Regulation of Talcum Powder in Cosmetics at Home and Abroad
Li Neng, Wang Erman
Shenzhen Intertek Quality Technology Service Co. Ltd., China
Johnson & Johnson was sued in many places in the United States because of its toilet powder containing talcum powder. Johnson & Johnson experienced seven similar lawsuits (3 wins and 4 defeats) up to now. Johnson &Johnson is deeply involved in the carcinogenesis scandalgate, involving a huge amount of indemnity.[1]In February,May, and November 2016, and May 2017, the amount of compensation was 72 million, 55 million, 70 million and 100 million USD respectively. Some talcum powders may contain asbestos, and asbestos is a known carcinogen.The two are easily confused. As a result, the safety of talcum powder is put in the spotlight and caused panic among consumers. The author collected and analyzed the toxicological information of talcum powder, and its regulations, to help regulators and relevant enterprises understand its hazards and promote consumers’awareness of talcum powder.
Toxicological information of Talcum Powder
Talc or Talcum Powder, CAS No. 14807-96-6, with the chemical formula Mg3(Si4O10)(OH)2or 3MgO ? 4SiO2?H2O, is a powder with a certain fineness by mechanical processing of talc ore. “GB/ T 15342-2012 talcum powder issued on Dec. 31, 2012, ”[2]indicates that talcum powder is divided into grind talcum powder, fine talcum powder and superfine talcum powder according to the size of its particle size; and is divided into 9 varieties according to its application, namely talcum powder for cosmetics,coating-paint, paper-making, plastics, rubber, cable,ceramics, waterproof material, and general-purpose talcum powder. Talcum powder is used widely in all types of cosmetics, particularly in powder cosmetics. It is used as an absorbent, filler, anticaking agent, opacifying agent or an opacifier in cosmetics. The hazardous substance it may contain is asbestos. Refer to Table 1 for the toxicological information of talcum powder.
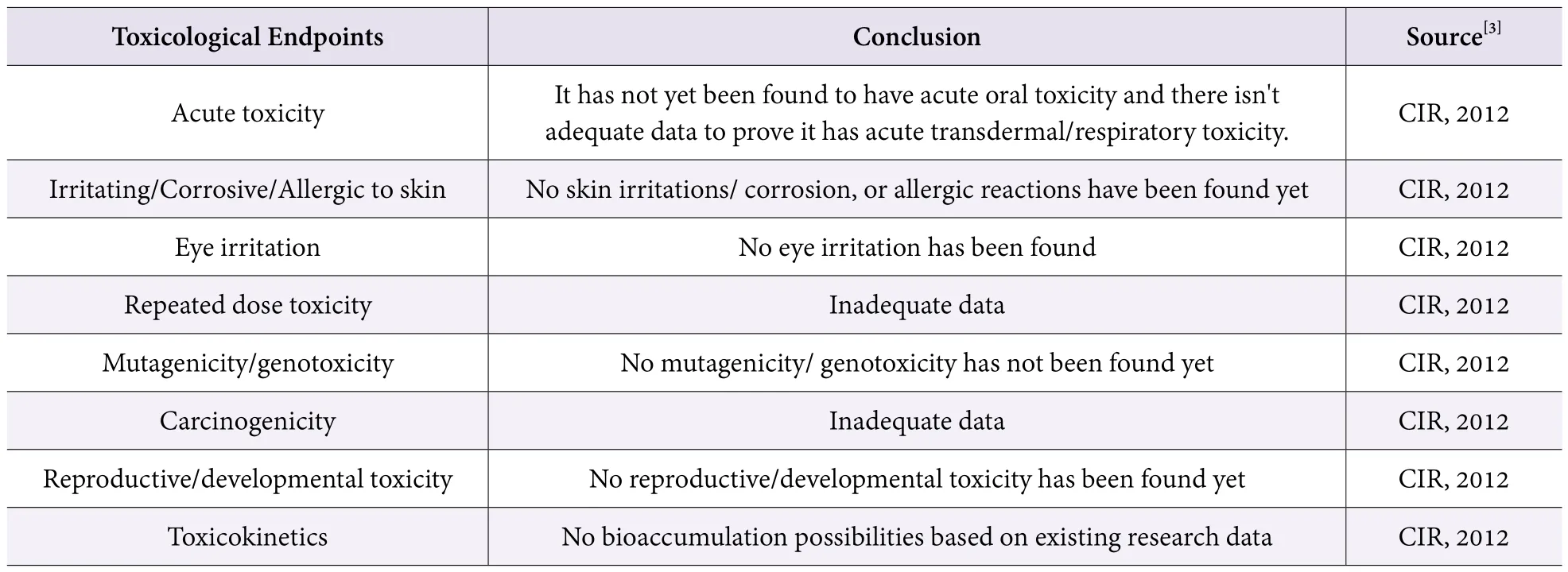
Table 1. Toxicological information on talcum powder
On the basis of what we have said, there is still no adequate data on the carcinogenicity of talcum powder,but it may contain asbestos which is a hazardous substance classified as 1A in carcinogenicity[4]by the EU Regulation 1272/2008 (CLP Regulation). IARC Monographs[5]published on May 19, 2015, by the International Agency for Research on Cancer, IARC,classified talcum powder into 3 categories: talcum powder containing asbestos fibers, classified as Class 1 in carcinogenicity; talcum powder not containing asbestos or asbestos fibers, classified as Class 3 in carcinogenicity(i.e., the carcinogenicity to humans cannot be classified yet); toilet powder containing talcum powder used for women’s private parts is classified as Class 2B in carcinogenicity (Maybe carcinogenic for humans).
Regulatory provisions
China
Safety and Technical Standards for Cosmetics (2015 Edition)[6], which entered into force on December 1,2016, includes talcum powder in the list of ingredients for limited use in cosmetics (No. 35), and specifies that the label should indicate “Keep away from the nose and mouth of children” when used in powder products for children under 3 years old; there is no need to label precautions when used in other products. In addition,Safety and Technical Standards for Cosmetics (2015 Edition), requires limits of harmful substances in the general requirements for cosmetics safety, and points out that asbestos should not be detected in cosmetics.A circular on the Provision of Method to Determine Asbestos in Powdered Cosmetics and Their Raw Materials (Temporary),[7]provides for the determination of asbestos in powdery cosmetics and their raw materials, and requests that a product applying for an hygiene administrative license for a special-purpose cosmetic, or PDF record for non-special-purpose cosmetics, if its formula contains talcum powder as a raw material, the applying unit shall submit a test report for asbestos impurity in the product when making application or filing for record. Requirements for Talcum Powder Used in Cosmetics[8]specifies the safety requirements and inspection methods of talcum powder,and requires that asbestos should not be detected in talcum powder.
GB/T 15342-2012 Talcum Powder specifies the requirements for the performance of talcum powder for powder cosmetics, including non-detection of asbestos minerals, and also stipulates that talcum powder for cosmetics is not graded as light, and other industrial talcum powder is divided into three grades—Grade I,Grade II and Grade III according to their physical and chemical properties. In addition, the main standards related to talcum powder are: Chemical Analyzing Method of Talcum GB/ T 15343, Physical Examination Method of Talcum GB/ T 15344, JC/T 534 packaging bag for talcum powder.
In February 2016, China Association of Fragrance,Flavor and Cosmetic Industries issued On the Use of Talcum Powder in Cosmetics,[9]which stipulated the cosmetic enterprise should strictly abide by relevant regulations when selecting talcum powder as a raw material,and in normal conditions, the products are considered safe and shall not pose a hazard to human health, given it is used in a normal, reasonable and foreseeable way.
The EU
Annex III of Regulation (EC) No.1223/2009 on Cosmetics[10]lists talcum powder as a substance of limited use in cosmetics, and the requirements for limited use are the same as those in China.
The United States
Cosmetic regulations in the US do not prescribe limited use of talcum powder. The FDA is of the opinion that talcum and asbestos are natural minerals that have been found, but unlike talcum, asbestos is a known carcinogen, and therefore talc contaminated with asbestos,in cosmetics, is considered unacceptable.[11]In order to prevent talcum from being contaminated by asbestos, it is necessary to carefully select talcum and take measures to adequately purify the ore.
In 2015, the United States Cosmetic Ingredient Assessment Panel (CIR) issued a Safety Assessment Report for Cosmetic Talcum Powder,[3]and it was considered safe to use talc at the current level of use (0. 000 5% to 100%)for cosmetic purposes, but no talcum can be used when the skin barrier disappears, or is obviously damaged. Table 2 shows the use of powder products in the report (Table 2).
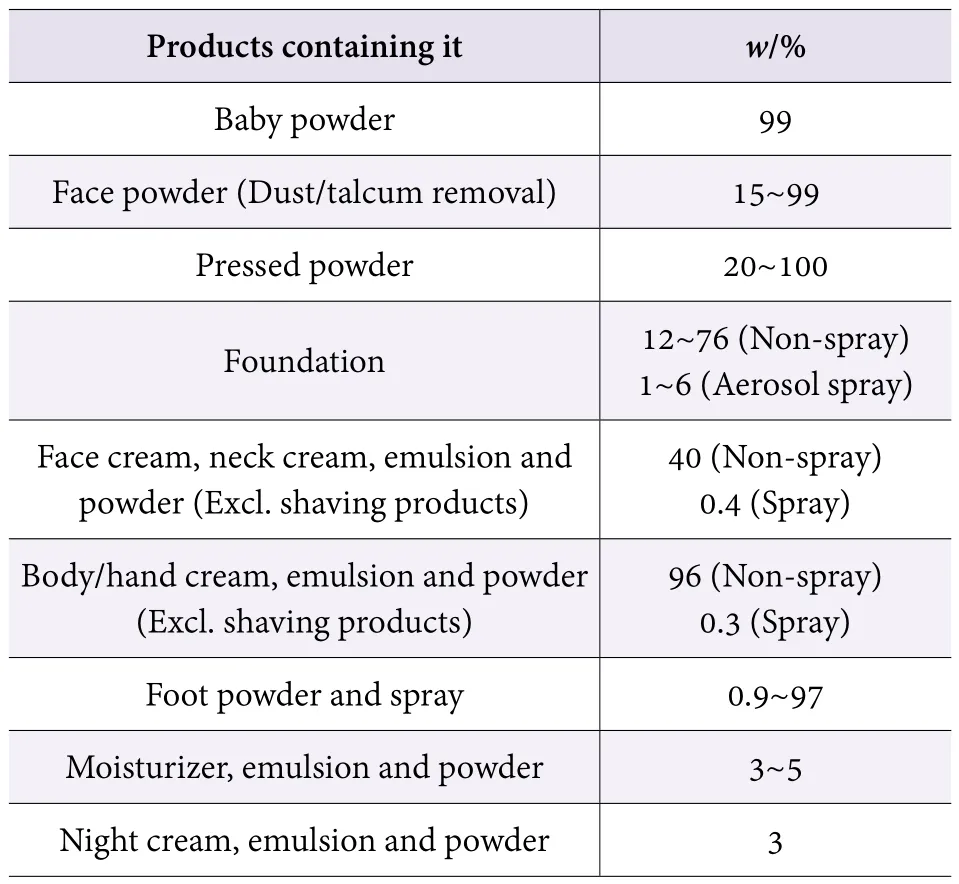
Table 2. Concentration of talcum powder in cosmetics
Canada
The list of prohibited and restricted cosmetics substances (Hotlist)[12]issued by Canada, includes talcum powder as a substance for limited use. In powder products used by infants and children, the warnings and precautions are: Avoid exposure to children and keep away from the nose and mouth of the child, to prevent breathing problems.
From the above-mentioned rules for the use of talcum powder laid down by different countries and regions, we can conclude that: if talcum powder is used in powder products used for children under 3 years old, the label should have corresponding warnings, but none of them sets the ceiling amount of talcum powder; another common stipulation is that talcum powder should not contain asbestos. The hazardous substance asbestos in talcum powder is listed as a banned substance, that is to say, it shall not be used as a raw material for making cosmetics.
Recommendations
In the opinion of the author, attention should be paid to the applicable population, requirements on the labeling, product containers, and skin conditions for products containing talcum powder, and it is not recommended for use on damaged skin, or skin short of a protective barrier. When talcum powder is used in a spray or an aerosol container, it is recommended not to use a very high concentration, otherwise it is likely to block the nozzle. It is certain that talcum powder complying with regulations does not pose a hazard to the health of a human body under normal, reasonable and foreseeable conditions of use, and it is not necessary for consumers to panic. However, they should pay attention to the exposure of skin, and take care not to use it on inappropriate areas.
From the toxicological information and regulations of talcum powder and its classification by IARC, talcum based talcum powder is used for female private parts and may cause cancer, but according to the existing research data,there is no association between talcum powder and ovarian cancer. According to the toxicological information, the carcinogenic potential of talcum powder is not sufficient.If it is used on other parts, the carcinogenic potential of talcum powder is trivial. The carcinogenicity of talcum powder shall be determined according to the impurities it contains and the parts of its use.
For the enterprises, when selecting talcum powder raw materials, they should observe corresponding regulatory requirements and requirements for raw material. The powder products containing talcum powder shall be subject to asbestos testing to ensure safety of the products.

[1] Chemical Watch. Johnson & Johnson to pay $110m in latest talcum powder lawsuit [EB/OL].[2017-05-05].https://chemicalwatch. com/55672/johnson-johnson-to-pay-110min-latest-talcum-powderlawsuit.
[2] General Administration of Quality Supervision, Inspection and Quarantine. GB/ T 15342-2012 Talcum Powder. Beijing:General Administration of Quality Supervision, Inspection and Quarantine, 2012.
[3] CIR. Safety Assessment of Talc as Used in Cosmetics [EB/ OL].[2012-12-18]. http://www. cir-safety.org/sites/default/files/talc122012tent_faa_final%20for%20posting.pdf.
[4] European Union. Commission Regulation (EC) No 1272/2008 on classification, labeling and packaging of substances and mixtures. Official Journal of the European Union 2008,L353:1-35.
[5] International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans[EB/ OL]. [2017-05-19]. http:// monographs.iarc.fr/ENG/Classification/ latest_ classif. php.
[6] State Food and Drug Administration. Safety and Technical Standards for Cosmetics (Version 2015). Beijing: State Food and Drug Administration, 2015.
[7] State Food and Drug Administration. Circular on the Provision of Method to Determine Asbestos in Powdered Cosmetics and Their Raw Materials (Temporary)[EB/OL][2009-04-16] [EB/OL].[2009-04-16]. http://www.sda.gov.cn/WS01/CL0846/37316.html.
[8] State Food and Drug Administration. Requirements for Talcum Powder Used in Cosmetics as a Raw Material[EB/OL].[2011-12-23]. http://www. sda. gov.cn/WS01/CL0846/68166.html.
[9] China Association of Fragrance Flavor and Cosmetic Industries“On the Use of Talcum Powder in Cosmetics[EB/OL]”.Read[2017-02-27]. http://www.caffci.org/xh_read.php?code=11&id=1335.
[10] European Union. Regulation(EC) No 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products. Official Journal of the European Union 2009, L342: 59-209.
[11] U.S. Food and Drug Administration. Talc[EB/OL]. [2014-03-19]. https://www.fda.gov/cosmetics/productsingredients/ingredients/ucm293184. htm.
[12] Health Canada. Cosmetic Ingredient Hotlist[EB/OL]. [2015-12-14]. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredienthotlist-prohibited-restricted-ingredients/hotlist.html.
 China Detergent & Cosmetics2018年1期
China Detergent & Cosmetics2018年1期
- China Detergent & Cosmetics的其它文章
- Reconstruction of 3D Skin Model and Its Application in Evaluation the Efficacy of Cosmetic Raw Materials and Active Intredients
- An In Vitro Biological Model for Evaluation of the Anti-Dandruff Performance of Hair Care Products
- Models for Assessing Pollution Damage of Asian Hair
- Technical Approaches and in-vitro Evaluation Methods for Scalp Care
- Technical Standard System Construction Plan for Surfactants and Detergents in the“13th Five-year Plan” Period
- A Summary on Chinese Daily Chemical Journals
