Technical Approaches and in-vitro Evaluation Methods for Scalp Care
Akira Nomachi, Yang Jianzhong
Asian Scalp Health Research Center, Kobe, Japan
Introduction
Scalp care products are drawing more and more attention in the global market. Among the entire globally newly launched hair care product in 2016, 22.3% of the products claimed scalp care benefits, significantly increased from 8.3% in 2006. Traditionally, scalp care products are designed mainly for “healing” scalp symptoms related to inflammation such as dandruff and itching. Recently, the trend is moving toward to preventing scalp from becoming unhealthy.
Inflammation is a primary indication of unhealthy scalp. Therefore, preventing scalp from inflammation should be the fundamental technical approach of scalp care. In this paper, we first analyze the potential causes of scalp inflammation, followed by possible technical approaches of prevention. In-vitro methods for measuring effectiveness of each approach will then be described based on our experimental learning.
Influencing factors of scalp health and technical approaches for scalp care
It is considered that suppression of inflammation of the scalp is essential for healthy scalp and perhaps“healthy hair” as well. Skin irritation is acute response mainly caused by foreign substances differing from skin sensitization based on immune response. Figure 1 illustrates factors that may cause scalp irritation. While delivering benefits of beauty and hygiene, some hair care products contain ingredients having skin irritating effect to a certain degree. Shampoo consisting of sulfatebased surfactants such as alkyl sulfate salts or alkyl ethoxy sulfate salts is considered irritating by excessively removing lipids or natural moisturizing factor from epidermis.[1,5]To maintain scalp heath, a first step of scalp care should be on limiting and minimizing the usage of irritative ingredients in hair care products to avoid scalp inflammation. As a technical approach, free of irritative ingredients as represented by “sulfate-free” is becoming more and more popular.
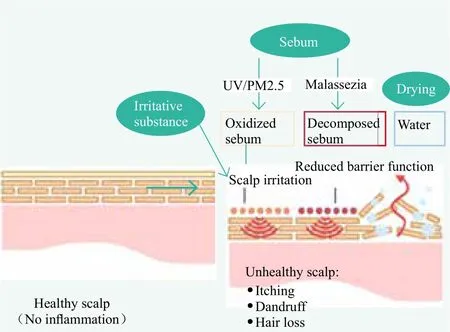
Figure 1. Factors affecting scalp health
The naturally occurring sebum is supposed to form a thin film on skin surface for protection purpose.Therefore, freshly secreted sebum is harmless to scalp.However, the sebum remaining on the scalp can be oxidized over time induced by ultraviolet rays and air pollution such as PM2.5, resulting in irritative lipid peroxide. It is reported that the oxidized squalene is remarkedly increased at sites of inflammatory scalp region.[6]
It is also known that unsaturated fatty acids produced by degradation of triglycerides in sebum caused by indigenous bacteria such as Malassezia are irritative.[7]Therefore,it is important to remove old sebum in a timely and efficient manner by washing the scalp with a mild shampoo before sebum is heavily converted to the irritative substances. Malassezia inhibition is also important and has been the key approach for antidandruff shampoos over the past decades. On the other hand, protecting scalp from exposure to UV and PM2.5 is considered as a relatively new technical direction for scalp care. Recently, researchers at Sunstar have found an active agent that selectively removes unsaturated fatty acids involved in inflammation of the scalp.[8]In the future,new technologies should emerge to selectively remove lipid peroxides.
The stratum corneum has an important role in the barrier function, and it controls prevention of moisture evaporation and penetration of foreign substances from outside.[9]Because the scalp has a lower barrier function than other parts of skin and is liable to cause skin troubles,it is particularly important to mitigate the reduction of barrier function.[10]Drying is another factor that negatively affects barrier function of the scalp. Therefore,moisturization of scalp to enhance defense power of the scalp is critical.
Physiological condition of scalp has significant impacts on hair condition. For example, promoting blood flow of scalp helps supply more oxygen and nutrient sources to hair follicles. There were few papers demonstrating the correlation between blood pressure and hair growth. More recently, Kao's researchers reported that scalp massage using pressing method transiently raised the blood flow rate of scalp, and consequently improved hair density and strength.[11]An ultimate goal of scalp care should be on hair growth promotion and hair loss prevention.
To promote hair growth as a part of scalp care,it is necessary to apply useful active substances to the scalp after removing negative factors from the scalp. Furthermore, influencing the hair cycle such as prolonging the growth period, suppressing transition to the regression phase, and transitioning from the resting period to the growing period is considered effective for hair growth.[12]However, the substance for controlling the hair cycle needs to be further identified with clearer mechanism understandings. In addition, it is considered important to control hair follicle stem cells existing in the bulge region to promote hair growth,[13]and the development of a method of controlling hair follicle stem cells is also critical. To prevent male pattern hair loss,suppression of DHT production or DHT induced TGFβ expression is considered effective.[14]Since finasteride used as male pattern hair loss preventive drug fails to show effect on hair loss of a female, it seems that the molecular mechanism of hair loss is different between male and female, and elucidation of its molecular mechanism is expected. Most recently, it was revealed that the expression of type 17 collagen present in hair follicle stem cells is decreased in hair follicle which was reported by Professor Nishimura at Tokyo Medical and Dental University.[15]It is expected that normalization of scalp environment and research on hair growth based on the scalp physiology will be developed in the future.
In summary, the potential technical approaches for scalp care include reducing irritation, improving scalp defense, and activating hair follicle cells as illustrated by Figure 2.
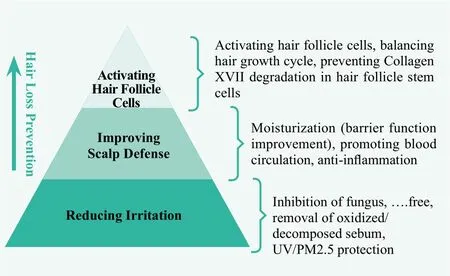
Figure 2. Technical approaches for scalp care
Methods for measuring scalp care effects
Skin irritation test
Evaluation method of skin irritation is adopted following OECD Test Guideline 439. This method is to evaluate the early stage of the inflammatory response caused by cell and tissue damage in vitro by using reconstructed human epidermal cells (RhE) mimic the biochemical and physiological characteristics of the epidermis.[16]Furthermore, when it is necessary to evaluate the production of inflammatory cytokines such as IL-1 alpha by treatment with a testing substance, the culture supernatant after incubation is collected and the protein is quantified by ELISA. Moreover, in order to investigate the influence of the testing substance, a tape stripping method can be used. It is also possible to examine whether protein extract from the stratum corneum is involved in producing inflammatory cytokine by using western blotting. This method has the advantage of obtaining information of skin turnover speed, keratinization state and inflammatory symptoms at the same time.
The RhE is LabCyte EPI-Model, each test substance is exposed to RhE for 15 minutes, and the test substance on the surface of the epidermis is washed with PBS. LabCyte EPI-Model is cultured for 42 hours after treatment and the cell viability is measured. MTT is used as a method for measuring cell viability. The living cells pass through the cell membrane and MTT is reduced by coenzyme riboflavin in the mitochondria to become formazan.Immerse RhE in 0.5 mg / mL MTT solution for 3 hours, collecting formazan extract with isopropanol and measuring OD value at 570 nm with an absorbance meter. After confirming the conditions for establishing the test, irritation is judged whether the cell viability is 50% or less.
In a real example of skin irritation test in our laboratory, as shown in Figure 3, RhE was stimulated with shampoo sample A and sample B for 15 minutes, and the number of viable cells after 42 hours was quantified by the MTT method. Water was used as a negative control and 5% sodium dodecyl sulfate (SDS) solution as a positive control, and sample A and B were adjusted to 1% with water. The cells treated with sample A were found to have a survival rate of 70.5 %. On the other hand, 93.2% of sample-B-treated cells were alive.
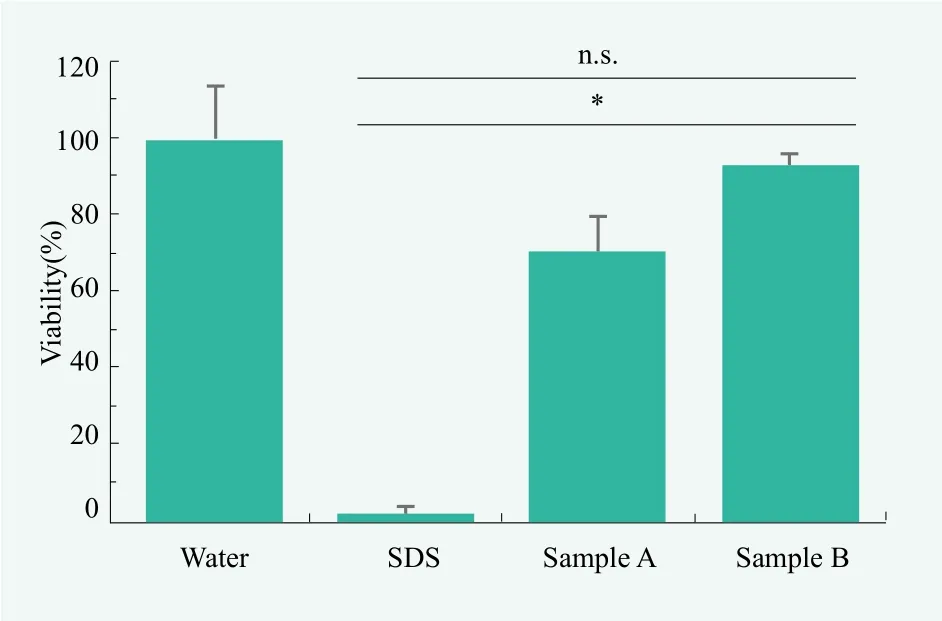
Figure 3. Evaluation of skin irritation by reconstructed human epidermal cells
This method is useful for screening immediate stimulus, though it is different from immune cell mediated inflammatory response.
Inflammatory state
Whether a testing substance contributes to the induction of inflammation at the cellular level can be quantified by the mRNA of the inflammatory cytokine IL-1 alpha after treating with the test substance using epidermal keratinocytes.
As an example of evaluating inflammation, we examined mRNA expression of inflammatory cytokine induced by UV irradiation or hydrogen peroxide treatment in keratinocytes[17]and quantifying ROS production.
Model sebum (MS): 48% Soybean oil, 13% Oleic acid, 12% Myristyl acid, 12% Squalene, 10% Paraffin wax, 3% Oleyl monoglyceryl, 2% Cholesterol stearate[18].HacaT cells were seeded on 24 well plates so that each cell became 4 × 103cells. On the day after cell seeding,the model sebum was treated at a concentration of 0.01%for 24 hours in a CO2incubator. After finishing culture,RNA was collected using RNeasy mini kit (QIAGEN).Thereafter, reverse transcription reaction was carried out using High Capacity cDNA Reverse Transcription Kits (Applied Bio) to prepare cDNA. Next, real time PCR was performed using FastStart Essential DNA Green Master (Roche). GAPDH was examined as an internal control and IL-1 alpa as an inflammatory related gene was examined.
Quantitation was performed on real-time PCR of IL-1 alpha mRNA after stimulation of hacaT cells with 0.01%model sebum for 24 hours. As a result in Figure 4, it was foun d that mRNA of IL-1 alpha increases 3.1 times after 24 hour stimulation. Moreover, pretreatment with 0.18 %dipotassium glycyrrhizate (G2K) for 1 hour results in suppression of MS-induced IL-1alpha mRNA expression.These results indicated that MS may be causing inflammation and G2K has the effect of suppressing inflammation as previously reported.[19]
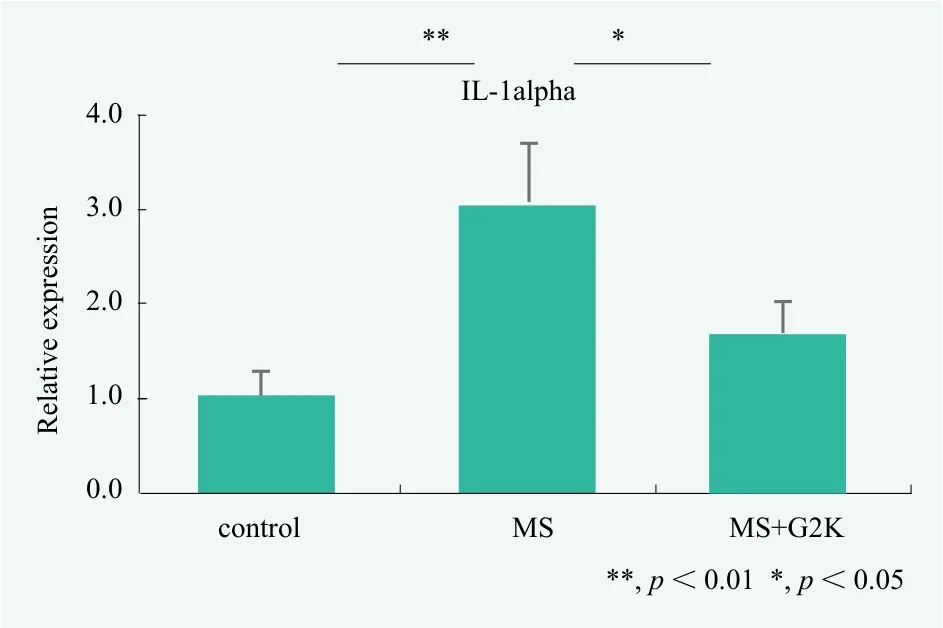
Figure 4. Evaluation of inflammation by Real Time PCR for IL-1 alpha

Methods for measuring hair growth and hair loss prevention effects
Hair growth is controlled by three stages in hair cycles,i.e. growth phase, retraction phase, and resting phase.FGF-7 and VEGF are well known as the genes involved in the growth phase. FGF-7 is secreted from dermal papilla cells and promotes proliferation by activating on hair matrix cells[20]. VEGF is also a secreted protein involved in angiogenesis, and is known that new blood vessels are formed to supply nutrition factors to dermal papilla cells.[21]So far, minoxidil is the only hair growth drug approved by FDA and is known to promote hair growth by promoting the transition from the resting stage to the growing stage of hair.[22]As a method for evaluating the hair growth effect, changing in expression of each gene when a test substance is treated on keratinocytes or dermal papilla cells is examined by Real-time PCR.The molecular mechanism of male pattern hair loss is converted to testosterone of male sex hormone to dihydrotestosterone by 5alpha reductase in dermal papilla cells.[23]Then this dihydrotestosterone induces the expression of TGTGFβ in dermal papilla cells, and the produced TGFβ is secreted extracellularly and acts on outer root sheath and keratinocytes to induce apoptosis.[24]Finasteride is thought to prevent male pattern hair loss by inhibiting the enzyme activity of 5 alpha reductase with FDA approved hair loss preventing drug. As a method for evaluating male pattern hair loss prevention, measuring the enzyme activity of 5 alpha reductase after treatment of a test substance, examining the expression of TGFβ, or investigating the influence of keratinocytes on TGFβ induced apoptosis.[25]
Hair growth
Human follicle dermal papilla cells (TAKARA) can be used. Cells are seeded on 24 well plates so that each cell became 5 × 103cells. The day after cell seeding, cells are stimulated with minoxidil at a final concentration of 10 μM for 3 hours. After culturing, RNA is collected using RNeasy mini kit (QIAGEN). Thereafter, reverse transcription reaction is carried out using High Capacity cDNA Reverse Transcription Kits (Applied Bio) to prepare cDNA. Next, real time PCR is performed using FastStart Essential DNA Green Master (Roche). GAPDH is examined as an internal control, and FGF-7 is examined as hair growth-related genes.
An experimental example is as follows. Epidermal keratinocytes or dermal papilla cells were stimulated with 10 μM minoxidil for 3 hours, and mRNA was isolated from the cells. Then, reverse transcription reaction was carried out, mRNA expression of FGF-7 was examined by real-time PCR after preparation of cDNA. As shown in Figure 5, minoxidil treated keratinocyte express IL-1alpha mRNA at a level of about 1.26 times versus the control.

As shown in Figure 6, cells were treated with finasteride suppressed the expression of DHT-induced TGFβ mRNA expression.
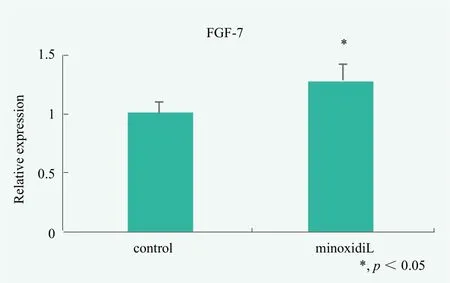
Figure 5. Evaluation of hair growth by keratinocytes stimulated with minoxidil
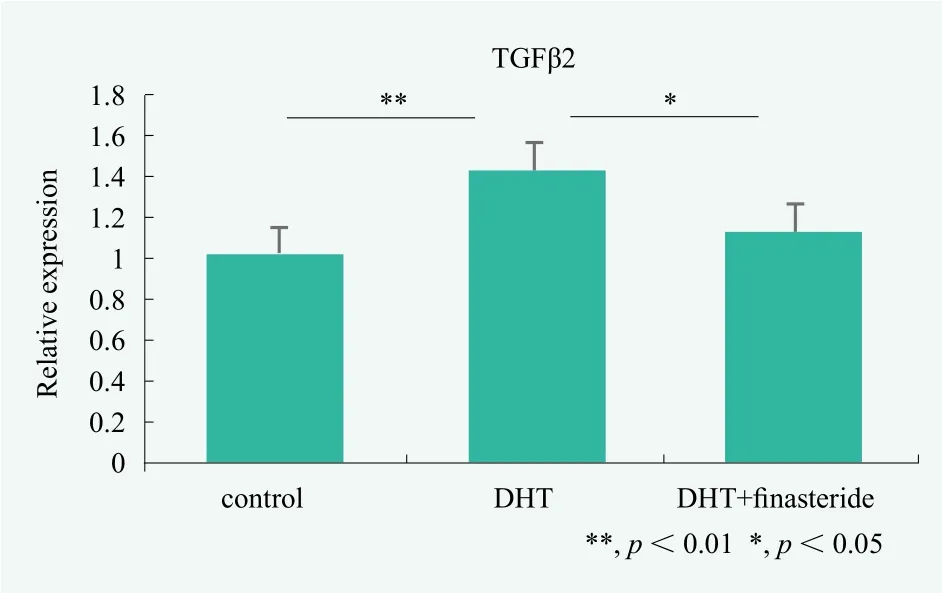
Figure 6. Evaluation of male pattern hair loss prevention by dermal papilla cells
Male pattern hair loss
As an example, human dermal papilla cells (TAKARA)were used. Cells were seeded on 24 well plates so that each cell became 5 × 103cell. The day after cell seeding,Finasteride was used at a final concentration of 200 nM for 1 hour. After the pretreatment, DHT was stimulated for 3 hours at a final concentration of 200 nM. Similarly,hair growth evaluation, mRNA was isolated from cultured cells, cDNA was prepared by reverse transcription reaction, and changing in gene expression was examined by real time PCR. The tested gene is GAPDH as an internal control and TGFβ as a hair loss related gene.
Conclusion
In order to maintain or promote scalp and hair health,it is necessary to first eliminate negative factors for the scalp by protecting scalp from excessive ultraviolet rays, pollution such as PM 2.5, unsaturated fatty acids produced by decomposed sebum, followed by improving scalp defense such as barrier function, blood circulation,and anti-inflammation. In vitro evaluation methods have been established for examining skin irritation, antiinflammation, barrier function, blood circulation, hair growth and male pattern hair loss prevention, respectively.The correlation between scalp health and hair condition needs to be further investigated in the future.
[1] Mehling A; Kleber M; Hensen H. Comparative studies on the ocular and dermal irritation potential of surfactants. Food Chem Toxicol. 2007, 5 (45), 747-758.
[2] Okasaka M; Kubota K;Yamasaki E; Yang J; Takata S. Evaluation of anionic surfactants effects on the skin barrier function based on skin permeability. Pharm Dev Technol 2018, 1 (23),1-6.
[3] Mizutani T; Mori R; Hirayama M; Sagawa Y; Shimizu K;Okano Y; Masaki H. Sodium lauryl sulfate stimulates the generation of reactive oxygen species through interactions with cell membranes. J Oleo Sci. 2016,65(12), 993-1001.
[4] Miyazawa K. Evaluation of haircare products; shampoo and rinse. J.Soc.Cosmet. Chem.Jpn. 1995,29(2), 95-105.
[5] Takino Y; Kawasaki Y. Development of low irritative surfactants. Fragrance Journal 1994,22(8), 67-73.
[6] Jourdain R; Moga; Vingler P; El Rawadi C; Pouradier F;Souverain L; Bastien P; Amalric N; Breton L. Exploration of scalp surface lipids reveals squalene peroxide as a potential actor in dandruff condition. Arch Dermatol Res. 2016, 308(4),153-63.
[7] Dawson TL Jr. Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J Investig Dermatol Symp Proc. 2007,12(2), 15-19.
[8] http://jp.sunstar.com/company/press/2018/0220.html.
[9] Egawa G and Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018, 67(1), 3-11.
[10] Zhai H; Fautz R; Bhandarkar S; Maibach HI. Human scalp irritation compared to that of the arm and back. Contact Dermatitis 2004, 51(4), 196-200.
[11] Soga H; Morita K; Arai K. Effects for scalp blood flow and properties from scalp massage. J.Soc.Cosmet.Chem.Jpn. 2014,48 (2), 97-103
[12] Pflaumbaum M; Farwick M; Mentel M; Kohler T. Preventing hair loss by balancing the hair cycle, strengthening the hair follicle and improving scalp health. EURO COSMETICS 2014,4, 12-16.
[13] Myung P; Ito M. Dissecting the bulge in hair regeneration. J Clin Invest 2012 ,122(2), 448-454.
[14] George FW; Russell DW; Wilson JD. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci U S A. 1991, 88(18), 8044-8047.
[15] Matsumura H; Mohri Y; Binh NT;Morinaga H;Fukuda M; Ito M; Kurata S. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016 Feb 5;351(6273): aad4395.
[16] Kandarova H; Willoughby JA;De Jong WH; Letasiova S;Milasova T; Bachelor MA; Breyfogle B; Handa Y; De la Fonteyne L; Coleman KP. Pre-validation of an in vitro skin irritation test for medical devices using the reconstructed human tissue model EpiDerm. Toxicol in Vitro. 2018 Feb 10.pii: S0887-2333(18)30044-4.

[17] Nasti TH; Timares L. Photochem Photobiol. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage. Photochem Photobiol 2012, 88(5), 1111-1125.
[18] Koyagi T; Hirohata R. Scalp care focusing on lipid peroxide.Fragrance Journal 2012, 40(10), 16-22.
[19] Ishida T; Ishida T; Yagi S; Irino Y; Nishiumi S; Miki I; Kondo Y;Mizuno S; Yoshida H; Azuma T; Yoshida M. Inhibitory effects of glycyrrhetinic Acid on DNA polymerase and inflammatory activities. Evid Based Complement Alternat Med. 2012.
[20] Iino M; Ehama R; Nakazawa Y; Iwabuchi T; Ogo M; Tajima M;Arase S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J Invest Dermatol 2007,127(6), 1318-1325.
[21] Yano K; Brown LF; Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest 2001, 107(4), 409-417.
[22] Messenger AG; Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol 2004,150(2), 186-194.
[23] George FW; Russell DW; Wilson JD. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci U S A. 1991, 88(18), 8044-8047.
[24] Hibino T; Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004, 35(1), 9-18.
[25] Shin HS; Park SY; Hwang ES; Lee DG; Mavlonov GT; Yi TH.Ginsenoside F2 reduces hair loss by controlling apoptosis through the sterol regulatory element-binding protein cleavage activating protein and transforming growth factor-beta pathways in a dihydrotestosterone-induced mouse model. Biol Pharm Bull 2014, 37(5), 755-763.
 China Detergent & Cosmetics2018年1期
China Detergent & Cosmetics2018年1期
- China Detergent & Cosmetics的其它文章
- Reconstruction of 3D Skin Model and Its Application in Evaluation the Efficacy of Cosmetic Raw Materials and Active Intredients
- An In Vitro Biological Model for Evaluation of the Anti-Dandruff Performance of Hair Care Products
- Models for Assessing Pollution Damage of Asian Hair
- Regulation of Talcum Powder in Cosmetics at Home and Abroad
- Technical Standard System Construction Plan for Surfactants and Detergents in the“13th Five-year Plan” Period
- A Summary on Chinese Daily Chemical Journals
