In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
Baskaran Xavier Ravi
1Shenzhen Key Laboratory of Southern Subtropical Plant Diversity, Fairylake Botanical Garden, Shenzhen & Chinese Academy of Sciences, Shenzhen 518 004, P. R. China
2Department of Botany, St. Joseph’s College, Tiruchirappalli, Tamil Nadu 620 002, India
In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
Baskaran Xavier Ravi1,2*
1Shenzhen Key Laboratory of Southern Subtropical Plant Diversity, Fairylake Botanical Garden, Shenzhen & Chinese Academy of Sciences, Shenzhen 518 004, P. R. China
2Department of Botany, St. Joseph’s College, Tiruchirappalli, Tamil Nadu 620 002, India
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Gametophyte Juvenile sporophytes 6-benzylaminopurine Murashige and Skoog basal media
Objective: An efficient in vitro protocol was established during the development of polyembryony in the spore derived gametophyte of Pteris tripartita Sw. Methods: Sterilized spores were germinated in half strength Murashige and Skoog (MS) basal media to produce gametophyte at in vitro condition. Three month old gametophytes were sub-cultured in half strength MS medium with 6-benzylaminopurine (BAP). All the inoculated cultures were incubated and noticed every month for polyembryony. Results: Multiple numbers (Di, Tri, Tetra, Hexa, and Octa) of the juvenile sporophyte per spore derived gametophyte were observed at 3 mg/L of BAP in half strength MS basal media, which augmented with 30% sucrose. Hexa polyembryony or juvenile sporophytes was noticed on both 3 and 4 mg/L of BAP, which developed 1.00 and 0.76 cm lengths, respectively. At 4 mg/L of BAP in MS culture medium, various numbers (Di, Tri, Tetra and Hexa) of juvenile sporophytes per gametophyte were formed and showed 1.03, 0.66, 0.53 and 0.76 cm of sporophyte lengths, correspondingly. Conclusion: An in vitro developed polyembryony or juvenile sporophyte exhibited normal growth in their morphological structure.
1. Introduction
In vitro propagation plays an imperative role in the conservation of plant species having pharmacological principles [1]. The successful uses of in vitro techniques producing pharmacologically important plants depend upon the establishment of an efficient method of regenerating a large number of plants [2]. Furthermore, tissue culture offers a unique advantage over conventional propagation methods of rapid multiplication of valuable genotypes, expeditious release of improved cultivars and production of disease-free plants, season independent production, germplasm conservation and facilitating their easy exchange. Development of an effective tissue culture approach for economically and medicinally important trees have the great potential for its mass production, germplasm conservation and genetic manipulations [3]. According to previous study [4], growth regulator concentrations are crucial to the control of plant growth and its morphogenesis. In 1719, Leeuwenhoek reported the polyembryony in orange seeds at first time, in which each contains two embryos. Even though many authors reported the formation of polyembryony in flowering plants such as Pterocarya fraxinifolia [5], maize [6], Commiphora wightii [7,8], Citrus sinensis [9], Mangifera indica [10], nine genera [11], Telfairia occidentalis [12], Citrus sp [13,14], olive [15], and few reports only available for pteridophyte lower group of vascular plants. In general, studying of polyembryony is very limited to occurrence, which plays an important role in the practical breeding effort in both origin and perpetuation of new forms [16]. Polyembryony has been documented in sexual ferns and usually attributed to multiple fertilizations [17-19]. According to previous study [20], 2–5 embryos were regularly developed on each gametophyte of Pteridium aquilinum (L.) Kuhn cultured in Moore’s medium containing dimethyl sulphoxide (DMSO) solvent. Development of multiple embryos in spite of multiple spermatozoid-egg fusions could be raised through the charisma of plant growth hormones and produced by the first sporophyte which either limit fertilization of other archegonia or may prevent the development of multiple embryos [21,22].
As a result of earlier reports on Pteridophyta such as, Matteuccia struthiopteris (L.) Tod, Onoclea sensibilis L., Dryopteris mollis Maxon. and Pteris longifolia L. gametophytes in cell culture media were developed polyembryony and two sporophytes was also observed [23]. Following ferns namely, Gleichenia sp [24-26], Osmunda sp [27], Angiopteris evecta (Forst.) Hoffm [28], Adiantum cuneatum Langsd. & Fisch [24], Helminthostachys sp [29], Botrychium lunaria (L.) Sw. [30],
Botrychium virginianum (L.) Sw. [31], Botrychium obliquum Muhl. [32], Pityrogramma chrysophylla (Sw.) Link [33], Aspidium thelypteris Sw. [34], Equisetum debile Roxb. Ex-Vaucher [35], E. laevigatum A. Braun [36] and Vittaria sp [37] were also developed polyembryony from a single gametophyte. The bio-accumulating fern species, Pteris tripartita Sw. is present in Sri Lanka, Indian islands, South India, Thailand, Malaysia, Indonesia, Philippines, Australia, Polynesia, Africa and Madagascar [38]. According to previous report [39], P. tripartita Sw. is a critically endangered fern among 414 species of threatened pteridophytes in India and also reported for the first time from Eastern Ghats [40]. The effects of heavy metals, sucrose, pH and hormones on spore germination percentage and their gametophyte growth, apogamous reproduction, antioxidant and its phytochemical studies of the frond extracts of P. tripartita Sw. has been reported in our earlier studies [41-44]. Due to its biological interest, the aim at this present investigation was to determine the most suitable growth regulator and its concentration to develop an optimized protocol for polyembryony induction using spore derived gametophyte of a critically endangered fern, P. tripartita Sw.
2. Materials and methods
2.1. Spore collection
Matured fertile sporophytes (fronds) of P. tripartita Sw. were collected in Alagar hills of Tamil Nadu and confirmed with the help of reference standards of the Centre for Biodiversity and Biotechnology (CCB), St. Xavier’s College, Palayamkottai (Tamil Nadu). The voucher specimen was also numbered (XCH 25403) and deposited at St. Xavier’s College Herbarium (XCH). Fertile sporophytes (fronds) of P. tripartita Sw. were dried at room temperature for two days to collect spores. Normally, fern spores lose their viability if stored at room temperature. Thus, the collected spores were preserved at low temperature (4 °C) for further studies.
2.2. Culture media
Merely, 5 mg of spores were scooped from the storage bag and immersed in distilled water for 2 h for imbibition to enhance the germination percentage. Then spores were sterilized with sodium hypochlorite (0.5% v/v in double distilled water) for 10 min. The sterilized spores were rinsed at least three times in double distilled water and centrifuged at 3 000 r/min for 3 min to collect them. Sterilized spores were sown in half strength Murashige and Skoog (MS) basal media with 30% sucrose, and the pH was adjusted to 5.6–5.8 with 0.1 mol/L NaOH or 0.1 mol/L HCl prior to the addition of 0.8% (w/v) agar for solidification. The cultures were maintained at (25 ± 2) °C for 16 h photoperiod of 40 μmol/(m2?s) irradiances provided with cool white fluorescent tubes and supplied with 55%–60% relative humidity (RH).
2.3. Subculture and induction of polyembryony
After 3 months, approximately 3 g of gametophytes were subcultured on 50 mL of half strength MS medium augmented with 6-benzylaminopurine (BAP) (1, 2, 3, 4, and 5 mg/L). The culture medium was regularly changed every month due to its depletion, and the same culture media was given for subculture. After 8 months, an efficacy of BAP in half strength MS medium on juvenile sporophyte proliferation was noticed during the culture period, and its numbers were counted. Under naked-eye appraisal, number of polyembryony was calculated by the number of juvenile sporophytes raised from a single gametophyte. Each culture was observed for juvenile sporophyte lengths and its number per gametophyte, and the total number of juvenile sporophytes (polyembryony) formed at all the gametophytes in each replicate.
2.4. Statistical analysis
All values are the means of three treatments, and each treatment consisted of 10 replicates. All data represented as mean ± SE of triplicate and analyzed using one way ANOVA test (SPSS 17.0 software, USA), with Duncan’s multiple range test [45] accompanied by P < 0.05 as the limit of significance.
3. Results
3.1. Polyembryony development
In our present study, Di, Tri, Tetra, Hexa, and Octa numbers of polyembryony or juvenile sporophytes were developed from a single gametophyte of bioaccumulation fern, P. tripartita Sw. Spore derived gametophytes of P. tripartita Sw. were grown in half strength MS basal media maintained for 3 months (Figure 1a). In our present study, Di to Octa numbers of polyembryony or juvenile sporophytes was induced in the midrib of gametophytes
after 5-month culture period. The numbers of juvenile sporophytes per gametophyte, its lengths, and the total number of polyembryony or juvenile sporophytes per culture treatment were counted, and their lengths were measured after 8-month of culture period (Table 1). Among five concentrations of BAP (1–5 mg/L), Di, Tri, Tetra, Hexa, and Octa numbers of polyembryony or juvenile sporophytes were observed from a single gametophyte at 3 mg/L of BAP. Of them, significant lengths (1.00, 0.70, 1.16, 1.00, 1.13 cm) were observed in Di, Tri, Tetra, Hexa, Octa polyembryony or juvenile sporophytes per gametophyte, respectively. Furthermore, Hexa and Octa polyembryony were observed from fewer cultures. Hexa polyembryony was obtained at 3 and 4 mg/L of BAP in MS culture media and showed 1.00 and 0.76 cm lengths of the juvenile sporophyte, correspondingly. Especially, Octa numbers of juvenile sporophytes were induced per gametophyte with 1.13 cm of length and were observed only at 3 mg/L of BAP (Figure 1c; Table 1). In addition, the formation of Penta and Hepta polyembryony or juvenile sporophytes per gametophyte has not been produced from any concentration of BAP hormone in half strength MS medium.
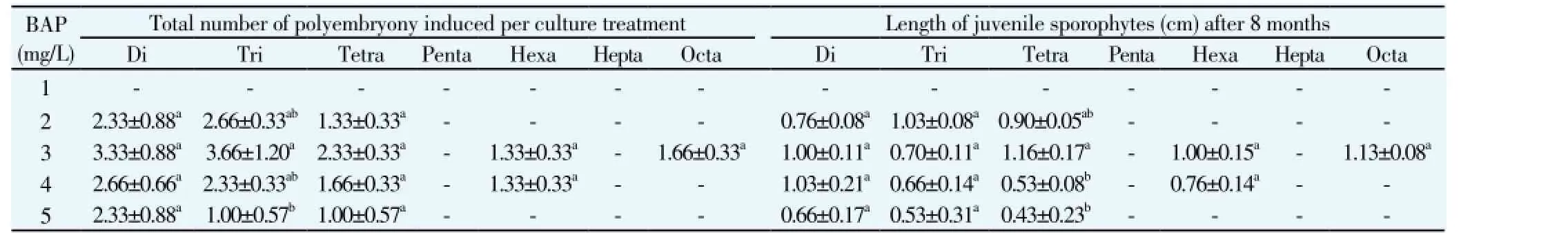
Table 1 Effects of BAP with half strength MS medium for polyembryony development on spore derived gametophyte of Pteris tripartita Sw.

Figure 1. Influence of cytokinin hormone (BAP) on the spore derived gametophyte of Pteris tripartite Sw. for polyembryony development.a) Three month old gametophytes in 1/2 strength MS basal medium; b) Formation of Di juvenile sporophytes in 4 mg/L BAP; c) Development of Octa juvenile sporophytes in 3 mg/L of BAP; d) Microscopic observation of Di juvenile sporophytes from a single gametophyte.
On the other hand, polyembryony was not induced in the lower concentration of BAP (1 mg/L). At 2 mg/L of BAP, Di, Tri and Tetra polyembryony per gametophyte were developed with 0.76, 1.03 and 0.90 cm lengths of juvenile sporophytes, correspondingly. Various total mean number of juvenile sporophytes (2.33, 2.66 and 1.33) or polyembryony was formed. At 4 mg/L of BAP, Di (Figure 1b), Tri, Tetra and Hexa polyembryony per gametophyte were produced with 1.03, 0.66, 0.53 and 0.76 cm of sporophyte mean lengths, respectively. Likewise, Di, Tri and Tetra numbers of polyembryony were noticed at 5 mg/L of BAP with 0.66, 0.53 and 0.43 cm mean lengths of shoots were observed, respectively. The formation of Di juvenile sporophytes (polyembryony) in a single gametophyte of P. tripartita Sw. was microscopically captured (Figure 1d). However, the half strength MS medium augmented with BAP hormone induced Di, Tri, Tetra, Hexa and Octa polyembryony in spore derived gametophytes of P. tripartita Sw. at prolonged culture. Various numbers of juvenile sporophytes or polyembryony were formed in a heart and cordate shaped gametophyte, and its sporophyte lengths have also been measured to develop an optimized protocol to induce polyembryony of a bio accumulating fern, P. tripartita Sw.
4. Discussion
Necrosis during in vitro culture could be abridged by continuous sub-culture in the similar medium beneficial for minimizing phenolic exudation and could also be improved explants survival [46,47]. In addition, a vascular lowered group of plants, ferns possess a number of secondary metabolites like total phenolic compounds. In general, the spore derived gametophyte (prothalli) of a fern ordinarily raises only one juvenile sporophyte. Occasionally, multiple numbers of juvenile sporophytes are produced per gametophyte and also have been recorded in earlier studies on Dryopteris mollis Maxon and Pteris longifolia L., in which the fern prothallus was divided carefully into two parts, and each portion can perform independently afterward to produce young plants [48]. According to previous studies [19,49], long delay of sporophyte formation, continuous gametophyte growth and polyembryony have been characterized a system of “l(fā)eaky lethality” in which the genetic load prevents the sporophyte formation until some fortuitous combination of factors such as egg cytoplasm, archegonia position, and gametophyte size allowed sporophytes formation. Occurrence of polyembryos might not be caused by multiple gametophyte growths of density culture experiments. However, polyembryony may also occur even in lowest density of culture conditions [50]. Therefore, polyembryos may arise from intergametophytic or intragametophytic mating in sexual ferns. Polyembryony could increase the probability of intergametophytic mating in sexual ferns through neighboring sporophytes and adjacent gametophytes, consequently [18,19,51]. According to earlier report, prothalli of Asplenium nidus L. could be multiplied successfully by a subsequent sub-culture in half strength MS medium with BAP at 1–4 mg/L [52].
Typically, prothalli of P. tripartita Sw. have developed a heartshaped gametophyte and also have midrib in the middle of two wings [43]. Generally, each gametophyte formed only single embryo on their midrib that nearby to apical meristem, but in some special cases, two embryos were grown close together in two apogamous ferns, P. cadieri Christ and P. grevilleana Wall after 3-month culture period [53]. From the midrib regions, more than one juvenile sporophyte has been raised directly from the ventral side of an apogamous gametophyte. Each gametophyte developed root system individually to absorb nutrients from the culture medium. The embryos were developed with scales and midribs on the first frond of juvenile sporophytes are the indicator of apogamy [53-57]. According to previous report, isolated gametophytes of Blechnum spicant underwent polyembryony in artificial culture media due to time delay, and their size of the gametophyte was also proliferated
[50]. The gametophytes of Matteuccia nodulosa Fernald, Dryopteris mollis Maxon, Osmunda claytoniana L., and Pteris longifolia L. was developed several sporophytes (11 sporophytes per gametophyte) in soil culture after 4 months [17]. In early study, significant spore germination percentages, gametophyte length and its width of P. tripartita Sw., were noticed in the MS culture medium augmented with 3 mg/L of BAP [43]. In our previous study, significant numbers of sporophytes, its length and root length of P. tripartita Sw. were raised from spore derived gametophytes at 4 mg/L of BAP [44]. Usually, cytokinins are very effective to promote direct shoot induction to plants, in which BAP is a widely used hormone for the organogenesis in plants owing to effectiveness and affordability. The main role of BAP is a bud breaking system which already reported on many angiosperms medicinal plants such as, Pelargonium capitatum (L.) Aiton [58], Populus ciliata Schur [59], Embelia ribes Burm.f [60], and Carthamus tinctorius L [61]. According to early reports, single gametophytes form numerous archegonia, and later produce only single sporophyte [62,63]. Occasionally, one gametophyte will form numerous sporophytes due to over maturation of gametophytes under prolonged cultivation that tend to be polyembryony [17,64]. Organic matter of the knop culture medium was found to induce Di and Tri polyembryony from a gametophyte of Thelypteris palustris Schott. The competition occurs to polyembryonic sporophytes to absorb the nutrients from the culture medium as well the light energy for photosynthesis [65].
Our results concluded that BAP, a cytokinin hormone is having significant capability to induce in vitro polyembryony in spore derived gametophytes of P. tripartita Sw.
Conflict of interest statement
The author declares that I have no conflict of interest.
Acknowledgement
The author dedicated this research works to my mentor, Rev. Dr. V. S. Manickam, SJ and also thanks Dr. V. Irudayaraj, who gives suggestion to improve the manuscript.
[1] Afolayan AJ, Adebola PO. In vitro propagation: a biotechnological tool capable of solving the problem of medicinal plants decimation in South Africa. Afr J Biotechnol 2004; 3: 683-687.
[2] Murch SJ, Krishna Raj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in-vitro regenerated St. John’s wort (Hypericum perforatum L. Cv. Anthos) plants. Plant Cell Rep 2000; 19: 698-704.
[3] Singh N, Meena MK, Patni V. In vitro rapid multiplication of a highly valuable medicinal plant Naringi crenulata (Roxb.) Nicolson. J Med Plants Res 2011; 5(31): 6752-6758.
[4] George E, Hall M, Klerk GJ. Plant propagation by tissue culture. Springer Netherlands Publishers 2007.
[5] Bouman F, Boesewinkel FD. On a case of polyembryony in Pterocarya fraxinifolia (Juglandaceae) and on polyembryony in general. Acta Bot Neerland 1969; 18: 50-57.
[6] Erdelska O, Vidovencova Z. Cleavage polyembryony in maize. Sex Plant Reprod 1992; 5(3): 224-226.
[7] Gupta P, Shivanna KR, Mohan Ram HY. Apomixis and polyembryony in the guggul plant, Commiphora wightii. Ann Bot 1996; 78(1): 67-72.
[8] Kawane A, Geetha KA, Reddy MN, Maiti S. Degree of polyembryony among the accessions of Commiphora wightii collected from different natural habitats of India. Curr Sci 2014; 107(3): 361-364.
[9] Koltunow AM, Hodaka T, Robinson SP. Polyembryony in Citrus. Plant Physiol 1996; 110: 599-609.
[10] Aron Y, Czosnek H, Gazit S. Polyembryony in Mango (Mangifera indica L.) is controlled by a single dominant gene. Hortscience 1998; 33(7): 1241-1242.
[11] Salomao AN, Allem AC. Polyembryony in angiospermous trees of the Brazilian cerrado and caatinca vegetation. Acta Bot Bras 2001; 15(3): 369-378.
[12] Onovo JC, Uguru MI, Obi IU. Implications of polyembryony on the growth and yield of fluted pumpkin (Telfairia occidentalis Hook. F.). J Trop Agri Food Envt Exten 2009; 8(2): 130-138.
[13] Pablo A, Jose J, Patrick O, Luis N. Polyembryony in non-apomictic citrus genotypes. Ann Bot 2010; 106: 533-545.
[14] Nakano M, Shimada T, Endo T, Fujii H, Nesumi H, Kita M, et al. Characterization of genomic sequence showing strong association with polyembryony among diverse Citrus species and cultivars, and its synteny aith Vitis and Polulus. Plant Sci 2012; 183: 131-142.
[15] Trapero C, Barranco D, Martin A, Diez CM. Occurrence and variability of sexual polyembryony in olive cultivars. Sci Hortic 2014; 177(2): 43-46. [16] Webber JM. Polyembryony. Bot Rev 1940; 6(11): 575-598.
[17] Mottier DM. Polyembryony in certain Polypodiaceae and Osmundaceae. Bot Gaz 1925; 80: 331-336.
[18] Klekowski EJJ. Populational and genetic studies of a homosporous fern-Osmunda regalis. Am J Bot 1970; 57: 1122-1138.
[19] Klekowski EJ. Evidence against genetic self-incompatibility in the homosporous fern Pteridium aquilinum. Evolution 1972; 26: 66-73.
[20] Sheffield E. Effects of dimethyl sulphoxide on the gametophytic and sporophytic phases of Pteridium aquilinum (L.) Kuhn. Ann Bot 1984; 54: 531-536.
[21] Duckett JG, Bell PR. Studies on fertilization in archegomate plants II egg penetration in Pteridhm aquilinum (L ) Kuhn. Cytobiologie 1972; 6: 35-50.
[22] Page CN. Experimental aspects of fern ecology. In, Dyer AF, editor. The experimental biology of ferns. London: Academic Press; 1979, p. 551-589.
[23] Austin E. Polyembryony developed under experimental conditions in certain polypodiaceous ferns. B Torrey Bot Club 1923; 50(3): 95-108.
[24] Atkinson GF. Two perfectly developed embryos on a single prothallium of Adiantum cuneatum. B Torrey Bot Club 1893; 20: 407-408.
[25] Rauwenhoff NWP. De geslachtsgeneratie der Gleicheniaceeen. Amsterdam: Johannes Müller; 1889.
[26] Rauwenhoff NWP. La generation sexuee des Gleicheniacees. Arch Neerland Aci 1891; 24: 157-231.
[27] Campbell DH. On the prothallium and embryo of Osmunda claytoniana L. and O. Cinnmomea L. Ann Bot 1892; 6: 49-94.
[28] Farmer JB. On the embryogeny of Angiopteris evecta. Ann Bot 1892; 6: 263-270.
[29] Lang WH. Studies in the morphology and anatomy of the Ophioglossaceae. II. Embryo of Helminthostachys. Ann Bot 1914; 28: 19-37.
[30] Bruchmann H. Ueber das Prithallium und die Sporenpflanze von Botrychium lunaria Sw. Flora 1906; 96: 203-230.
[31] Jeffrey EC. The gametophyte of Botrychium virginianum. T Can Inst 1918; 5: 265-295.
[32] Campbell DH. The gametophyte and embryo of Botrychium obliquum Muhl. Ann Bot 1921; 35: 141-158.
[33] Czaja AT. Ueber Befruchtung, Bastardierung und Geschlechtertrennung bei Protallien homosporer Farne. Z Bot 1921; 13: 545-589.
[34] Buchholz JT. Developmental selection in vascular plants. Bot Gaz 1922; 73: 249-286.
[35] Kashyab SR. Structure and development of the prothallium of Equisetum debile. Ann Bot 1914; 28: 173-181.
[36] Walker, Elda R. The gametophytes of Equisetum laevigatum. Bot Gaz 1921; 71: 378-391.
[37] Goebel K. Morphologische und biologische Studien. II. Zur Keimungsgeschichte einiger Farne. Ann Jard Bot Buitenzorg 1888; 7: 74-119.
[38] Fraser-Jenkins CR. Rare and threatened pteridophytes of asia endangered species of India– the higher IUCN categories. Bull Natl Mus Nat Sci Ser B 2012; 38(4): 153-181.
[39] Chandra S, Fraser-Jenkins CR, Kumari A, Archana S. A summary of the status of threatened pteridophytes of India. Taiwania 2008; 53(2): 170-209.
[40] Benniamin A. Pteris tripartita Sw. A new record for Eastern Ghats. Inter J
Biol Technol 2011; 2(1): 14-15.
[41] Baskaran X, Jeyachandran R. Evaluation of antioxidant and phytochemical analysis of Pteris tripartita Sw. A critically endangered fern from South India. J Fairy Lake Bot Gard 2010; 9(3): 28-34.
[42] Baskaran X, Jeyachandran R. In vitro spore germination and gametophyte growth assessment of a critically endangered fern: Pteris tripartita Sw. Pteridol Res 2012; 1(1): 4-9.
[43] Baskaran X, Jeyachandran R, Melghias G. In vitro spore germination and gametophytic growth development of a critically endangered fern Pteris tripartita Sw. Afr J Biotechnol 2014; 13(23): 2350-2358.
[44] Baskaran X, Geo Vigila A, Rajan K, Jeyachandran R. Apogamous sporophyte development through spore reproduction of a South Asia’s critically endangered fern: Pteris tripartita Sw. Asian Pac J Reprod 2015; 4(2): 135-139.
[45] Duncan DB. Multiple range and multiple F test. Biometrics 1955; 11: 1-42.
[46] Gill RIS, Gill SS. In vitro exudation of phenol in Eucalyptus. Indian Forest 1994; 120: 504-509.
[47] Tiwary SK, Tiwary KP, Siril EA. An improved micropropagation protocol for teak. Plant Cell Tiss Org 2002; 71: 1-6.
[48] Austin E. Polyembryony developed under experimental conditions in certain polypodiaceous ferns. B Torrey Bot Club 1922; 50: 95-108.
[49] Klekowski EJ. Genetic load in Osmunda regalis populations. Am J Bot 1973; 60: 146-154.
[50] Cousens MI. Gametophyte ontogeny, sex expression, and genetic load as measures of population divergence in Blechnum spicant. Am J Bot 1979; 66: 116-132.
[51] Lloyd RM. Reproductive biology and evolution in the Pteridophyta. Ann Mo Bot Gard 1974; 61: 318-331.
[52] Khan S, Raziq M, Kayani HA. In vitro propagation of bird’s nest fern (Asplenium nidus) from spores. Pak J Bot 2008; 40(1): 91-97.
[53] Chao YS, Liu HY, Huang YM, Chiou WL. Reproductive traits of Pteris cadieri and P. Grevilleana in Taiwan: Implications for their hybrid origin. Bot Stud 2010; 51: 209-216.
[54] Steil WN. Apogamy, apospory, and parthenogenesis in the pteridophytes. Bot Rev 1939; 5: 433-453.
[55] Kanamori K. Apogamy in ferns with special reference to the apogamous embryogenesis. Sci Rep Tokyo Kyoiku Daigaku 1972; 15: 111-131.
[56] Moore SJ, Hsieh TH, Huang YM, Chiou WL. Diplazium maonense Ching. A poorly known species of the Athyriaceae (Pteridophyta) in Taiwan. Taiw J Forest Sci 2002; 17: 113-118.
[57] Huang, YM, Chou HM, Hsieh TH, Wang JC, Chiou WL. Cryptic characteristics distinguish diploid and triploid varieties of Pteris fauriei (Pteridaceae). Can J Bot 2006; 84: 261-268.
[58] Hassanein A, Dorion N. Efficient plant regeneration system from leaf discs of zonal (Pelargonium x hortorum) and two scented (P. Capitatum and P. Graveolens) geraniums. Plant Cell Tiss Org 2005; 83: 231-240.
[59] Thakur AK, Srivastava DK. High-efficiency plant regeneration from leaf explants of male Himalayan poplar (Populus ciliata Wall.). In vitro Cell Dev B 2006; 42: 144-147.
[60] Raghu AV, Geetha SP, Balachandran GMI, Ravindran PN. Direct shoot organogenesis from leaf explants of Embelia ribes Burm. F.: a vulnerable medicinal plant. J Forest Res 2006; 11: 57-60.
[61] Sujatha M, Dineshkumar V. In vitro bud regeneration of Carthamus tinctorius and wild Carthamus species from leaf explants and axillary buds. Biol Plantarum 2007; 51(4): 782-786.
[62] Ganders FR. Heterozygosity for recessive lethal in homosporous fern populations: Thelypteris palustris and Onoclea sensibilis. Bot J Linn Soc 1972; 65: 211-221.
[63] Masuyama S. Reproductive biology of ferns (in Japanese). Hojo Shokan, Tokyo 1984.
[64] Mottier DM. Behavior of certain fern prothallia under prolonged cultivation. Bot Gaz 1927; 83: 244-267.
[65] Sakamaki Y, Ino Y. Gametophyte contribution to sporophyte growth on the basis of carbon gain in the fern Thelypteris palustris: effect of gametophyte organic matter production on sporophytes. J Plant Res 2007; 120: 301-308.
ment heading
10.1016/j.apjr.2016.06.012
*Corresponding author: Xavier ravi Baskaran, Shenzhen Key Laboratory of Southern Subtropical Plant Diversity, Fairylake Botanical Garden, Shenzhen & Chinese Academy of Sciences, Shenzhen 518 004, P. R. China.
E-mail: xbaskaran@yahoo.com
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
- Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
- The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
- Evaluation of the academic achievement of rural versus urban undergraduate medical students in pharmacology examinations
