Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
Davood Mehrabani, Marzieh Tajedini, Amin Tamadon*, Mehdi Dianatpour,3, Fatemeh Parvin, Shahrokh Zare, Farhad Rahmanifar
1Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Graduated from School of Veterinary Medicine, Shiraz University, Shiraz, Iran
3Department of Medical Genetics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
4Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
Establishment, characterization and cryopreservation of Fars native goat fetal fibroblast cell lines
Davood Mehrabani1, Marzieh Tajedini2*, Amin Tamadon1*, Mehdi Dianatpour1,3, Fatemeh Parvin1, Shahrokh Zare1, Farhad Rahmanifar4
1Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Graduated from School of Veterinary Medicine, Shiraz University, Shiraz, Iran
3Department of Medical Genetics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
4Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Cell culture
Fibroblast
Cryopreservation
Fetus
Goat
Objective: To biologically develop and evaluate the caprine fetal fi broblast cell cultures before and after freezing. Methods: Goat fetuses (ages 51, 53 and 55 d) were collected from slaughterhouse. Their skin was cut into small pieces (1 mm3) and cultured in DMEM and FBS. When reaching 80%–90% conf luence, cells were passaged. Cells of the 8thpassage were cultured in 24-well plates (1.5×105 cells/well) for 9 d and three wells were counted every day. The average cell counts at each time point were plotted against day number and the population doubling time (PDT) was determined. Then, 42 vials of cells (2×106cells/mL) were frozen. Samples were thawed and cultured after 1 month. Cell viability and PDT were evaluated after thawing. Results: After eight passages, the goat fetal fi broblast cells had a latent phase of about 48 h and after an exponential phase, cells entered the plateau phase on day 5. Before freezing, PDT was about 22 h and after thawing it was about 28 h. Conclusions: The goat fetal fi broblast cell culture can be established using the adherent culture method and can be cryopreserved, too. After thawing, growth and viability indices of these cells were acceptable.
1. Introduction
The variability of animal genetic resources is an important determinant of maintenance of biodiversity in farm livestock species. If these genetic resources are not protected from the extinction, not only they will be lost forever, but also research focused on the thorough explanation of biological mechanisms underlying proliferative activity, genetic stability, replicative senescence, physiological aging of cultured nuclear donor somatic cells and the subsequent epigenetic reprogramming of their cell nuclei both in the oocytes reconstructed by somatic cell nuclear transfer (SCNT) and in the resultant cloned embryos will have not been completed. Therefore, there is an urgent need to start protecting of endangered animals[1]. The present practical options for ex situ and in vitro conservation of endangered species are the protection of individual animals, semen cryopreservation, embryo, or oocyte freezing and vitrification, ovarian and testicular slices cryopreservation, whole ovary cryopreservation, somatic cells cryopreservation (as cell culture or as tissue slices and up to whole animal), stem cell cryopreservation and genomic libraries. These gametes, cells, and tissues freezing can only be performed for a limited number of species and needs customized techniques for each species[2]. Somatic cells cryopreservation is an alternative option for maintaining of genetic diversity in endangered animals in vitro[3]. In addition, cloning techniques have been developed for conservation of animal genetic materials using somatic cells as an attractive resource[4]. The development of somatic cell cloning technology in farm livestock species and the establishment of somatic cell banking for the purposes of recovery of endangered mammalian breeds and species threatened with extinction appear to be especially important. For each animal, tissue samples can be frozen and stored in liquid nitrogen as a method of choice for the rapid establishment of emergency cell banks.
The development of fibroblast cell banks, particularly for endangered species can provide an excellent resource for biological research and preserve valuable genetic materials[5]. Fibroblasts have been cultured from diff erent species and tissues and havevarious applications including feeder layer of embryonic stem cells, nuclear transfer in cloning, tissue engineering, and wound healing researches. Isolation of ear marginal or fetal skin fi broblasts using adherent culture have been established for some species to develop fi broblast cell bank. Fibroblast cell banks establishment have been reported for some ruminant breeds such as Simmental cattle[6], Luxi cattle[7], Ujumqin sheep[8], Texel sheep[9], Mongolian sheep[10], Jining black grey goat[5,11], Taihang black goat[12], Liaoning cashmere goat[13], and Cashmere goat[14], as well as for laboratory animals such as guinea pig[15].
Goat is an important livestock species contributing to milk, meat and wool production[16]. Initial goat domestication is documented in the highlands of Zagros Mountains, Iran, at 10 000 calibrated calendar years ago[17]. Iran is one of the ten countries in goat keeping in the world with 25.7 million heads. About 30% of all goats in Iran are kept in Fars Province by migrating nomads and villagers[18]. Southern Zagros Mountains cover Fars Province. To preserve this valuable genetic resource, establishment of fi broblast banks have been proposed as a practical method. The purpose of this study was the establishment and in vitro evaluation of fi broblast cultures from skin of goat fetus.
2. Materials and methods
2.1. Fetus collection and skin preparation and culture
Six gravid uteruses of Fars native goat were collected from Shiraz Slaughterhouse, Iran and transported on ice to the laboratory. Seven fetuses (5 single and 1 twins) were dissected out using sterilized scissor and forceps. Sex of fetuses was visually determined. Linear measurement of the crown-rump length (straight distance between the occiput and the distal end of os coccygeus) to the nearest mm was recorded. Fetal age was estimated using the following equation[19]:
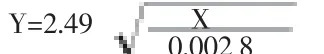
where X = crown-to-rump length (mm) and Y = age (d).
Fetuses were washed 4 to 5 times in sterile phosphate buff ered saline (PBS; Gibco, cat. no. 18912–014, UK) containing 1% penicillin and streptomycin (Sigma cat. no. P-4687 and S-1277, St. Louis, USA). Slices of fetal skin were removed using sterilized forceps and were cut into small pieces (1 mm2). Skin pieces were cultured in 88% Dulbecco’s modified Eagles medium (DMEM; Gibco cat. no. 12800-116) containing 10% fetal bovine serum (FBS; Gibco, cat. no. 10270-106), 1% penicillin and streptomycin, and 1% L-glutamine (Sigma cat. no. G5840) and were cultured at 37 °C in an incubator with 5% CO2and saturated humidity. The medium was replaced after 48 h. When fi broblast cells reached 80%–90% confl uence. The cells were harvested using 0.25% trypsin (Gibco cat. no. 15090-046). Fetal goat fi broblasts were passaged 8 times.
2.2. Cryopreservation and reseeding
In each passage, cells at the logarithmic growth phase were collected and counted with a hemocytometer, and then resuspended in freezing solution containing 10% dimethyl sulfoxide (DMSO; MP Bio cat. no. 196055) and 90% FBS, at a density of 2×106cells/ mL. The cell suspension was aliquoted into sterile plastic cryovials that were labeled with the fetus number, sex, freezing serial number, and the date. The vials were sealed and kept at -20 °C for 60 min to equilibrate the DMSO and then they were transferred to -70 °C for 24 h, and finally transferred to liquid nitrogen for long-term storage[20]. The cryovials were removed from the liquid nitrogen and quickly thawed in a 37 °C water bath. When the ice clump was almost thawed, 1 mL of cell culture medium (88% DMEM, 10% FBS, 1% penicillin and streptomycin, and 1% L-glutamine) was added, the vials were centrifuged at 240 ×g and the cells were transferred into fl asks with gently blown into uniform single cell suspension, and cultured at 37 °C and 5% CO2.
2.3. Cell viability
Before freezing and after thawing, viability was determined using the trypan blue exclusion test (0.4% trypan blue in PBS). The number of nonviable cells was determined by counting of 1 000 cells and then subtract the number of stained cells from the total and calculate unstained cells proportion (percent) from the total after 1 months of cryopreservation[21].
2.4. Growth curve analysis
Cells of the 8thpassage before and after freezing were seeded in 24-well plates at a density of approximately 1.5×105cells per well, cultured for 8 d, and counted every day (3 wells each time). The mean cell numbers at each time point were then plotted against time using GraphPad Prism version 5.01 for Windows (GraphPad software Inc., San Diego, CA, USA). Population doubling time (PDT) was determined based on this curve[22].
2.5. Karyotype analysis
The chromosomes were prepared, fixed, and stained following standard method[23]. Cells of the 8thpassage were harvested when reaching 50%–70% confl uence. After hypotonic treatment using 0.075 mol/L KCl (Merck, cat. no. 1.04936.1000, Dormstadt, Germany), fixation by acetic acid (Merck, cat. no. SAAR1021020LC) and methanol (Merck, cat. no. 1.02447.0500) (1:3), and Giemsa and Leishman staining (v:v, 1:3), chromosome number was counted for 50 metaphases under an oil immersion objective (×100) using a light microscope (Olympus IX51, Japan).
2.6. Statistical analysis
The mean and SE of counted cells in growth curve analysis before freezing and after thawing were subjected to the Kolmogorov-Smirnov test of normality and then were compared using independent sample t-test (SPSS for Windows, version 11.5, SPSS Inc, Chicago, Illinois). Values with P ≤ 0.01 were considered signifi cantly diff erent.
3. Results
3.1. Fetal age estimation
Three fetuses with ages of 51 (male), 53 (male), and 55 (female) d where selected based on crown-to-rump length (Table 1).
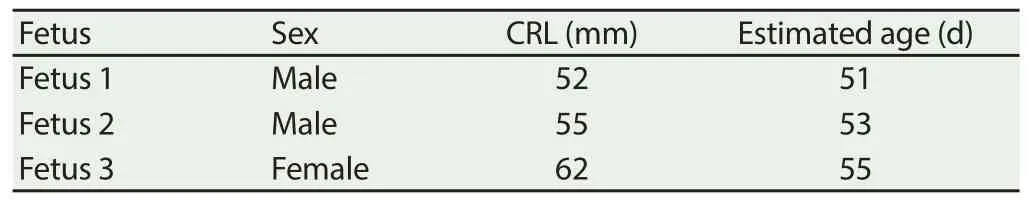
Table 1 Estimated age of goat fetuses using crown-to-rump length measurement.
3.2. Morphological observation
At about 4–5 d after the tissue explants adhered to the flasks, fi broblast-like cells were observed sprouting from the margins of these tissue pieces (Figure 1a). The cells showed typical fusiform morphology with centrally located oval nuclei. The cells covered the bottom of the fl asks within 3–4 d and formed a monolayer. The cells had fi broblastic characteristics with turgor vitalis cytoplasm, fi broblast-like radiating, and fl ame-like migrating patterns (Figures 1b, 1c and 1d).

Figure 1. Morphology of the Fars native goat fi broblast cells in vitro.
3.3. Growth curve analysis and cell viability
The growth curve of the Fars native goat fetal fi broblasts exhibited a typical “S” shape (Figure 2) and PDT was about 22 h before freezing and 28 h after thawing. In fresh and frozen-thawed samples, the latent phase was about 1 d, a result of trypsinization. This was followed by an exponential phase of 5 d before freezing and 3 d after thawing, which gave way to the stationary phase afterwards. There was no signifi cant diff erence between cell concentrations in each day before and after freezing (P > 0.01). Viability of the culture was (89.78±4.63)% before freezing and (88.32±5.17)% after thawing.
3.4. Karyotype analysis
The chromosome number of the Fars native goat fetal fi broblasts was n = 60, comprising 58 autosomal and 2 sex chromosomes (Figure 3). For 50 metaphases of the eighth passage, the chromosome numbers per metaphases were counted, and the results showed that 98% of the cells were diploid, supporting the conclusion that the cell culture was reproducibly diploid.
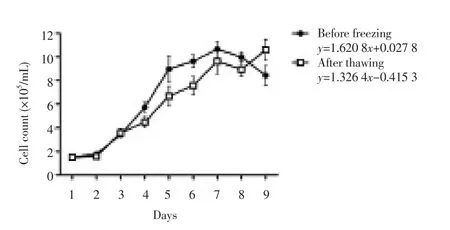
Figure 2. The growth curve and trend line equation of the 8thpassages of the Fars native goat fetal fi broblasts before and after thawing (n = 3 fetus or 9 counted well/d; mean±SE).
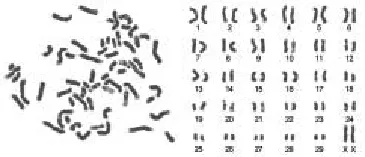
Figure 3. Chromosomes at metaphase (left) and karyotype (right) of the Fars native goat fetal fi broblasts.
4. Discussion
The fetal fi broblast-like cell culture from the Fars native goat was established using the adherent culture method and these cells were frozen following 8 passages. Somatic cells storage may be an option for in vitro conservation of species[3]. Somatic cell cryopreservation for every species is a cheap and fast way, and the method of choice for the rapid creation of cell banks. Fibroblasts may be trypsinized and adhered more easily and more readily than epithelial cells[24]. Because of these characteristic, a culture of pure fibroblast may be obtained after two to three passages[5, 25, 26]. Morphology, as the most important qualitative parameter of epidermal tissue reconstitution was evaluated by light microscopy. In our study, the cells had fi brous characteristics with turgor vitalis cytoplasm, and during growth, they showed typical fi broblast-like morphology as radiating, fl ame-like or whirlpool migrating shapes. Consistent with our fi ndings in the Luxi cattle, fi broblasts, could be seen migrating from the tissue pieces fi ve to 12 d after explanting[7].
Analysis showed that the population doubling time (PDT) for subculturing fi broblast-like cells with high rate of proliferation was approximately about 22 h before freezing and 28 h after thawing in accordance with the reports of Singh et al. who established three fibroblast cell lines from lower edge ear skin samples of healthy dairy goats with a population doubling time of 25 h withoutfreezing[27].
Genetic stability of cell cultures is the most important aspect when preserving genetic resources. The cells must maintain the same diploid character as cells in vivo. In vitro cultured cells that keep their division capability but diff erentiation appears after successive cell divisions, so they cannot be used for breed conservation. A n = 60 frequency of 98% indicated that the Fars native goat fetal fi broblast cultures were stably diploid in accordance with the previous reports in goats[5,12-14,27-29]. Although hypodiploid and hyperdiploid cells, and some polyploid cells may emerge in the cultures with increasing passaging[30], the incidence of such cells was still very small in our study (below 2%). Hence, there was seldom a chromosome number variation in the Fars native goat fi broblasts.
It is not uncommon for cells to cease growth and show changes in biological characteristics or lose their diplont properties with time in cultures due to a variety of stimuli and factors. Effective measures are thus required to ensure diploid stability in cultures of cells that are used for preserving valuable genetic resources. The genetic characteristics of the cells may be changed by in vitro culture conditions after many passages, so a minimal number of passages are recommended to conserve them.
A fi broblast-like cell culture was established from explanted fetal skin tissue of the Fars native goat using standard tissue adherent culture and continuous passaging following trypsinization. We conclude that cell quality was similar among the cell cultures. We contend that our cell bank makes a valuable contribution to the preservation of the genetic resources of the Fars native goat and provides useful biomaterial for future studies in cell biology, medicine, genomics, postgenomics, and both genetic and embryonic engineering.
Declare of interest statement
There is no confl ict of interest.
Acknowledgments
This research was fi nancially supported by the Shiraz University Vice-Chancellor for Research, the Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
[1] Guan WJ. The construction and identifi cation of the cell bank of species of domestic animal on the brink of extinct. Rev China Agric Sci Technol 2002; 6(5): 66-677.
[2] Woolliams JA, Wilmut I. New advances in cloning and their potential impact on genetic variation in livestock. Anim Sci 1999; 68: 245-256.
[3] Corley-Smith GE, Brandhorst BP. Preservation of endangered species and populations: a role for genome banking, somatic cell cloning, and androgenesis? Mol Reprod Dev 1999; 53(3): 363-367.
[4] Wu CX. The theory and technology of the conservation of animal genetic resources-the specy foundation of animal agricultural continuing development in 21 century. J Yunnan Univ 1999; 21: 7-10.
[5] Li XC, Yue H, Li CY, He XH, Zhao QJ, Ma YH, et al. Establishment and characterization of a fi broblast cell line derived from Jining Black Grey goat for genetic conservation. Small Ruminant Res 2009; 87(1-3): 17-26.
[6] Li LF, Yue H, Ma J, Guan WJ, Ma YH. Establishment and characterization of a fi broblast line from Simmental cattle. Cryobiology 2009; 59(1): 63-68.
[7] Liu C, Guo Y, Guan W, Ma Y, Zhang HH, Tang X. Establishment and biological characteristics of Luxi cattle fi broblast bank. Tissue Cell 2008; 40(6): 417-424.
[8] Na RS, Zhao QJ, Su XH, Chen XW, Guan WJ, Ma YH. Establishment and biological characteristics of Ujumqin sheep fibroblast line. Cytotechnology 2010; 62(1): 43-52.
[9] Li LF, Guan WJ, Li H, Zhou XZ, Bai XJ, Ma YH. Establishment and characterization of a fibroblast cell line derived from Texel sheep. Biochem Cell Biol 2009; 87(3): 485-492.
[10] Liu CQ, Guo Y, Guan WJ, Ma YH. Establishment and characterization of a fi broblast cell line derived from Mongolian sheep. Anim Sci J 2011; 82(2): 215-222.
[11] Bai C, Wang D, Su X, Zhang M, Guan W, Ma Y. Establishment and biological research of the Jining Grey goat fibroblast line. Turk J Vet Anim Sci 2012; 36(6): 659-667.
[12] Guan WJ, Ma YH, Zhou XY, Liu GL, Liu XD. The establishment of fi broblast cell line and its biological characteristic research in Taihang black goat. Rev China Agric Sci Technol 2005; 7: 25-33.
[13] Hu PF, Guan WJ, Li XC, Zhang WX, Li CL, Ma YH. Study on characteristics of in vitro culture and intracellular transduction of exogenous proteins in fi broblast cell line of Liaoning cashmere goat. Mol Biol Rep 2013; 40(1): 327-336.
[14] Islam MS, Zhou H. Isolation and characterization of putative epidermal stem cells derived from Cashmere goat fetus. Eur J Dermatol 2007; 17(4): 302-308.
[15] Mehrabani D, Mahboobi R, Dianatpour M, Zare S, Tamadon A, Hosseini SE. Establishment, culture and characterization of Guinea pig fetal fi broblast cells. Vet Med Int 2014; 2014(2): 510328-510328.
[16] Kumar De A, Malakar D, Akshey YS, Jena MK, Dutta R. Isolation and characterization of embryonic stem cell-like cells from in vitro produced goat (Capra hircus) embryos. Anim Biotechnol 2011; 22(4): 181-196.
[17] Zeder MA, Hesse B. The initial domestication of goats (Capra hircus) in the Zagros Mountains 10,000 years ago. Science 2000; 287(5461): 2254-2257.
[18] Feedlot performance and carcass characteristics of Fars native goats. In: Eilami B, editor. Proceedings of 7th International Conference on Goats; France; 2000.
[19] Karen AM, Fattouh E-SM, Abu-Zeid SS. Estimation of gestational age in Egyptian native goats by ultrasonographic fetometry. Anim Reprod Sci 2009; 114(1): 167-174.
[20] Takashima A. Establishment of fi broblast cultures. Curr Protoc Cell Biol 2001; 2-1: 1-12.
[21] Freshney RI. Culture of specific cell types. In: Freshney RI, editor. Culture of Animal Cells: Wiley Online Library; 2005: 375-420.
[22] Mehrabani D, Rahmanifar F, Mellinejad M, Tamadon A, Dianatpour M, Zare S , et al. Isolation, culture, characterization, and adipogenic diff erentiation of heifer endometrial mesenchymal stem cells. Comp Clin Pathol 2015; 24(5): 1-6.
[23] Asadi-Yousefabad S-L, Khodakaram-Tafti A, Dianatpour M, Mehrabani D, Zare S, Tamadon A, et al. Genetic evaluation of bone marrow-derived mesenchymal stem cells by a modifi ed karyotyping method. Comp Clin Pathol. 2015; 24(6): 1361-1366.
[24] Ren FL, Li Y, Zhang Y. In vitro cultivation and freezing of bovine skin fi broblast cells. Scalper Mag 2002; 28: 8-10.
[25] Zhou XM, Ma YH, Guan WJ, Wen J, Li H. Establishment and characteristics of a Beijing fatty chicken embryo fibroblast cell line. Chinese J Anim Vet Sci 2005; 36: 209-215.
[26] Li GF, Li Y, Zhao ZS, Li DQ. The fi broblast culture of sheep ear in vitro. China Herbivores. 2003; 23(4): 5-7.
[27] Singh M, Sharma AK, Yadav P. Characterization of gsf289: A fi broblast cell line derived from goat ear skin explants. J Biotech Res 2011; 3(1): 1-6.
[28] Zhan T, Tian Y, Lou M, Liu J, Wang Y, Zhao X. The genetic mechanism of intersexuality in milk goats of Saanen breed of Xinong. Acta Genet Sin 1994; 21(5): 356-361.
[29] Singh M, Sharma A. Outgrowth of fibroblast cells from goat skin explants in three diff erent culture media and the establishment of cell lines. In vitro Cell Dev Biol Anim 2011; 47(2): 83-88.
[30] Men ZM, Liu X, Ma HM, Han JL. Karyotype analysis of Lanzhou fattailed sheep. J Gansu Agric Univ 2002; 37(2): 158-160.
ent heading
10.1016/j.apjr.2016.04.013
*Corresponding author: Amin Tamadon, Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Tel/fax: +98 71 3234 1025
E-mail: amintamaddon@yahoo.com
Marzieh Tajeddini, Graduated from School of Veterinary Medicine, Shiraz University, Shiraz, Iran.
Tel/fax: +98 71 3234 1025
E-mail: ta_marzieh@yahoo.com
Foundation project: This research was financially supported by the Shiraz University Vice-Chancellor for Research, the Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
 Asian Pacific Journal of Reproduction2016年3期
Asian Pacific Journal of Reproduction2016年3期
- Asian Pacific Journal of Reproduction的其它文章
- A rare cause of infertility: A late complication of female genital mutilation
- Corpus callosum agenesis: Role of fetal magnetic resonance imaging
- Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG)
- Somatic embryogenesis and in vitro flowering in Hybanthus enneaspermus (L.) F. Muell.-a rare multipotent herb
- Pollutant exposure in Manila Bay: Effects on the allometry and histological structures of Perna viridis (Linn.)
- Changes in sperm characteristics of the three main breeds of sheep in Algeria after dietary supplementation
