The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
Ali Olfati, Gholamali Moghaddam, Nasroallah Moradi Kor, Behzad Baradaran
1Department of Animal Science, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
2Department of Reproduction Physiologies, Iranian Society of Physiology and Pharmacology, Tehran, Iran
3Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
Ali Olfati1*, Gholamali Moghaddam1, Nasroallah Moradi Kor2, Behzad Baradaran3
1Department of Animal Science, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
2Department of Reproduction Physiologies, Iranian Society of Physiology and Pharmacology, Tehran, Iran
3Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Amniotic fluid Ghezel ewes Placental Pregnancy toxemia Trace minerals
1. Introduction
Maintenance of health is an important key to profitable farm animal reproduction. Therefore, keeping newborns alive and healthy may be the greatest management challenge facing farm owners. Important strategies for meeting these challenges include making sure that the placenta and fetus are in good condition throughout the pregnancy period (especially during late pregnancy). Trace mineral evaluation can play an important role in the survey of reproductive status and diagnosis of various diseases. Evaluation of trace mineral concentrations in body tissues and blood [1] is assumed as an easy, safe, proper and low cost method to determine the normal status in animals [2,3]. Therefore, understanding the normal values, especially in the amniotic fluid (AF), would be a useful index in determination of the physiological aspects of health and pregnancy toxemia in ewes. Gestation is a period of rapid growth and differentiation for both dam and fetus. Each fetus is completely dependent on its mother via the placenta for its supply of essential trace minerals [4]. Thus, many of the circulatory and transport properties of the ewe placenta are similar to those of the human placenta and as such, the pregnant sheep offers an excellent model in which to study the development of AF [5,6].
In recent years, among various researches (clinical medicine, biology, environmental studies, physiology and nutrition) the topic of trace minerals has received a great deal of attention. Additionally, the measurement of trace minerals is attracting increasing interest in medicine and veterinary sciences, because deviations in trace element uptake and/or metabolism are known to be related to certain diseases [7,8]. These minerals can be commonly found not only in the environment but also in diet. When animal receives trace minerals or intakes of them are deficient, maternal transfer of these essential minerals to the fetus are insufficient for processing normal
Objective: to investigate the trace mineral concentrations (Mg, Se, Zn, Cu and Fe) in amniotic fluid (AF) in 40 pregnancy toxemia Ghezel ewes at the time of parturition phase and its association with placental traits. Methods: Animals were treated with controlled internal drug release for 14 d and injected 400 IU pregnant mare serum gonadotropin at the time of controlled internal drug release removal. After the detection of estrus by use of teaser rams, ewes were hand-mated. Ewes were classified as having subclinical pregnancy toxemia on the basis of beta-hydroxy butyrate (BHBA) results (BHBA > 0.86 mmol/L). Results: The overall mean AF traces of mineral concentrations were 3.13 ng/mL, 22.1 μg/dL, 134.7 μg/dL, 122.5 μg/ dL and 166.6 μg/dL, respectively. There was a significant positive correlation between placental efficiency and Zn concentration in AF ewes (r = 0.633, P < 0.01), while the relationship was significantly negative between total volume of amniotic fluid and Fe concentration in AF ewes (r = 0.717, P < 0.01). In this research, no relationship between Se, Mg and Cu trace minerals was observed in AF ewes with placental traits ewes. Results of laboratory analyses demonstrated no relationship between BHBA concentrations and placental traits (P > 0.01), except for placental weight (r = 0.808, P < 0.01). Also, no significant correlation was detected between BHBA with the above trace minerals. Conclusions: Overall, determinations of these trace minerals in the AF ewes could have been used to obtain information on nutritional and reproductive status for the diagnosis of pregnancy toxemia in Ghezel ewes.
development (such as differentiation, activation, vitamin synthesis, hormone production and performance of the numerous functions of immune cells) and there may be abnormalities in the majority of body development.
Taking into consideration all the above information, the effects of reproductive status on the amniotic chemistry in ewes has not been described adequately anywhere in the world. Thus, the main hypothesis of this study was the identification of the trace mineral concentration at AF in the pregnancy toxemia Ghezel ewes at the time of parturition phase and its association with placental traits that may be useful to provide and predict some advantages to producers.
2. Materials and methods
2.1. Hormonal drugs
Controlled internal drug release (CIDR) with 30 mg of progesterone, a progestagen analogue (InterAg, Hamilton, New-Zealand), pregnant mare serum gonadotropin (PMSG) (folligon; Intervet International B.V, Boxmeer, the Netherlands), beta-hydroxy butyrate (BHBA) (Randox, UK, BT264QY), Venoject? (Sterile Terumo Europe, Leuven, Belgium) and commercially available kits for analysis of trace minerals (Pars Azmon, Karaj, Iran) were purchased from Invert Drug Industry (Tehran, Iran).
2.2. Animals, housing and diets
This experiment was performed on 40 clinical pregnancy toxemia Ghezel ewes (2–5 years old, weighing 40–50 kg) maintained in Animal Reproduction Research Station of Tabriz University, located in Tabriz province, Iran (38°07? N and 46o29? E) from June to December 2015 during breeding season. Ambient temperature during the experiment ranged from 20 to 26 °C with annual rainfall in this region ranging from 212 to 244 mm. The sheep obtained food by pasture grazing in summer; while in winter they were fed hay and fodder mix applied in rations of 220–270 g per sheep. In addition, salt-licks and mineral mixes were offered as well in the shed.
The ewes were treated with CIDR for 14 d and received 400 IU PMSG injection at the time of CIDR withdrawal. After the detection of estrus by use of teaser rams, ewes were hand-mated. Ewes were classified as having subclinical pregnancy toxemia on the basis of BHBA results (BHBA > 0.86 mmol/L) [9].
2.3. Sampling
Pregnant ewes were placed in an individual birth box around their estimated parturition date. Placenta was collected immediately after delivery and weighed fresh on a digital scale and the placental traits including placental weight (PW) and cotyledon number (CN) were measured and recorded. Placental efficiency (PE) was defined as the ratio of total birth weight (g) to placental weight (g). Cotyledon length (CL), cotyledon depth (CD) and cotyledon width (CW) were measured with an electronic digital caliper that randomly selected 10 cotyledons from each placenta. Total volume of amniotic fluid (TVAF) was measured using a graduated cylinder. The AF was collected by disposable syringes (10 mL) and stored in labeled plastic tubes at 20 °C until analysis of trace minerals.
2.4. Assay of trace minerals
Forty samples of AF were collected from pregnancy toxemia Ghezel ewes at the time of parturition. In the laboratory, these samples were centrifuged at 4 000 r/min for 10 min at approximately 22 °C to remove cellular debris, and stored at -20 °C for later analyses. The AF samples were analyzed for various trace minerals (Mg, Se, Zn, Cu and Fe) by using commercially available kits by atomic absorption spectrometry machine (Shimatzo, Japan). Standard commercial kits were used for analysis and the procedures were adopted as recommended by the manufacturer of these kits.
2.5. Assay of BHBA
A total of 5 mL blood was collected at the time of parturition by jugular venipuncture. Jugular blood samples were collected in vacuum tubes, early in the morning before feeding. To assay BHBA concentration, the serum was separated by centrifugation at 4 000 r/min for 10 min and samples were first frozen in -20 °C and then assessed in groups of 40 samples by atomic absorption spectrophotometric method using Runbut kit.
2.6. Statistical analysis
Means, Pearson correlation coefficient (for continuous data), analysis of variance (ANOVA) by general linear model and regression analyses were performed by using the Statistical Analysis System software [10].
3. Results
3.1. Trace minerals measurements
The trace mineral concentrations of AF that were obtained from pregnancy toxemia Ghezel ewes at the time of parturition were determined. The overall mean AF trace mineral concentrations were (3.13±2.05) (range: 21.18–40.01) ng/mL, (22.10±325.11) (range: 7.07–15.41) μg/dL, (134.70±63.28) (range: 72.01–196.25) μg/dL, (122.55±98.08) (range: 49.02–73.87) μg/dL and (166.67±89.20) (range: 47.09–285.07) μg/dL.
3.2. Placental traits
Table 1 presents the mean, standard error of mean and normal range of placental traits in the pregnancy toxemia Ghezel ewes at the time of parturition.
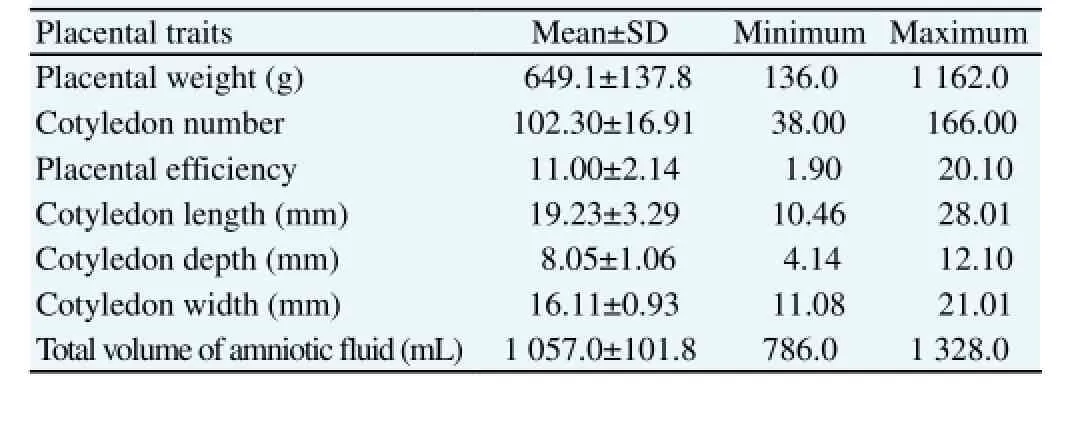
Table 1 Descriptive statistics of placental traits in the pregnancy toxemia Ghezel ewes (n = 40).
3.3. Correlation among trace minerals concentrations of AF and placental traits
Table 2 contains the correlations between the various trace minerals and placental traits in the pregnancy toxemia Ghezel ewes. There was significant positive correlation between PE and Zn concentration in AF ewes (r = 0.633, P < 0.01), while the relationship was significantly negative between TVAF of fetus and Fe concentration in AF ewes (r = -0.717, P < 0.01). In this research, for AF ewes no relationship was found between Se, Mg and Cu trace minerals with placental traits in ewes.
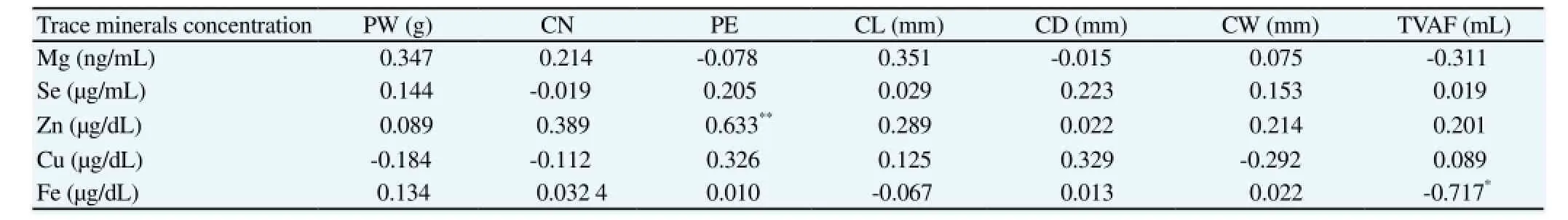
Table 2 Correlation among trace minerals concentrations of AF and placental traits in Ghezel ewes (n = 40).
3.4. Correlation among trace minerals concentrations of AF and BHBA
In our study, no correlation was detected between BHBA with trace minerals in pregnancy toxemia in Ghezel ewes (r = 0.425 for Mg, r = 0.314 for Se, r = -0.255 for Zn, r = 0.105 for Cu, r = 0.310 for Fe). Results of laboratory analyses demonstrated that there were no relationships between BHBA concentrations and placental traits (P > 0.01) (r = 0.231 for CN, r = -0.060 for PE, r = 0.359 for CL, r = 0.402 for CD, r = 0.352 for CW, r = 0.026 for TVAF), except for PW (r = 0.808, P < 0.01).
4. Discussion
The physiological importance of trace minerals in AF ewes is not well documented. To the best of our knowledge, no studies have investigated the relationship between trace mineral concentrations of AF with placenta traits in the pregnancy toxemia Ghezel ewes. Thus, the main objective of this study was the investigation of the trace minerals concentration at AF in the pregnancy toxemia Ghezel ewes at the time of parturition phase and its associated placental traits during breeding season in the north-west of Iran (Tabriz).
Metabolism of trace minerals plays a significant role in the physiological function (e.g., coenzyme activity) of the various reproduction phases. Also, trace minerals are essential for the normal progression of maternal and fetal tissue growth and metabolism in pregnancy. This experiment shows that during late pregnancy trace minerals pass into and accumulate in AF ewes. Trace minerals are transported to the offspring (lambs) along two pathways: via the placenta during the foetal stage, and with the colostrum at the neonatal phase, according to the mother’s availability. The present experiment confirms that trace minerals are present in AF ewes. This fact confirms that these minerals can cross the placental barrier in the sheep.
Analysis to determine the range of trace minerals in AF is uniquely susceptible to extreme errors because trace minerals are normally present in very low concentrations in the body, unless special precautions are taken during collection, storage, and analysis. According to the above facts, it is important to determine the trace element concentrations in ewes AF having physiological disorders such as pregnancy toxemia. Although potentially harmful effects of trace minerals are generally well-known, limited studies are available regarding the investigation of the relationship between these minerals and diseases [11]. Knowledge of trace minerals in AF of ewes would be a key factor in effective control of exposure and an improved knowledge of the potential role of trace minerals in pregnancy toxemia. The authors of this paper emphasize that the primary problem with the interpretation of achieved results of AF analysis is the lack of previous information on normal ranges of trace elements in AF ewes (especially in the pregnancy toxemia Ghezel ewes).
Zn has a vital role in the health, production and reproduction performances of animals [12] and is essential in cell survival, metabolic reactions, oxidative and reductive and metal enzymes. Significant positive relationships were recorded between PE and Zn concentrations in AF ewes (r = 0.633, P < 0.01). Uterine capacity is described by the total placental mass which a female can carry to term [13]. PE was developed as an indicator of uterine capacity [14]. PE has been commonly used for livestock species which produce larger birth type, such as pig and sheep [15,16]. The concentration of Zn in AF was very low in our study (134.70±63.28 μg/dL). This concentration was in agreement with the data of Schlabritz-Loutsevitch et al. [17], which measured (130±13) μg/dL Zn concentrations in pregnant baboons AF. Forage Zn is not sufficient and supplements in forages and concentrate are necessary to provide the daily requirements of production and reproduction trials [18]. Moreover, recent demonstrations that indicate supplementation of individuals with mild Zn deficiency decreases the prevalence or severity of several common infectious diseases provides hope that improvement of the trace element status of individuals with primary and secondary deficiencies represents an efficacious means for increasing immune competence [19].
Fe is important in cell survival, metabolic process, oxidation and
reduction, hemoglobin, myoglobin, enzyme activity and ferritin .
According to our results, there was significantly negative correlation between TVAF and Fe concentration in AF ewes (r = -0.717, P <0.01). This negative correlation might be caused by the high demand for Fe by fetus at the time of parturition phase. Iron in milk is poor and in roughages is not enough to meet the daily requirements and therefore must be added in food and concentrate. One of the reasons for low Fe in the AF of this study is perhaps the mineral competition in food and lack of concentrate feeding.
Cu is often one of the most limiting trace minerals for the fetus and neonate for the process of normal development. Also, Cu is an essential trace element that plays an important role in the biochemical reactions of the body; however, its requirements and interaction with other minerals are not clearly understood [20]. The role of Cu in hematopoiesis [21] and contrast with molybdenum and sulfur is clearly discerned in ruminants [22]. Hefnawy et al. reported that there were significant positive relationships between maternal plasma Cu concentrations with AF in Pelibuey sheep [23]. A great number of factors such as species, breed, sex, age, disease, rearing conditions, diet, reproductive status and seasonal variations (e.g., pregnancy and climate), can have an effect on the trace minerals values in AF. Thus, the above mentioned indexes are crucial in diagnosis of the pathological conditions. Besides, Konyali et al. also suggests that placental traits are affected by the birth type rather than the sex of the kids [24]. Cu, Zn and Fe are essential heavy metals and any imbalance in their interactions during pregnancy could have a major impact on fetal growth and development [25].
An optimal level of Mg in serum is effective in lamb’s growth and health, and thus prevents the occurrence of diseases related with hypomagnesemia [26]. Hypomagnesemia can occur in suckling foals the same as in calves. Hypomagnesemia is observed during the colic, acute diarrhea, digestive and respiratory disorders [27,28] in adult lambs. Since mare’s milk is poor in Mg and does not have adequate storage in the body, the continuation of Mg in daily diet is very essential.
As mentioned in our study, no highly significant correlations were observed between BHBA with trace minerals in pregnancy toxemia Ghezel ewes. We have not been able to find a similar model of our study to compare our results. The reason for these results in pregnant ewes could be related to either high fat metabolism during late pregnancy or nutritional management. Ketone bodies resulted from fat catabolism where glucose did not meet the energy requirements. In the absence of glucose and oxaloacetic acid in Krebs cycle, BHBA increases in serum and results in the presence of ketone body in milk, urine and fetal fluids called subacute [29] and acute pregnancy toxemia [30]. Stressful parturitions in ewes may cause variations in AF trace minerals concentration, specifically in those ewes suffering from pregnancy toxemia. Apparently, the stress of the pregnancy results in mobilization and some loss of trace minerals from the AF stores. Finally, understanding the relationship among minerals has practical applications.
The strong relations between the BHBA concentration and the PW
show, and increased PW led to prolonged duration of secretions of BHBA from AF in the pregnancy toxemia Ghezel ewes. Obtained data demonstrated for the first time that BHBA concentrations were correlated with PW.
Trace minerals deficiencies and imbalances are often cited as causes of poor reproduction. Numerous recent studies [31-33] have shown that the trace minerals requirements of human and animal increase during late pregnancy due to the rapid growth of the fetus. Thus, if ewes do not receive at least half of the required metals during this period, the occurrence of pregnancy toxemia may be maximized. This suggests that the supplemented minerals protect the body against tissue damage and abnormalities. The AF can be considered as a valuable marker of this prenatal exposure to exogenous factors. AF usually reflects the distribution of the minerals in the body (dams or fetus) at the moment the specimen is collected. When intakes of trace minerals are deficient in dams, maternal transfer of these minerals to the fetus is insufficient for optimum development and acute disorders to the central nervous system, skeleton, muscular and metabolism results. Therefore, AF analysis can provide important information to a clinician that may not be readily available with blood analysis.
The authors recommend that AF samples must be taken from various species of animals at different times of the year and in different reproductive status so that proper reference values are obtained. Also normal range of trace minerals in AF would increase the ewe’s fecundity and improve the health of neonatal lambs (minimizing the occurrence of various diseases). Finally, future investigations should be made on the correlations between various enzyme activity in AF and placental traits in human and livestock.
In conclusion, taking the results together suggests that there is an ordinary relationship between the placental traits and AF trace minerals in the pregnancy toxemia Ghezel ewes at the time of parturition in the northwest of Iran. Finally, the data in the present paper support the idea that ewes are excellent models for studying the physiology of AF trace minerals in humans.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to acknowledge Immunology Research Center, Tabriz University of Medical Sciences that has provided excellent assistance with the preparation of this research. We would like also to thank all of the members of our laboratories (Immunology Research Center, Tabriz University of Medical Sciences) for their scientific contributions during these years. This work was supported by the Department of Animal Science, Faculty of Agriculture, University of Tabriz, Iran, with the research
mentorship program (Dr. Gholamali Moghaddam) of higher education thesis program.
[1] Biricik H, Ocal N. Seasonal changes of some mineral status in mares. J Equine Vet Sci 2005; 25: 346-348.
[2] Hansen SL, Schlegel P, Legleiter LR, Lloyd KE, Spears JW. Bioavailability of copper from copper glycinate in steers fed high dietary sulfur and molybdenum. J Anim Sci 2008; 86: 173-179.
[3] Samia M, Bouabdellah B, Miloud H. Serum electrolytes variation in Arabian mares. Global Vet 2004; 10: 520-523.
[4] Abdelrahman MM, Kincaid RL. Deposition of copper, manganese, zinc, and selenium in bovine fetal tissue at different stages of gestation. J Dairy Sci 1993; 76: 3588-3593.
[5] Olfati A, Moghaddam G, Moradi Kor N, Bakhtiari M. The relationship between progesterone and biochemical contents of amniotic fluid with placenta traits in Iranian crossbred ewes (Arkhar-Merino×Ghezel). Asian Pac J Trop Med 2014; 7(Suppl 1): 162-166.
[6] Moradi Kor N, Akbari M, Olfati A. The effects of different levels Chlorella microalgae on blood biochemical parameters and trace mineral concentrations of laying hens under heat stress condition. Inter J Biometeorol 2015; 59(244): 1-6.
[7] Burguera J, Burguera M. Recent on-line processing procedures for biological samples for determination of trace minerals by atomic spectrometric methods. Spectrochim Acta Part B at Spectrosc 2009; 64: 451-458.
[8] Awad M, Lilly E. Trace minerals in smokers and non-smoker’s urine samples. Juba Uni J Arts and Sci 2010; 9: 27-40.
[9] Bani Ismail ZA, Al-Majali AM, Amireh F. Al-Rawashdeh OF. A metabolic profile in goat does in late pregnancy with and without subclinical pregnancy toxemia. Vet Clin Pathol 2008; 37(4): 434-437.
[10] SAS Institute Inc. SAS users guide. Cary, NC: SAS Institute Inc.; 2003.
[11] Momen AA, Khalid MAA, Elsheikh MAA, Hag Ali DM. Assessment and modifications of digestion procedures to determine trace minerals in urine of hypertensive and diabetes mellitus patients. J Health Special 2013; 1(3): 122-128.
[12] Radostits OM, Blood DC, Henderson JA. Veterinary medicine. 8th edition. London: Bailliere & Tindall Publication, Ltd.; 2007.
[13] Wilson ME, Biensen NJ, Ford SP. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J Anim Sci 1999; 77: 1654-1658.
[14] Wilson ME, Ford SP. Comparative aspects of placental efficiency. Reprod Supplement 2001; 58: 223-232.
[15] Mesa H, Safranski TJ, Johnson RK, Lamberson WR. Correlated response in placental efficiency in swine selected for an index of components of litter size. J Anim Sci 2003; 81: 74-79.
[16] Dwyer CM, Calvert SK, Farish M, Donbavand J. Pickup HE. Breed, litter and parity effects on placenta weight and placentome number, and consequences for the neonatal behavior of the lamb. Theriogenology 2005; 63: 1092-1110.
[17] Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, Jenkins SL, Frost PA, McDonald TJ, et al. Normal concentrations of essential and toxic minerals in pregnant baboons and fetuses (Papio species). J Med Primatol 2004; 33: 152-162.
[18] Farah A, Akbar LL, Zafar Q, Ahmad I, Riaz H. Serum mineral profile in various reproductive phases of mares. Pak Vet J 2013; 33: 296-299.
[19] Failla ML. Trace minerals and host defense: Recent advances and continuing challenges. J Nut 2003; 133: 1433-1447.
[20] Solaiman SG, Maloney MA, Qureshi M, Davis G, D`Andres G. Effects of high copper supplements on performance, health, plasma copper and enzymes in goats. Small Rumin Res 2001; 41: 127-139.
[21] Laven RA, Lawrence KE, Livesey CT. The assessment of blood copper status in cattle: a comparison of measurements of caeruloplasmin and elemental copper in serum and plasma. N Z Vet J 2007; 55: 171-176.
[22] Hansen SL, Schlegel P, Legleiter LR, Lloyd KE, Spears JW. Bioavailability of copper from copper glycinate in steers fed high dietary sulfur and molybdenum. J Anim Sci 2008; 86: 173-179.
[23] Hefnawy AE, Shousha SM, Abu Kora SY, Tortora-Perez J. Effect of gestation and maternal copper on the fetal fluids and tissues copper concentrations in sheep. Vet Res 2011; 4(1): 9-12.
[24] Konyali A, T?lü C, Da G, Sava T. Factors affecting placental traits and relationships of placental traits with neonatal behavior in goat. Anim Reprod Sci 2007; 97: 394-401.
[25] Barone A, Ebesh O, Harper RG, Wapnir RA. Placental copper transport in rats: effects of elevated dietary zinc on fetal copper, iron and metallothionein. J Nut 1998; 128: 1037-1041.
[26] Grace ND, Pearce SG, Firth EC, Fennessy YPF. Content and distribution of macro- and micro-elements in the body of pasture-fed young horses. Aust Vet J 2008; 43: 45-52.
[27] Mohri E, Fernandes WR, Mirandola RMS, Kubo G, Ferreira RR, Oliveira GV, et al. Reference values on serum biochemical parameters of Brazilian donkey (Equusasinus) breed. J Equine Vet Sci 2003; 23: 358-364.
[28] Durie L, Van Loon G, Hesta M, Bauvens C, Deprez P. Hypocalcemia caused by primary hypoparathyroidism in a 3 month old filly. J Vet Inter Med 2010; 24: 439-442.
[29] Lacetera N, Bernabucci U, Ronchi B, Nardone A. Effects of subclinical pregnancy toxemia on immune response in sheep. Am J Vet Res 2001; 62: 1446.
[30] Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary medicine. 9th edition. London: Harcourt Publishers Ltd.; 2006.
[31] Yahaya MI, Ezeh GC, Musa YF, Yelwa AS. Analysis of heavy metals concentration in road side’s soil in Yauri, Nigeria. Afr J Pure Appl Chem 2010; 4(3): 22-30.
[32] Koureas M, Tsakalof A, Tsatsakis A. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Let 2012; 210: 155-168.
[33] Llop S, Casas L, Santa ML, Esplugues A. Prenatal and postnatal residential usage of insecticides in a multicenter birth cohort in Spain. Sci Total Environ 2013; 445-446 C: 273-280.
ment heading
10.1016/j.apjr.2016.06.003
*Corresponding author: Ali Olfati, Department of Animal Science, Faculty of Agriculture, University of Tabriz, Iran.
Tel: +98-9195966273
Fax: +98-4116698260
E-mail: A.Olfati65@gmail.com; Aajbs@casrp.co.uk
Foundation project: This work was supported by the Department of Animal Science, Faculty of Agriculture, University of Tabriz, Iran, with the research mentorship program (Dr. Gholamali Moghaddam) of higher education thesis program.
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
- Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
- Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
- Evaluation of the academic achievement of rural versus urban undergraduate medical students in pharmacology examinations
