Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
Reda I. El-Sheshtawy, Gamal A. El-Sisy, Walid S. El-Nattat
Animal Reproduction and Al Department, Veterinary Division, National Research Centre, Dokki, Giza, Egypt
Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
Reda I. El-Sheshtawy, Gamal A. El-Sisy, Walid S. El-Nattat*
Animal Reproduction and Al Department, Veterinary Division, National Research Centre, Dokki, Giza, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form Accepted
Available online
Pomegranate juice Semen Cattle bull Cryopreservation
Objective: To study the effect of adding different concentrations of the pomegranate juice (PJ) to the cattle bull semen extender on post-thawing semen quality. Methods: Semen was collected from fivw cattle-bulls at weekly intervals for 5 weeks at the Semen Freezing Center, General Organization for Vet. Services, Ministry of Agriculture. Semen samples were diluted in Tris-citric acid-egg yolk-fructose extender and divided into six aliquots, the 1st served as control while PJ was supplemented at 10%, 20%, 30%, 40% and 50% in the aliquot 2, 3, 4, 5 and 6 respectively. Diluted semen samples were subjected to cooling and cryopreservation and stored in liquid nitrogen (LN2). Sperm motility in chilled semen (over 10 d) and post-thawing sperm parameters, including individual motility, alive sperm, membrane integrity, and total sperm abnormality were assessed. Results: Obtained results clearly demonstrated that the addition of 10% PJ in the chilled extended cattle semen proved to be beneficial for maintaining sperm motility percentage. On the other hand, the addition of 40% and 50% PJ failed to preserve motility all over the 10 d. Also, supplementation of extender with 10%–20% PJ significantly increases the post-thaw motility and viability as compared with control group. Conclusions: Supplementation of bull semen extender with 10% and 20% PJ provides good chilling and improved frozenthawed semen quality.
1. Introduction
Vegetables and fruits are of the natural sources that maintain life through their contents of multiple active remedies compounds. Pomegranate is one of the beneficial fruits in medicinal treatment [1,2]. Some authors have investigated mainly the antioxidant activities of its polyphenols [3,4].
Populations in the Middle East used pomegranate (Punica granatum) as a herbal medicine [5]. Tezcan et al. showed that fructose and glucose were the major sugars and that citric and malic are the major acids [4]. The nutritive value of the pomegranate fruit has been demonstrated by Virgili and Marino [6] who explained that daily consumption of 250 mL of PJ covers about 50% of the daily requirements of vitamins A, C and E. Moreover, the fruit contained antioxidant polyphenols that present half of the fruit’s antioxidant ability for counteracting the free radicals [7]. The strong antioxidant capacity of PJ was useful in fighting certain cancers [8-10]. Besides many experimental studies described PJ in improving semen quality [11,12] and erectile dysfunction in male patients [13]. El Ghazzawy et al. mentioned that PJ had the ability to counteract structural changes in the rat epididymis caused by plasticizer Bisphenol, which interfere with its function and contribute to infertility, via increasing the number of caudal epididymal sperm, decreasing sperm abnormalities and improving male fertility [14]. Administration of pomegranate extract could improve sperm characteristics and antioxidant activity in adult male Wistar rats [12] and men [15]. Al-Daraji revealed that supplementation of semen diluent with PJ significantly improved storage ability of roosters’ semen and increased the protective effects against lipid
peroxidation during liquid storage of roosters’ semen for up to 36 h [16].
Hence, the present study was designed to investigate the effect of pomegranate juice when incorporated as a semen extender for maintaining the quality of chilled and frozen-thawed cattle bull semen.
2. Materials and methods
2.1. Semen collection and initial evaluation
Five mature cattle-bulls reared at the Semen Freezing Center, General Organization for Vet. Services, Ministry of Agriculture, Abbasia, Egypt, were included in this study. Semen was collected from these five cattle-bulls using an artificial vagina at weekly intervals for 5 weeks. The semen samples were transferred to the adjacent lab within few seconds and initially evaluated for volume (in a graduated tube), sperm motility and live sperm percent. The neat semen samples with more than 70% motility and 80% morphologically normal spermatozoa were admitted to freezing procedure. The ejaculates were pooled in order to have sufficient semen for a replicate and to eliminate the bull effect. The semen was given a holding time for 10 min at 37 °C in a water bath before dilution.
2.2. Pomegranate processing
Pomegranates with intact peel were sold from the Egyptian market. They were washed, peeled and the red grains were collected in a clean dish. The grains were squeezed with gauze to obtain a clear watery juice. The juice was filtered and stored at -18 °C till used [1]. characteristics (motility %, alive %, abnormality % and membrane integrity (hypoosmotic swelling test HOST) %).
2.3. Semen processing
A basic control extender (Tris-citric acid-egg yolk-fructose [TCYF]) was prepared according to Foote [17]. TCYF / PJ (pomegranate juice enriched extender, PJEE) [v:v] (0.5/4.5 (10%), 1.0/4.0 (20%), 1.5/3.5 (30%), 2.0/3.0 (40%) and 2.5/2.5 (50%)) were prepared and centrifuged to discard any precipitate. Semen samples were diluted in TCYF (control, 0% PJEE) and the former concentrations of PJEE to ensure 60 million motile spermatozoa / mL, cooled slowly up to 5 °C and equilibrated for 4 h. Semen was packed into 0.25 mL polyvinyl French straws (IMV, France). After equilibration periods, the straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen (LN2) for 10 min and were then dipped in liquid LN2.
2.4. Semen quality assessment
The assessment was undertaken on after freeze-thawing of bull spermatozoa. Also, sperm motility was evaluated for raw semen, 2 h after cooling and chilled semen daily up to 10 d. Frozen straws were thawed at 37 °C/ 30 s. The parameters studied were subjective semen
2.4.1. Subjective motility
Subjective motility was assessed using a phase-contrast microscope (100x magnification), with a warm stage maintained at 37 °C. A wet mount was made using a drop of semen placed directly on a pre-warmed slide and covered by a pre-warmed cover slip under the same temperature conditions. Sperm motility estimations were performed in three different microscopic fields for each semen sample. Visual motility was assessed microscopically with closed circuit television system [18].
2.4.2. Live and abnormal spermatozoa (%)
This was evaluated using eosin-Nigrosin stained smear as described by Sidhu and Guraya [19]. Two hundred spermatozoa were assessed.
2.4.3. Sperm membrane integrity
Sperm membrane integrity was assessed using the hypo-osmotic swelling test (HOST) [20]. Two hundred spermatozoa were assessed and the percentage of spermatozoa with curled tails (swollen/ intact plasma membrane) was calculated.
2.5. Statistical analysis
Statistical analysis data were analyzed using the SPSS (2005) computerized program v. 14.0 to calculate the analysis of variance (ANOVA) [21] for the different parameters between control and additives replications. A significant difference between means was calculated using Duncan’s multiple range test at P < 0.05.
3. Results
Data analysis revealed a gradual decline concerning the motility percentage of spermatozoa chilled at 5 °C for the control (0% PJEE) and the first treatment (10% PJEE) from the 1st day to the 10th day. On the contrary, the four other treatments (20% to 50% PJEE) abruptly declined at the 7th and 10th days. The use of 10% PJEE for extended chilled cattle semen showed the highest significant (at least P < 0.058 1) motility percentage all over the 10 d. The worst results were obtained on using the 40% and 50% PJEE (Table 1).
Concerning to frozen-thawed semen, the present study revealed that supplementation of extender with 10% and 20% PJ increased the post-thaw motility [(53.89±2.61)% and (51.67±1.67)%, respectively], and these values are significantly (P < 0.000 1) higher than the control group [(45.56±1.55)%]. The addition of 10% PJ increased the live percentage count [(69.00±0.58)%], this value was significantly (P < 0.000 1) higher than the control group [(58.33±0.33)%] (Table 2). No significant (P < 0.429 5) differences could be detected between groups in sperm membrane integrity and total sperm abnormality %.
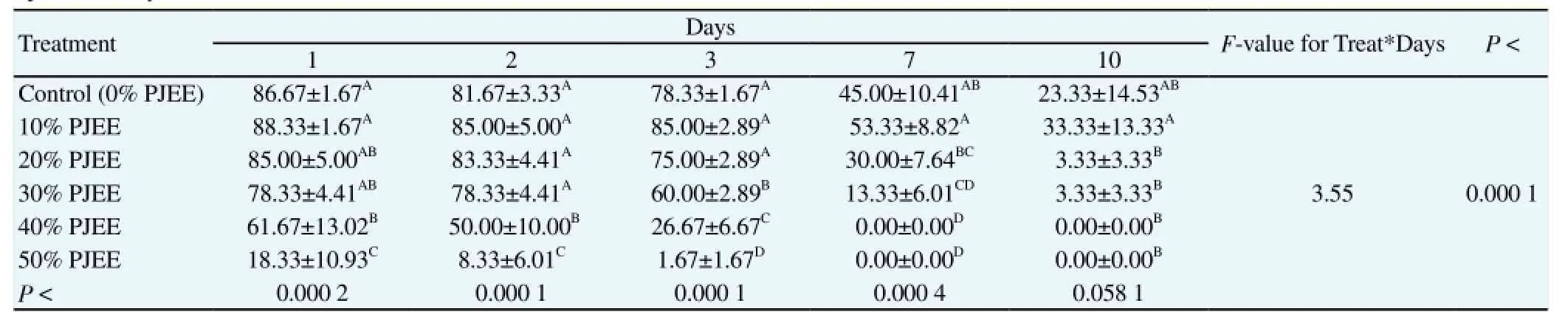
Table 1 Sperm motility % of chilled semen in PJEE in cattle bulls.
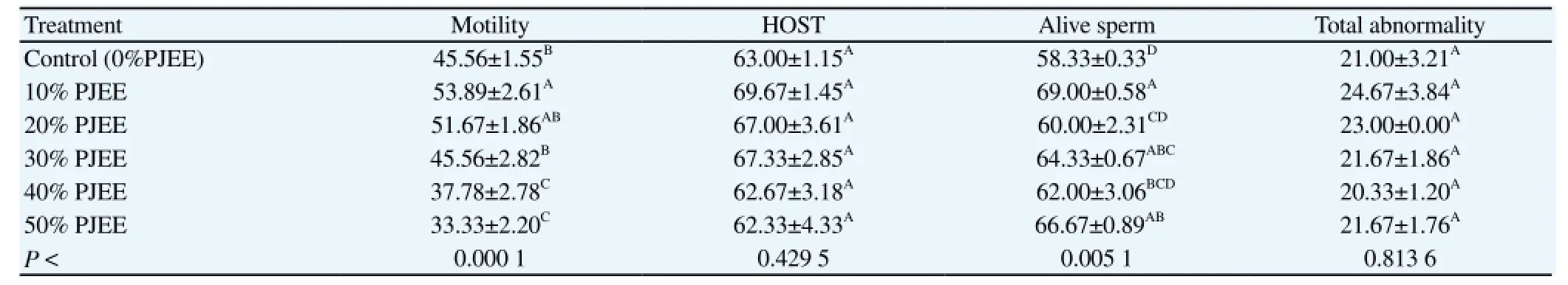
Table 2 Effect of PJEE on post thawing characteristics of cattle bulls extended semen (%).
4. Discussion
Pomegranate is known for its antioxidant activity, both in vivo and in vitro [3,4]. Fresh juice contains high amount of vitamin C, and polyphenolic compounds (anthocyanins, punicalagin, ellagic and gallic acid) [4]. The main objective of the currrent study was to add PJ to cattle semen extender for chilling and cryopreservation and to assess its effect on sperm motility, functional sperm membrane integrity, viable sperm and total sperm abnormality %. The PJ concentrations were chosen after pre-experiments we conducted, and according to the limited available data [16]. In the current study, PJ has been added to TCYF extender at different concentrations (10%, 20%, 30%, 40% and 50%).
Obtained results clearly demonstrated that PJ resulted in a gradual decline concerning the motility percentage of spermatozoa chilled at 5 °C for the control (0% PJEE) and the first treatment (10% PJEE) from the 1st day to the 10th day. The addition of 10% PJEE for chilled cattle semen proved to be beneficial for maintaining sperm motility percentage (at least, P < 0.058 1) all over the 10 d. On contrast, the concentrations of 40% and 50% PJEE did not preserve motility. Similarly, Al-Daraji revealed that the inclusion of PJ (2 mL, 4 mL/ 100 mL) into rooster semen extender resulted in significant (P < 0.05) decreases the dead, the abnormal and the acrosomal integrity % of spermatozoa when semen samples were examined before storage or after certain storage periods (12, 24 or 36 h) [16]. The previous author attributed these positive results to the potent antioxidant activity of PJ.
With regard to frozen-thawed semen, the present study revealed that supplementation of extender with 10% and 20% PJ significantly increased the post-thaw motility (P < 0.000 1) as compared with the control group. The addition of 10% PJ significantly increased the live percentage (P < 0.000 1) than the control group. During freezing, there is progressed production of reactive oxygen species (ROS) [22] that cause changes in function and structure of sperm membrane in concomitant with an alteration in antioxidant defense systems [23], including a reduction in intracellular GSH content [24]. To counteract the destructive effects of ROS, seminal plasma possesses an antioxidant system that seems to be very relevant to the protection of sperm [25]. Unfortunately, this antioxidant capacity of spermatozoa is very limited to protect itself against ROS, compared with somatic cells. The addition of antioxidant to the freezing and thawing medium with antioxidants could be useful to improve the viability and subsequent fertilizing capacity of frozen-thawed farm animal’s spermatozoa [26]. A lot of studies were carried out on the addition of different antioxidants in extenders to protect spermatozoa against detrimental effects of ROS [27,28]. Halvorsen et al. reported that pomegranate fruit contained very high concentrations of antioxidants (11.33 mmol / 100 g) [29]. Flavonoids and other phenolic compounds appear to have antioxidant activities that are several times higher than those of vitamins E and C [30]. Longtin concluded that the antioxidant capacity of PJ is dependent not only on vitamin C content but also other antioxidant-rich like tannins and flavonoids compounds [31]. However, he suggested that the antioxidant capacity of PJ is a function of the combined action of a number of constituents. In addition, fresh PJ contains 10% total sugars, and 1.5% pectin, ascorbic acid, polyphenolic, flavonoids and the principal amino acids (glutamic and aspartic acid) [32,33]. These phytochemicals may act as antioxidants, and modulate bacterial
populations in the body or media [34]. Moreover, Roger reported that PJ is also packed with vitamins A, C, and E, all of which boost sexual libido in men and women [35]. Gangwar et al. indicated that vitamin C at the level of 56.78 mmol/L can be used as an antioxidant in semen diluent in a routine freezing process for better postthaw recovery of buck semen [36]. Vitamin C is naturally present in seminal plasma to scavenge and decrease numerous disruptive free radical processes, including lipid peroxidation [37]. The addition of vitamin C in an extender could possibly improve sperm function by reducing cell damage through its continuous radical-scavenging action. Alpha-tocopherol, one of the main sperm antioxidants, was found to be abundant in spermatozoa membrane [38,39] and protect sperm motility from oxidative damage [40]. Addition of 20 mmol/L of L-glutamine to ram semen extender prevented injuries to sperm and improved the post-thaw semen characteristics [41]. In conclusion, PJ supplementation improved sperm motility of chilled semen and postthaw sperm motility, membrane integrity and viability and decreased total sperm abnormalities of cattle cryopreserved semen.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are greatly indebted to the National Research Center for sponsoring this work through the project entitled: “Conservation of the genetic resources of local male breeds using natural additives for semen extenders”, also thanks are due to Dr. Gharieb A. El-Morsy and all staff of Artificial Insemination Center and Dr. Abd El-Hamid El-Sokary in the General Organization for Veterinary Services, Ministry of agriculture, Egypt, for their kind assistance during this study.
[1] Aviram M, Dornfield L, Rosenblatt M, Volkova N, Kaplan M, Coleman R. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J ClinNutr 2000; 71: 1062-1076.
[2] Aviram M, Rosenblatt M, Gaitani D, Nitecki S, Hoffman A, Dornfield L. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis (CAS) reduces common carotid intima-media thickness (IMT), blood pressure and LDL oxidation. Clin Nutr 2004; 23: 423-433.
[3] Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Muraleedharan G, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 2005; 16(6): 360-367.
[4] Tezcan F, Ozguven MG, Diken T, Ozcelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem 2009; 115: 873-877.
[5] Siegfried NL, Hughes G. Herbal medicine, randomised controlled trials and global core competencies. S Afr Med J 2012; 102(12): 912-913.
[6] Virgili F, Marino M. Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity. Free Rad Biol Med 2008; 45(9): 1205-1216.
[7] Seeram NP, Aviram M, Yanjun Z, Henning SM, Feng L, Mark D, et al. Comparison of antioxidant potency of commonly consumed polyphenolrich beverages in the United States. J Agric Food Chem 2008; 56(4): 1415-1422.
[8] Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett 2008; 269(2): 262-268.
[9] Khan G, Gorin M, Rosenthal D, Pan Q, Bao L, Wu Z, et al. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr Cancer Ther 2009; 8(3): 242-253.
[10] Sashi G, Dobroslawa B, Muntha K, Guoyi M, Shabana I, Daneel F. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem 2010; 58(4): 2180-2187.
[11] Türk G, Sónmez M, Aydin M, Yüce A, Gür S, Yüksel M, et al. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr 2008; 27: 287-296.
[12] Mansour SW, Sangi S, Harsha S, Khalee MA, Ibrahim ARN. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pac J Trop Biomed 2013; 3(7): 563-568.
[13] Forest CP, Padma-Nathan H, Liker HR. Efficacy and safety of pomegranate juice on improvement of erectile dysfunction in male patients with mild to moderate erectile dysfunction: a randomized, placebo-controlled, double-blind, crossover study. Int J Impot Res 2007; 19(6): 564-567.
[14] El Ghazzawy IF, Meleis AE, Eman F, Solaiman FA. Histological study of the possible protective effect of pomegranate juice on bisphenol-A induced changes of the caput epididymal epithelium and sperms of adult albino rats. Alex J Med 2011; 47: 125-137.
[15] Fedder MDK, Jakobsen BH, Giversen I, Christensen LP, Parner ET, Fedder J. An extract of pomegranate fruit and galangal rhizome increases the numbers of motile sperm: A prospective, randomised, controlled, double-blinded trial. PLoS One 2014; 9(9): e108532.
[16] Al-Daraji HJ. The use of pomegranate juice for counteract lipid peroxidation that naturally occurred during liquid storage of roosters’semen. Pharmacognosy Commu 2015; 5(1): 70-76.
[17] Foote RH. Fertility of bull semen at high extension rates in Tris buffered extenders. J Dairy Sci 1970; 53: 1475-1477.
[18] Graham EF, Schmehl MKL, Maki-Laurila M. Some physical and chemical methods of evaluating semen. In: Proc. 3rd NAAB Tech. Conf. Artif. Insemin. Reprod.; 1970 April 12–14: Milwaukee, WI: National Association of Animal Breeders, Columbia, MO; 1970, p. 44-48.
[19] Sidhu KS, Guraya SS. Buffalo bull semen morphology, biochemistry, physiology and methodology. Ludhiana: USA Publishers and Distributors; 1984, p. 152-154.
[20] Jeyendran RS, Vander Ven HH, Perez Pelaez M, Crabo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70: 219-228.
[21] Snedecor GW, Cochran WG. Statistical methods. Ames: Iowa State University Press; 1967.
[22] Ball BA, Medina V, Gravance CG, Baumber I. Effect of antioxidant on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5°C. Theriogenology 2001; 56: 577-569.
[23] Bilodeau JF, Blanchette S, Gagnon IC, Sirard MA. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 2001; 56: 275-286.
[24] Gadea J, Selles E, Marco MA, Coy P, Matas C, Romar R, et al. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004; 62: 690–701.
[25] Alvarez JG, Storey BT. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: Its effect on sperm motility. Biol Reprod 1982; 27: 1102-1108.
[26] Gadea J, Gumbo D, Novass C, Zquezf AZ, Grullol A, Gardo GC. Supplementation of the dilution medium after thawing with reduced glutathione improves function and the in vitro fertilizing ability of frozenthawed bull spermatozoa. Andrology 2007; 7: 1-10.
[27] Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet Brno 2007; 76: 383-390.
[28] Bucak MN, Atessahin A, Yuce A. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze–thawing process. Small Rum Res 2008; 75: 128-134.
[29] Halvorsen B, Holte K, Myhrstad MCW, Barikmo I, Havattum E, Remberg SF, et al. A systematic screening of total antioxidants in dietary plants. J Nutr 2002; 132: 461-471.
[30] Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr 1998; 68: 1081-1087.
[31] Longtin R. The pomegranate: Nature’s power fruit? J Nat Canc Inst 2003; 95(5): 346-348.
[32] Blesbois E, Grasseau I, Hermier D. Changes in lipid content of fowl spermatozoa after liquid storage at 2 to 5 °C. Theriogenology 1999, 52: 325-334.
[33] Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001; 158: 195-198.
[34] Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 1999; 70: 475-490.
[35] Roger C. New research confirms health benefits from pomegranate juice. [Online]. Available from: http:// www.mirror.co.uk/2005. [Accessed on Day/Month, Year].
[36] Gangwar C, Kharche SD, Ranjan R, Kumar S, Goel AK, Jindal SK, et al. Effect of vitamin C supplementation on freezability of Barbari buck semen. Small Rum Res 2015; 129: 104-107.
[37] Anane R, Creppy EE. Lipid peroxidation as pathway of aluminum cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase and catalase and vitamins E and C. Hum Exp Toxicol 2001; 20: 477-481.
[38] Suleiman SA, Ali ME, Zaki Z, Elmalik E, Nasr M. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 1996; 17: 530-537.
[39] Surai P, Noble R, Sparks N, Speake B. Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. J Reprod Fert 2000; 120: 257-264.
[40] Rooke J, Shao C, Speake B. Effects of feeding tuna oil on the lipid composition of pig spermatozoa and in vitro characteristics of semen. Reproduction 2001; 121: 315-322.
[41] Sangeeta S, Arangasamy A, Kulkarni S, Selvaraju S. Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels after-freeze and post-thawed ram semen. Anim Reprod Sci 2015; 161: 82-88.
ment heading
10.1016/j.apjr.2016.06.001
*Corresponding author: Walid S. El-Nattat, Animal Reproduction and AI dept., Veterinary Research Division, National Research Centre, Dokki, Giza 12622, Egypt.
E-mail: elnattat2003@yahoo.com
Tel: 202 33371635
Fax: 202-37601877
Foundation project: This work was carried out in Semen Freezing Center, General Organization for Vet. Services, Ministry of Agriculture, Abbasia, and National Research Centre, Giza, Egypt.
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
- Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
- The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
- Evaluation of the academic achievement of rural versus urban undergraduate medical students in pharmacology examinations
