Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
Reda I. El-Sheshtawy, Diya A. El-Badry, Gamal A. El-Sisy, Walid S. El-Nattat*, Amal M. Abo Almaaty
1Animal Reproduction and Al Department, Veterinary Division, National Research Center, Dokki, Giza, Egypt
2Department Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research Center, Giza, Egypt
Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
Reda I. El-Sheshtawy1, Diya A. El-Badry2, Gamal A. El-Sisy1, Walid S. El-Nattat1*, Amal M. Abo Almaaty1
1Animal Reproduction and Al Department, Veterinary Division, National Research Center, Dokki, Giza, Egypt
2Department Artificial Insemination and Embryo Transfer, Animal Reproduction Research Institute, Agriculture Research Center, Giza, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Arabian stallion Semen Preservation Honey
Objective: To investigate the effect of extender supplementation with different concentrations of a honey bee on post-thawed sperm motility, viability index, membrane and acrosome integrities in Arab stallion. Methods: Five ejaculates from each of four Arabian stallions were subjected to cryopreservation with a modified INRA-82, without any supplementation (control) or supplemented with 1%, 2%, 3%, 4% and 5% honey bee. After thawing, all samples were maintained at 37 °C, while analyses were performed at 0, 1, and 2 and 3 h. Sperm motility %, viability index %, membrane integrity % and acrosome integrity % of each sample was determined by conventional laboratory methods. Results: Relative to the control group, supplementation with honey (2%, 3% and 4% significantly improved (P < 0.01 at least) post-thaw sperm motility, viability index (P < 0.001 at least) and had a positive effect on membrane integrity and intact acrosome % (P < 0.001 at least) at 0, 1, 2 and 3 h post-thawing. For all semen parameters, the lower concentration of honey (1%) and higher concentration (5%) did not show significant differences (P > 0.05) compared with the control. Conclusion: Honey bee supplementation in Arab stallion semen extenders provided a better protection of sperm parameters against cryopreservation injury, in comparison to the control groups.
1. Introduction
There are worldwide renewed research interest, in improving reproductive performance in livestock artificial insemination (AI) programs and maximizing the use of AI sires with valuable genomes. Such progress can be made through improvement of semen collection, analysis, processing and cryopreservation [1]. Semen cryopreservation induces certain detrimental structural effects on spermatozoa as a result of its exposure to thermal, mechanical, chemical osmotic and oxidative stress [2] during freeze-thawing. These changes resulted in reduced sperm motility, plasma membrane functionality and acrosome integrity [3], leading to diminished spermatozoa fertilizing ability [4]. There is an international interest concerning the application of natural medical sources in animal production fields. The beneficial effects of honey bee (HB) in reproductive health protection have been strongly evidenced by many authors [5-7]. This effects are mainly attributed to its nutrient-rich content, e.g., sugars such as fructose and glucose; minerals such as magnesium, potassium, calcium, sodium chloride, sulphur, iron and phosphates, caffeic acid, caffeic acid phenethyl ester (CAPE) and flavonoid glycones; as well as vitamins B1, B2, C, B6, B5 and B3 [5,6]. HB has a potent antioxidant and antibacterial properties [7,8]. HB flavonoids possess free radicals (FR) scavenging activity, thereby inhibiting FR-induced DNA damage [9]. Olayemi et al. concluded that the addition of honey to egg yolk extender improve the motility and live dead ratio and viability of liquid storage goat semen [10]. Fakhrildin and Alsaadi concluded that supplementation of honey bee (10%) to cryoprotectant solution show enhancement sperm parameter post-thawing in man [11]. Ogretmen and Inananobserved that using the honey in cryomedia
is effective for cryopreservation especially about hatching success of egg fertilized by the frozen-thawed sperm of common carp [12]. El-Sheshtawy et al. mentioned that addition of 10% HB solution to cattle bull semen extender improved sperm motility in chilled and frozen semen and also improved the conception rate [13]. Jerez-Ebensperger et al. reported a significant high ram sperm quality values when extender contained pasteurized egg yolk and honey at 0 and 2 h after thawing [14]. Jerez-Ebensperg et al. concluded that the addition of honey to ram extender reduces sperm deterioration when stored at 4 °C [15]. Up to date, there is a lack of data concerning the effect of the addition of honey to semen extender on post-thawing semen quality of Arab stallion. The present study aimed to determine the effects of addition of honey to semen extender on post-thawing sperm quality in Arab stallion.
2. Materials and methods
2.1. Preparation of extender
Modified INRA-82 [mINRA-82] described by El-Badry et al. [16] was used as base and control extender. This extender consists of 25 g/L glucose monohydrate, 1.5 g/L lactose monohydrate, 1.5 g/ L raffinose pentahydrate, 0.4 g/L potassium citrate monohydrate, 0.3 g/L sodium citrate dihydrate, 4.76 g HEPES, pH 7.0, 500 mg/L gentamycin, 0.035% SDS and 0.15% skim milk. Aliquots of mINRA-82 extenders were supplemented with different concentrations of bee honey.
2.2. Honey Bee mINRA-82 (Apis mellifera lamarckii) extender (HEMI)
Honey solution was prepared by adding 1 mL honey to 9 mL bidistilled water to obtain a honey solution of 10% concentration. This solution is added to mINRA-82 aliquots in concentrations of 0.5/4.5 [1% honey enriched mINRA-82 (HEMI 1%)], 1/4 (HEMI 2%), 1.5/3.5 (HEMI 3%), 2/3 (HEMI 4%), 2.5/2.5 (HEMI 5%) mL (v/v) of honey / mINRA-82 to obtain a final volume of 5 mL in each tube [13].
2.3. Animals and semen collection
On a once weekly collection schedule, five ejaculates per stallion were obtained from four Arabian stallions, aged 8–14 years, and individually housed at Police Academy stud, Cairo, Egypt. At the time of collection, early in the morning, a mare in estrus was used as a mounted animal. Semen was collected using a lubricated and pre-warmed (45 to 50 °C) Colorado model artificial vagina with an inline filter to separate the gel fraction.
2.4. Processing of semen
Immediately following collection, the gel-free portion of the ejaculate was evaluated for volume and progressive motility, and concentration was determined with a hemocytometer. Only ejaculates with at least 60% progressively motile sperm and 2.5×108sperm cell/mL were used for freezing. The semen was extended 1:1 (semen: extender) in INRA-82 extender that had been warmed to 38 °C. The diluted samples were placed into 15 mL tubes and centrifuged for 10 min at 400 ×g [17]. At least 95% of the supernatant was removed [18] and each pellet was re-suspended with modified INRA-82 (containing 5% glycerol and 15% egg yolk) with honey (1%, 2%, 3%, 4%, 5%) and without honey (0%, control) to a final sperm concentration of 1.0×108motile sperm/mL. Each aliquot was cooled slowly to 5 °C over 1 h under aerobic conditions and then incubated at 5 °C for 30 min [19]. The extended semen was drawn into 0.5 mL straws (Minitube, Germany) and sealed thermally and placed 4 cm above liquid nitrogen in the vapor phase in foam box for 10 min before being plunged into the liquid phase [20]. The straws were then stored in goblets on canes and kept immersed in liquid nitrogen. For thawing, two straws per treatment were warmed in a water bath at 38 °C for 30 s.
2.5. Evaluation of frozen-thawed semen
Spermatozoa motility was examined and recorded using a prewarmed stage of phase contrast microscope (× 200) just after 0, 1, 2 and 3 h post-thawing for frozen-thawed semen. The post-thawing viability indices were estimated according to Milovanov [21]. The percentage of HOS-positive cells and acrosomal integrity in each sample was determined according to Nie and Wenzel [22] and Wells and Awa [23], respectively.
2.6. Statistical analysis
One way analysis of variance and Duncan’s multiple range tests (using SPSS program version 16.0) were done for the obtained data of frozen-thawed semen qualities after transformation of percentages to their corresponding arcsin values [24]. P < 0.05 was considered as statistically significant.
3. Results
The effects of different concentrations of honey in extenders on post-thawing Arab stallion sperm motility at different time are reported in Table 1. There were significant differences among different concentrations in total motility at 0, 1, 2 and 3 h postthawing. A significant increase in the percentage of total motile sperm was observed in the presence of 2%, 3% and 4% bee honey as compared with control (0%). Enrichment of the basic extender (mINRA-82) for horse with the bee honey (HEMI 3%) had significantly (P < 0.01 at least) enhanced post-thawing (0, 1, 2 and 3 h) sperm motility % in comparison with the control and HEMI (1% and 5%) as shown in Table 1. While, the enrichment with HEMI 2%
(at 0, 1 and 2 h post-thawing) and HEMI 4% (at 0, 1, 2 and 3 h after thawing) have non-significantly differed from the HEMI 3% results at 0, 1, 2 and 3 h post-thawing as shown in Table 1.
In the same consent, there were also significant differences among extenders in viability index, membrane integrity (HOS-positive cells) and acrosome integrity at different times post-thawing as shown in Table 2. The enrichment of the mINRA-82 with the bee honey (HEMI 3%) showed the highest significant (P < 0.001 at least) mean records concerning the viability index, the HOS-positive cells and the acrosome integrity in comparison with the means recorded for the control and HEMI (1%, 2% and 5%) as shown in Table 2. A nonsignificant (P < 0.001 at least) difference was found between the mean records of the HEMI 3% and 4% as revealed in Table 2.
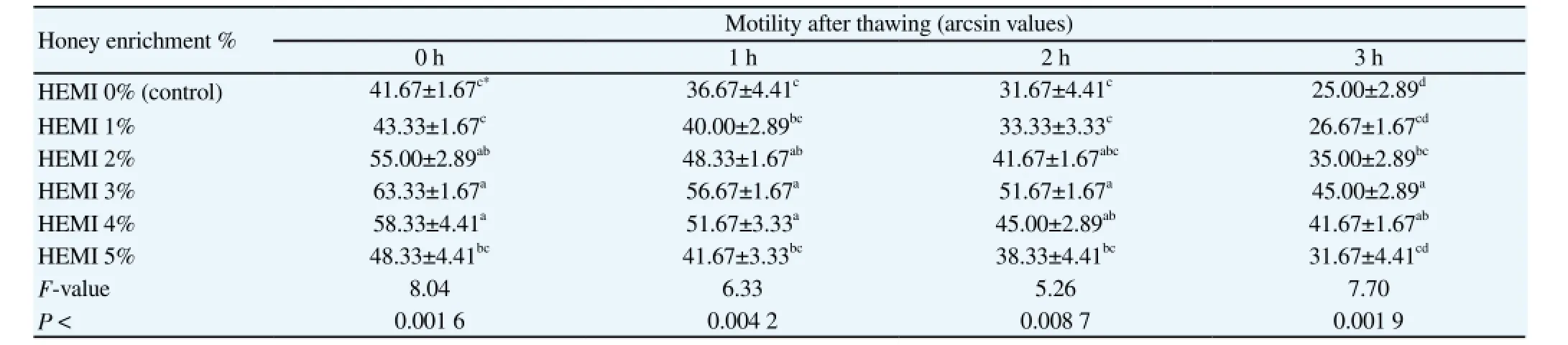
Table 1 Effect of honey addition to modified INRA-82 extender on sperm motility percentage of Arabian horse.
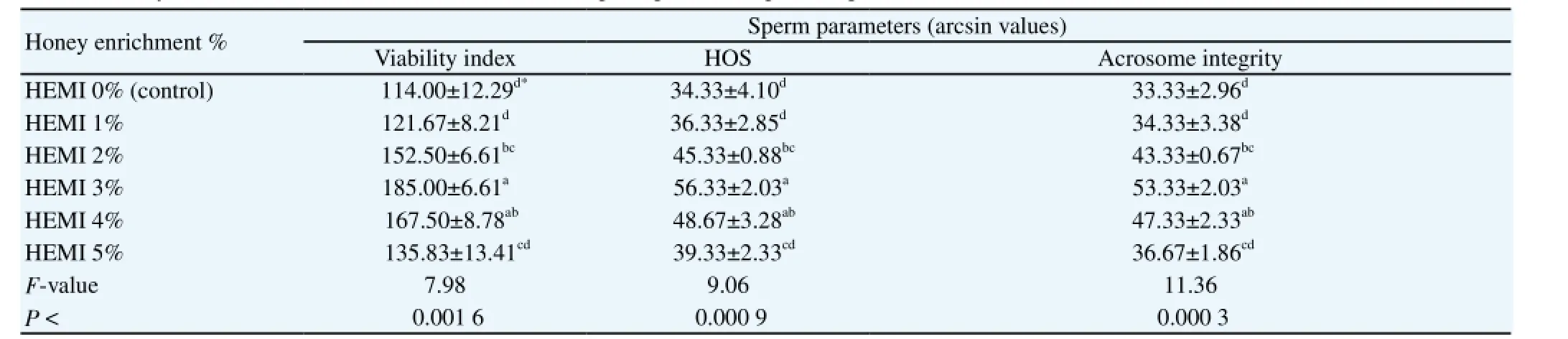
Table 2 Effect of honey addition to modified INRA-82 extender on sperm parameters percentage of Arabian horse.
4. Discussion
In the present study, we utilized honey bee in Arab stallion semen extender as an energy substrate, none permeate cryoprotectants and for its beneficial antioxidant effect on sperm membrane stability. Honey has been used with cryoprotectant extender for Arab stallion at different concentrations 1%, 2%, 3%, 4% and 5%. The results of the current study concluded that the incorporation of different concentrations of the honey bee to freezing extender increased sperm motility, viability index, and membrane and acrosome integrities after Arab stallion sperm cryopreservation. There were significant high sperm motility values when extender contained honey (2%, 3% and 4%) at 0, 1, 2 and 3 h after thawing than control (0%). The viability index, percentage of sperm with intact membrane and intact acrosome were significantly higher in most honey concentrations (2%, 3% and 4%) than control. The enrichment of the mINRA-82 with the honey bee (HEMI 3%) showed the highest significant mean records concerning the sperm motility, viability index, the HOS-positive cells and the acrosome integrity in comparison with the means recorded for the control and HEMI (1%, 2% and 5%). These results are in agreement with Olayemi et al. [10]; Jerez et al. [25] and El-Sheshtawy et al. [13] who reported that the presence of honey in cooling and freezing extenders increased spermatozoa motility and improved sperm quality in goat, ram and cattle bull semen, respectively. Also, Fakhrildin and Alsaadi indicated that the supplementation of honey (10%) to human semen cryoprotectant solution results in enhancement of sperm quality post-thawing [11]. Recently, Jerez-Ebensperger et al. concluded that there were significant high ram sperm quality (total motile spermatozoa, acrosome integrity, and HOST) values when extender contained honey at 0 and 2 h after thawing and fructose can be replaced by honey in ram semen extender, without detrimental effects on sperm quality after cryopreservation [14]. From a physical point of view, honey will not freeze solid at very low temperatures. The viscosity of honey increases and will become thick and condense with decreasing temperature. Honey has a glass transition between
-42 and -51 °C. Below this temperature, honey enters a glassy state and will become an amorphous solid (non-crystalline) and prevent ice crystal formation [26]. Besides, Honey has unique chemical properties where it contains a mixture of 25 sugars accounting for approximately 95% to 97% of its dry matter (mainly fructose and glucose). Along with saccharides, a huge number of other bioactive substances such as organic acids, enzymes, antioxidants, and vitamins are present in honey in traces [27]. Such composition provides various nutritional, biological and pharmacological effects in living cells, i.e., antimicrobial (antiviral, antifungal and antibacterial), antioxidant and antitoxins, anti-inflammatory, antimutagenic activities [27,28]. Fructose and glucose act as extracellular non-penetrating cryoprotectants due to their high molecular weight and maintain the osmotic balance [29]. In addition, sugars provide the main energy source that spermatozoa require developing their metabolic processes [30]. Nowadays, there is some evidence of antioxidant effects of honey [5-8]. HB flavonoids possess FR scavenging activity, thereby inhibiting FR-induced DNA damage [9]. Studies in different species: cattle [13], ovine [14,15], goat [10], common carp [12] and human [11] determined the antioxidant properties of honey on sperm cryopreservation. In conclusion, this study indicates that honey bee plays an important role in protecting the post-thaw spermatozoa motility, viability, membrane integrity in the cryopreserved Arab stallion spermatozoa through its unique nutritive value, energy, antibacterial and antioxidant effects.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgment
The authors are greatly indebted to the National Research Center for sponsoring this work through the project entitled: “Conservation of the genetic resources of local male breeds using natural additives for semen extenders” code no. 10120801, and duly acknowledge the staff and workers at Police Academy stud, Cairo, Egypt providing the logistics support for conducting this research work.
[1] Hossain MS, Johannisson A, Wallgren M, Nagy S, Siqueira AP, Rodriguez-Martinez H. Flow cytometry for the assessment of animal sperm integrity and functionality: state of the art. Asian J Androl 2011; 13: 406-419.
[2] Holt WV, Head MF and North RD. Freeze-induced membrane damage in ram spermatozoa is manifested after thawing: observations with experimental cryomicroscopy. Biol Reprod 1992; 46(6): 1086-1094.
[3] Woelders H, Matthijs A, Engel B. Effects of trehalose and sucrose, osmolality of the freezing medium and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiology 1997; 35: 93-105.
[4] Tekin N, Uysal O, Akcay E, Yavas I. Effects of different taurine doses and freezing rate on freezing of ram semen. Ankara Univ Vet Fak Derg 2006; 53: 179-184.
[5] Orsolic N, Basic I. Cancer chemoprevention by propolis and its polyphenolic compounds in experimental animals. In: Singh VK, Govil JN, Arunachalam C, editors. Recent progress in medicinal plants. USA: Studium Press LLC; 2007, p. 55-113.
[6] Estevinho L, Pereira A, Moreira L, Dias L, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 2008; 46: 3774-3779.
[7] Zoheir KMA, Harisa GI, Abo-Salem OM, Sheikh Fayaz Ahmad SF. Honey bee is a potential antioxidant against cyclo-phosphamide induced genotoxicity in albino male mice. Pak J Pharm Sci 2015; 28(3): 973-981.
[8] Aljady AM, Kamaruddin MY, Jamal AM, Mohd Yassin MY. Biochemical study on the efficacy of Malaysian honey in infected wounds: An animal model. Med J Islam Acad Sci 2000; 13(3): 125-132.
[9] Chen CH, Weng M, Wu CH, Lin J. Comparison of radical scavenging activity, cytotoxiceffects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. Evidence-based Complement Altern Med 2004; 1(2): 175-185.
[10] Olayemi FO, Adenigi DA, Oyeyemi MO. Evaluation of sperm motility and viability in honey included egg yolk based extenders. Glob Vet 2011; 7(1): 19-21.
[11] Fakhrildin MB, Alsaadi RA. Honey supplementation to semen-freezing medium improves human sperm parameters post-thawing. J Family Reprod Health 2014; 8(1): 27-31.
[12] Ogretmen F, Inanan BE. Evaluation of cryoprotective effect of Turkish pine honey on common carp (Cyprinus carpio) spermatozoa. Cryo Letters 2014; 35(5): 427-437.
[13] El-Sheshtawy RI, El-Nattat WS, Sabra HA, Ali AH. Effect of honey solution on semen preservability of local breeds of cattle bulls. Wld Appl Sci J 2014; 32(10): 2076-2078.
[14] Jerez-Ebenspergera RA, Lu?oa V, Olacireguia M, Gonzáleza N, Blasb I de, Gila L. Effect of pasteurized egg yolk and rosemary honey supplementation on quality of cryopreserved ram semen. Small Rum Res 2015; 130: 153-156.
[15] Jerez-Ebensperger R, Gil L, Gonzales N, De Blas I. The combined effect of use of honey, garlic (Allium Sativum L.) and skimmed milk as an extender for chilling sheep semen. Cryo Letters 2015; 36(4): 243-251.
[16] EL-Badry DA, Anwer AM, Rawash ZM. Effect of different concentrations of sodium dodecyl sulfate, egg yolk and glycerol on the freezability and DNA integrity of Arabian stallion spermatozoa. Assiut Vet Med J 2014; 60(141): 29-35.
[17] Cochran JD, Amann RP, Froman DP, Pickett BW. Effects of centrifugation, glycerol level, cooling to 5°C, freezing rate and thawing rate on the post-thaw motility of equine sperm. Theriogenology 1984; 22:
25-38.
[18] Loomis PR. Advanced methods for handling and preparation of stallion semen. Vet Clin North Am Equine Pract 2006; 22: 663-676.
[19] Crockett EC, Graham JK, Bruemmer JE, Squires EL Effect of cooling of equine spermatozoa before freezing on post-thaw motility: Preliminary results. Theriogenology 2001; 55: 793-803.
[20] Cristanelli MJ, Amann RP, Squires EL, Pickett BW. Effects of egg yolk and glycerol level in lactose-EDTA-egg yolk extender and of freezing rate on the motility of frozen-thawed stallion spermatozoa. Theriogenology 1985; 23: 25-38.
[21] Milovanov VK. Biology of Reproduction and Artificial Insemination of Farm Animals. Monograph. Selkohz. Lit. J. and Plakatov, Moscow; 1962.
[22] Nie GJ, Wenzel JGW. Adaptation of the hypo-osmotic swelling test to assess functional integrity of stallion spermatozoal plasma membranes. Theriogenology 2001; 55: 1005-1018.
[23] Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci 1970; 53: 327-332.
[24] Snedecor GW, Cochran WG. Statistical methods. 8th edition. Ames, IA, USA: Iowa State Univ. Press; 1989.
[25] Jerez R, Gil L, González N, Lu?o V, Olaciregui M, Martínez F, et al. Rosemary honey as natural energetic source on refrigeration ram semen. Reprod Dom Anim 2013; 48: 1.
[26] Kantor Z, Pitsi G, Thoen J. Glass transition temperature of honey as a function of water content as determined by differential scanning calorimetry. Agric Food Chem 1999; 47: 2327-2330.
[27] Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: A review. J Am College Nutr 2008; 27: 677-689.
[28] Manyi-Loh CE, Clarke AM, Ndip RN. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr J Microbiol Res 2011; 5: 844-852.
[29] Meryman HT. Cryoprotective agents. Cryobiology 1971; 8: 173-183.
[30] Gil L, Mascaró F, Mur P, Gale I, Silvia A, González N, et al. Freezing ram semen: the effect of combination of soya and rosemary essences as a freezing extender on post-thaw sperm motility. Reprod Domest Anim 2010; 45: 91.
ment heading
10.1016/j.apjr.2016.06.004
*Corresponding author: Walid S. El-Nattat, Animal Reproduction and AI dept., Veterinary Research Division, 13 National Research Centre, Dokki, Giza, Egypt.
Work: 202 33371635
Fax: 202-37601877
E-mail: elnattat@gmail.com
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
- Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
- The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
- Evaluation of the academic achievement of rural versus urban undergraduate medical students in pharmacology examinations
