weight in grams to shell volume in cubic centimeters multiplied to a constant 1 000[14].
CI (g/cm3) = Total dry soft tissue weight (g)/Shell volume (cm3) x 1 000.
2.4. Histopathological assessment
Six green mussels from LPP and eight from BC were used for the assessment of histological structures. Gonads and guts with digestive glands were dissected out and were placed in 10% buff ered formalin. The samples were brought to the Philippine Kidney Dialysis Foundation Institute for histopathological slide preparation. Each organ was stained with hematoxylin and eosin. Histological slides were observed under an inverted microscope at 200 x and 400 x. The histopathological structure of gut epithelium and digestive gland were scored based on the following observed characteristics: 0 – no recognizable lesions; 1 – few lesions; 2 – adequate number of lesions; and 3 – several/many lesions[17].
Furthermore, gonads were assessed based on the following developmental stages: Stage I – undiff erentiated gonad; Stage II – early development stage; Stage III – mature stage; Stage IV – spawning stage; and Stage V – post-spawning stage[18].
2.5. Statistical analysis
Statistical differences of data on allometric parameters among groups were analyzed using t test. Data were presented as mean±SD (standard deviation), and were signifi cant at P-values < 0.05. The statistical test was conducted using Microsoft Excel.
3. Results
3.1. Allometric parameters
Table 1 shows that there are no signifi cant diff erences in the shell length (SL), shell width (SWI), shell volume (SV), and condition index (CI) of green mussels in LPP and BC. However, signifi cantlylower mean shell height (SH), shell weight (SWE), total wet soft tissue weight (WWE), and total dry soft tissue weight (DWE) were recorded in green mussels collected from LPP compared to samples collected from BC.
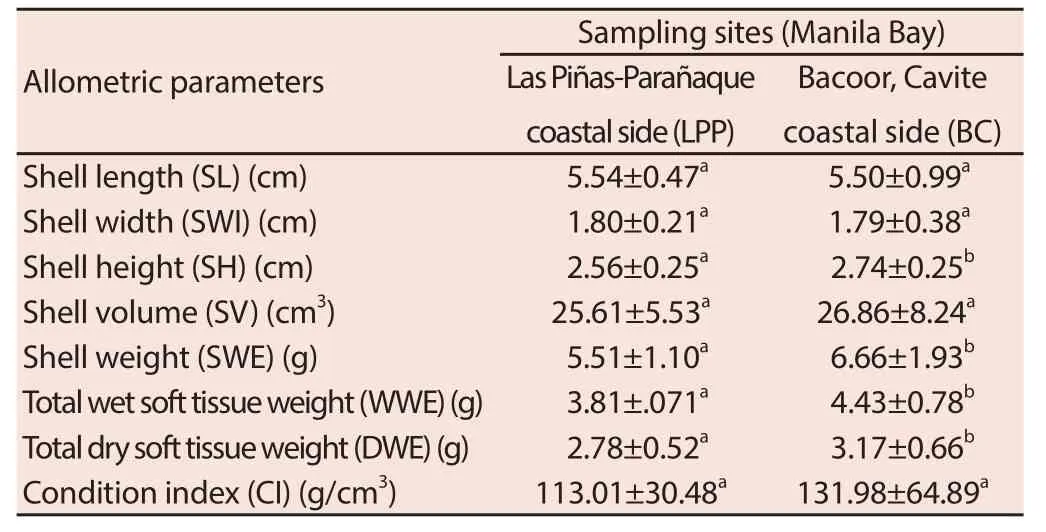
Table 1 Allometric parameters of P. viridis collected from two sampling sites in Manila Bay (n = 24).
3.2. Histopathological assessment
The percentage of green mussels in each gonad developmental stages is shown in Table 2. There is a higher percentage of male green mussels with advanced gonad developmental stage from BC compared to samples from LPP. It was recorded that 100.00% of male green mussels from BC is on the spawning stage (Stage 4), whereas 33.33% of males from LPP are in developing stage (Stage 2) and only 66.67% are in spawning stage (Stage 4). Male green mussels in the developing stage have gonads in early spermatogenesis[18]. Representative photomicrographs of male gonads from each sampling sites are shown in Figure 1.Same observations were also recorded in female green mussels. Among the collected samples from BC, about 20% of females are in developing stage (Stage 2), 60% in spawning stage (Stage 4), and 20% in post-spawning stage. In LPP samples, 66.67% of females are in developing stage (Stage 2), and only 33.33% are matured (Stage 3). Females in stage 2 have gonads in early vitellogenesis, and mussels in Stage 3 have gonads in late vitellogenesis[18]. Representative photomicrographs of female gonads from each sampling site are shown in Figure 2.
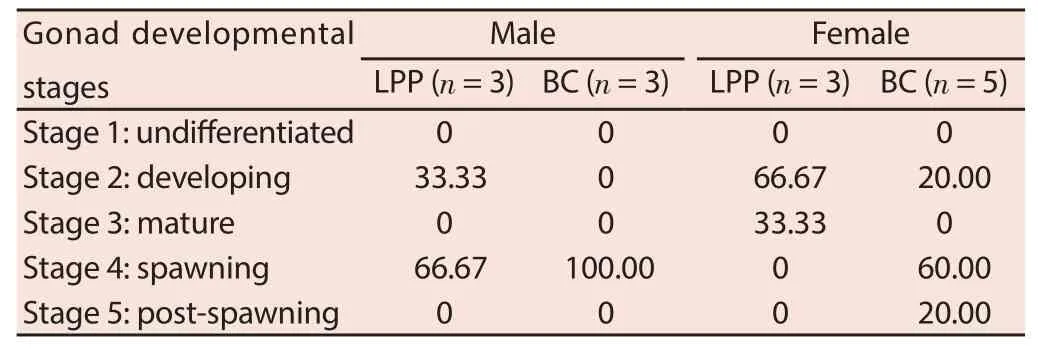
Table 2 Percentage of green mussels in each gonad development stages collected from two sampling sites in Manila Bay (%).

Figure 1. Male gonads of green mussels from two sampling sites at 200 x magnifi cation.
Figure 2. Female gonads of green mussels from two sampling sites at 200 x magnifi cation.
Table 3 shows the percentage of green mussels in each histopathological scoring of gut epithelium and digestive gland. Samples from both Manila bay coastal areas have lesions in the gut epithelium and digestive glands. However, there is a higher percentage of green mussels from BC with gut epithelium having lesions and abnormal histological structure compared to samples from LPP. About 33.33% of green mussels from BC have gut epithelium with adequate number of lesions (score 2) and 16.67% of mussels with several lesions (score 3). No green mussels from LPP have several lesions, however the percentage of samples with adequate number of lesions from this sampling site is similar to BC. Representative photomicrographs of gut epithelium from each sampling site are shown in Figure 3.
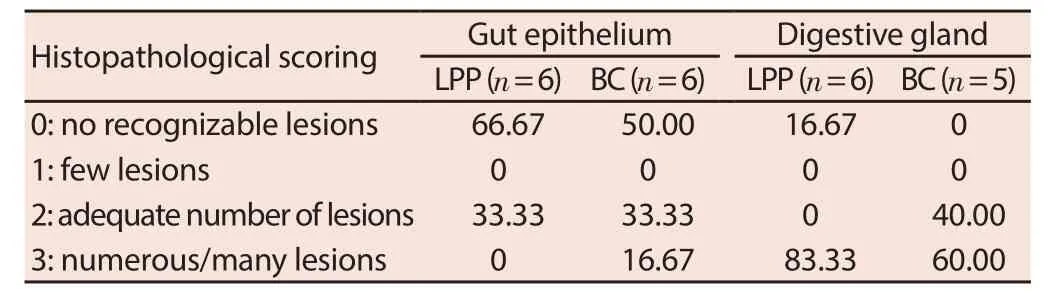
Table 3 Percentage of green mussels in each histopathological scoring of gut epithelium and digestive gland (%).

Figure 3. Gut epithelium of green mussels from two sampling sites at 200 x and 400 x magnifi cation.
In contrast to the fi ndings in gut epithelium, severe cases of lesions and necrotic tissues were observed in the digestive glands of green mussels from LPP. About 83.33% of green mussels from this site have digestive glands with several lesions (Stage 3) compared to the 60% of green mussels from BC. However, about 40% of green mussels from BC have digestive glands with adequate number of lesions. Representative photomicrographs of digestive glands from each sampling site are shown in Figure 4.
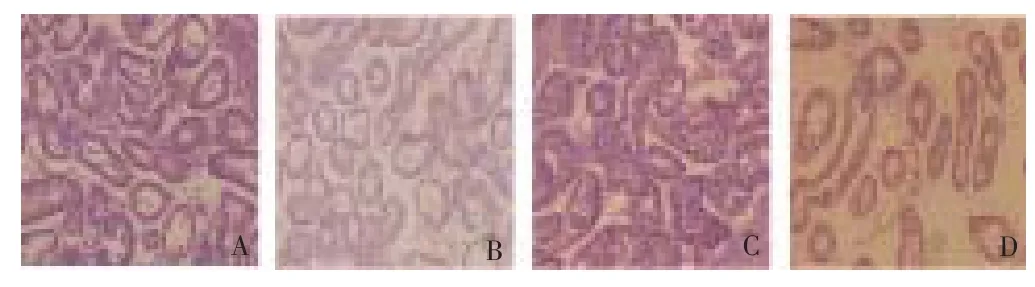
Figure 4. Digestive glands of green mussels from two sampling sites at 400 x magnifi cation.
4. Discussion
Growth was seen affected by the water quality of Manila Bay, specifi cally shell and total soft tissue weight. One of the factors that tremendously aff ected growth and development of mussels and other bivalves is oxygen depletion in the water. Substantial increments in organic loads entering the bay through excessive urban emissions of nutrients (nitrogen and phosphorus), could lower the concentration of dissolved oxygen at the bottom, specifi cally in the inner bay area[5]. Other factors that cause hypoxia and anoxia include heavy metals incidences, frequent blooms of harmful microalgae and persistent red tides caused by dinofl agellates[19]. The lower allometric parameters of P. viridis from LPP compared to BC can be due to a more hypoxic and anoxic marine environment.
High levels of heavy metals in the bodies of water can be the major factor for the decreased growth of green mussels. According to a study, P. viridis collected from Muar, Peninsular Malaysia had lower total dry weight of soft tissues and condition index than those collected from Sebatu, Peninsular Malaysia[14]. These results could probably be connected to the higher levels of cadmium, copper, and lead in the coastal sediments of Muar compared to Sebatu. Moreover, a reported study mentioned that the sublethal exposure of the freshwater swan mussel (Anodonta cygnea) to chromium, copper, and lead resulted to severe changes in shell composition and morphology[20]. This report strengthens the results of this study, because the heavy metals that were mentioned are detected in Manila bay, which could probably alter the shell dimensions of mussels. Organic contaminants such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs), which are present in Manila Bay, were also reported to affect the bivalve’s growth and development. These contaminants aff ect primarily the rates of respiration and carbon turnover in mussels, which have high correlation to inhibited growth[21].
Pollutants in both sampling sites of Manila Bay can cause endocrine disruption. These chemicals that include heavy metals and organic pollutants disrupt the activities of androgen and estrogen. Delays in the gonad developmental stages of mussels in this study are the result of the irregular activities of estrogen and androgen receptors[22]. It must be noted that the eff ects on hormonal activity depends upon the level of chemical pollutants in the environment. It was reported that aside from the adverse effects of organic contaminants to growth, these pollutants disrupt normal reproduction, fecundity and embryonic development of blue mussel (Mytilus edulis) and soft shell clam (Mya arenaria)[21]. Heavy metals, which are endocrine disruptors, accumulate in the gonads, adductor muscle, mantle, and foot of mussels[23]. It was mentioned in a study that the mussels (Mytilus edulis) collected from the polluted and contaminated waters of Bothnian Sea had abnormal histopathological alterations in the gonads, resulting to abnormal reproduction and spawning[24]. Specifi cally, it was observed that follicles were in low quality and sperm cells undergo cytolysis. These reports support the fi ndings of this study that pollutants have deleterious eff ects to gonadal development of mussels.
Aside from gonads, the gut and digestive glands are the most delicate organs to pollutant exposure. Pollutants and contaminants in Manila bay cause degeneration of cells resulting to lesions and abnormal histological structure of these organs[17]. The digestive glands and gut are the major organs of metabolism and detoxification. The lysosomes in the cells of digestive glands act as major defense lines against the toxic eff ects of pollutants. However, if the digestive glands cannot manage massive levels of contaminants, defects are observed on the functions of lysosomes[25]. Aside from delayed and abnormal gonadal development, mussels collected from the polluted waters of Bothnian Sea were reported to have abnormal histopathological alterations in the digestive diverticula[24]. Cytoplasmic erosions were observed in the digestive cells of these mussels. Furthermore, mussels collected from the polluted water of Basque coast, which has recorded levels of inorganic and organic contaminants, had infl amed and damaged digestive glands[26]. These reports support the fi ndings of this study that pollutants could aff ect the histostructure of the gut and digestive gland
In general, this study demonstrated that the chemical pollutants and contaminants in Manila Bay, which were already identifi ed by other research studies, have detrimental eff ects to some of the growth parameters of green mussels that include shell height, weight, and total soft tissue weight. Moreover, pollutant exposure resulted to delays in gonadal development, and caused lesions and abnormal histological structure of digestive glands and gut in green mussels
Declare of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
We would like to thank Mrs. Eugenia V. Guerra and Mrs. Laprizal Castueras, the principal and science head teacher of Las Pi?as National High School, for supporting this research study and for providing us assistance in our research needs.
[1] Duruibe JO, Ogwuegbu MOC, Egwurugwu JN. Heavy metal pollution and human biotoxic eff ect. Int J Phys Sci 2007; 2(5): 112-118.
[2] Molina VB, Espaldon MVO, Flavier ME, Pacador EP. Bioaccumulation in Nile Tilapia (Oreochromis niloticus) from Laguna de Bay, Philippines. J Environ Sci Manage 2011; 14(2): 28-35.
[3] Deka S, Mahanta R. A study on the eff ect of organophosphorus pesticide malathion on hepato -renal and reproductive organs of Heteropneustes fossilis (Bloch). Sci Probe 2012; 1(1): 1-13.
[4] Velasquez IB, Jacinto GS, Valera FS. The speciation of dissolved copper, cadmium, and zinc in Manila Bay, Philippines. Mar Pollut Bull 2002; 45: 210-217.
[5] Chang KH, Amano A, Miller TW, Isobe T, Maneja R, Siringan FP, et al. Pollution study in Manila Bay: eutrophication and its impact on plankton community. In: Obayashi Y, Isobe T, Subramanian A, Suzuki S, Tanabe S, editors. Interdisciplinary Studies on Environmental Chemistry-Environmental Research in Asia. 2009: 261-267.
[6] Sia Su G, Martillano KJ, Alcantara TP, Ragragio E, De Jesus J, Hallare A, et al. Assessing heavy metals in the waters, fi sh, and macroinvertebrates in Manila Bay, Philippines. J Appl Sci Environ Santitation 2009; 4(3): 187-195.
[7] Sia Su GL, Ramos GB, Sia Su MLL. Bioaccumulation and histopathological alteration of total lead in selected fi shes from Manila Bay, Philippines. Saudi J Biol Sci 2013; 20: 353-355.
[8] Santiago EC. The levels and distribution of polycyclic aromatic hydrocarbons (PAH) contamination in bottom sediments in Manila Bay. Sci Diliman 1997; 9(1 & 2): 16-28.
[9] Kim JW, Isobe T, Ramaswamy BR, Chang KH, Amano A, Miller TM, et al. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography-tandem mass spectrometry. Chemosphere 2011; 85: 751-758.
[10] Kim JW, Isobe T, Chang KH, Amano A, Maneja RH, Zamora PB, et al. Levels and distribution of organophosphorus fl ame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ Pollut 2011; 159: 3653-3659.
[11] Tsangaris C, Kormas K, Strogyloudi E, Hatzianestis I, Neofitou C, Andral B, et al. Multiple biomarkers of pollution eff ects in caged mussels on the Greek coastline. Comp Biochem Physiol C Toxicol Pharmacol 2010; 151(3): 369-378.
[12] Putri LSE, Prasetyo AD, Arifin Z. Green mussel (Perna viridis L.) as bioindicator of heavy metal pollution at Kamal Eastuary, Jakarta Bay, Indonesia. J Environ Res Develop 2012; 6(3): 389-396.
[13] Dailianis S. Environmental impact of anthropogenic activities: the use of mussels as a reliable tool for monitoring marine pollution. In: McGevin LE, editor. Mussels: Anatomy, Habitat and Environmental Impact. New York: Nova Science Publishers, Inc.; 2011: 43-72.
[14] Yap CK, Al-Barwani SM. A comparative study of condition indices and heavy metals in Perna viridis populations at Sebatu and Muar, Peninsular Malaysia. Sains Malaysiana 2012; 41(9): 1063-1069.
[15] Bura M, Nagy RM, Hevesi C, Dunea IB. Morphometric studies on Anodonta anatina Bivalve Population from the Dognecea Lake. Lucrari Stiintifice Zootehnie Si Biotehnologii 2011; 44(2): 9-12
[16] Thejasvi A, Shenoy C, Thippeswamy S. Morphometric and lengthweight relationships of the green mussel, Perna viridis (Linnaeus) from a subtidal habitat of Karwar Coast, Karnataka, India. Int J Recent Sci Res 2014; 5(1): 295-299.
[17] Mantecca P, Vallati G, Bacchetta R. Histologic changes and micronucleus induction in the Zebra mussel Dreissena polymorpha after paraquat exposure. Histol Histopathol 2006; 21: 829-840.
[18] Saavedra L, Leonardi M, Morin V, Qui?ones R. Induction of vitellogeninlike lipoproteins in the Aulacomya ater under exposure to 17β-estradiol. Revista de Biologia Marina y Oceanografia 2012; 47: 429-438
[19] Prudente MS, Ichihashi H, Tatsukawa R. Heavy metal concentrations in sediments from Manila Bay, Philippines and inflowing rivers. Environ Pollut 1994; 86: 83-88.
[20] Lima ML, Freitas S, Pereira L, Gouveia E, Hinzmann M, Checa A, et al. Ionic regulation and shell mineralization in the bivalve Anodonta cygnea (swan mussel) following heavy-metal exposure. Can J Zool 2012; 90(2): 267-283.
[21] McDowell JE, Lancaster BA, Leavitt DF, Rantamaki P, Ripley B. The eff ects of lipophilic organic contaminants on reproductive physiology and disease processes in marine bivalve molluscs. Limnology Oceanogr 1999; 44(3): 903-909.
[22] Bayen S, Gong Y, Chin HS, Lee HK, Leong YE, Obbard JP. Androgenic and estrogenic response of green mussel extracts from Singapore’s coastal environment using a human cell-based bioassay. Environ Health Persp 2004; 112(15): 1467-1471.
[23] Ponnusamy K, Sivaperumai P, Suresh M, Arularasan S, Munilkumar S, Pal AK. Heavy metal concentration from biologically important edible species of bivalves (Perna viridis and Modiolus metcalfei) from Vellar Estuary, South East Coast of India. J Aquaculture Res Develop 2014; 5(5).
[24] Sunila I. Histopatholical changes in the mussel Mytilus edulis L. at the outlet from a titanium dioxide plant in Northern Baltic. Annales Zoologici Fennici 1986; 23: 61-70.
[25] Krishnakumar PK, Casillas E, Varanasi U. Effect of environmental contaminants on the health of Mytilus edulis from Puget Sound, Washington, USA. I. Cytochemical measures of lysosomal responses in the digestive cells using automatic image analysis. Marine Ecol Progr Ser 1994; 106, 249-261
[26] Cuevas N, Zorita I, Costa PM, Franco J, Larreta J. Development of histopathological indices in the digestive gland and gonad of mussel: intergration with contamination levels and eff ects of confounding factors. Aquatic Toxicol 2015; 162: 152-164.
ent heading
10.1016/j.apjr.2016.03.002
*Corresponding author: Mark Anthony C. Mamon, Science Department, Las Pi?as National High School, Las Pi?as City, Philippines
E-mail: markcatedralmamon@yahoo.com
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
- Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
- Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
- Changes in sperm characteristics of the three main breeds of sheep in Algeria after dietary supplementation
