Phenol Wastewater Degradation by Electrocatalytic Oxidation with RuO2-IrO2-SnO2/Ti Anodes in the High Gravity Field
Gao Jing; Yan Junjuan; Liu Youzhi; Zhang Dongming; Chen Lijia
(Shanxi Province Key Laboratory of High Gravity Chemical Engineering, North University of China, Taiyuan 030051)
Abstract: A novel high gravity multi-concentric cylinder electrodes-rotating bed (MCCE-RB) was developed for the electrocatalytic degradation of phenol wastewater in order to enhance the mass transfer with the self-made RuO2-IrO2-SnO2/Ti anodes. The influences of electric current density, inlet liquid circulation f lowrate, high gravity factor, sodium chloride concentration, and initial pH value on phenol degradation efficiency were investigated, with the optimal operating conditions determined. The results showed that under the optimal operating conditions covering a current density of 35 mA/cm2, an inlet liquid circulation f lowrate of 48 L/h, a high gravity factor of 20, a sodium chloride concentration of 8.5 g/L, an initial pH value of 6.5, a reaction time of 100 min, and an initial phenol concentration of 500 mg/L, the efficiency for removal of phenol reached 99.7%, which was improved by 10.4% as compared to that achieved in the normal gravity f ield. The tendency regarding the change in efficiency for removal of phenol, total organic carbon (TOC), and chemical oxygen demand (COD) over time was studied. The intermediates and degradation pathway of phenol were deduced by high performance liquid chromatography (HPLC).
Key words: high gravity; RuO2-IrO2-SnO2/Ti anode; electrocatalytic oxidation; phenol wastewater; degradation pathway
1 Introduction
As a new process intensification technology, the high gravity technology can effectively enhance the mass transfer of electrochemical oxidation[1]. In recent years, a coupling technique between the high gravity and the electrochemical oxidation has the advantage of high efficiency and environmental compatibility, and has been used in the petrochemical wastewater treatment[2], hydrogen production by water electrolysis[3], chlor-alkali reaction[4-5], and materials preparation by electrodeposition[6]. In this paper, a high gravity electrocatalytic reactor with multi-concentric cylindrical electrodes-rotating bed (MCCE-RB) was developed to couple the high gravity technology with the electrocatalytic oxidation technology and degrade the phenol contained in wastewater. The rotating anode plate in the MCCE-RB was used to rotate the concentric cylindrical anodes to create a high gravity f ield, and then to enhance the mass transfer process in the electrocatalytic oxidation reaction[7].
In addition, the efficiency of electrocatalytic oxidation also depends closely on the performance of anodes. Currently, more attention has been paid to the titaniumbased coating anodes because of their excellent electrocatalytic performance. Among these anodes, the RuO2-IrO2-SnO2/Ti anode has been used for electrocatalytic oxidation of phenol and is proved to be suitable for the degradation of phenol[8-12]. However, process intensification is also important to improve the degradation of phenol and however, there are only few researches related with this work.
In order to enhance the mass transfer during the electrocatalytic oxidation of phenol, MCCE-RB was developed to degrade the simulated phenol wastewater with the RuO2-IrO2-SnO2/Ti anodes for the first time in this paper. The effects of current density, inlet liquid circulation f lowrate, high gravity factor, sodium chloride concentration, and initial pH value on the efficiency for removal of phenol were investigated and the optimal operating conditions were determined. The changes in phenol removal efficiency, TOC and COD with the time were investigated under the optimal operating conditions. HPLC was adopted to infer the degradation pathway of phenol. The purpose of this work is to provide an efficient electrocatalytic method for effective treatment of the phenol-containing wastewater from petrochemical units.
2 Experimental
2.1 Preparation of RuO2-IrO2-SnO2/Ti anode
The self-made RuO2-IrO2-SnO2/Ti anode was prepared by thermal oxidation of RuO2-IrO2-SnO2coating on the cylindrical titanium substrate. The titanium substrate was polished, washed with 5% NaOH solution, and immersed in 10% boiled oxalic acid solution for 10 h of etching. Then, the titanium substrate was cleaned with distilled water. H2IrCl6, RuCl3, and SnCl4at a certain molar ratio were dissolved in a proper amount of isopropanol solution and mixed to prepare the coating solution. The coating solution was brushed evenly onto the titanium substrate, evaporated completely at 95°C for 10 minutes in an oven, pyrolyzed at 450°C for 15 min in a muff le furnace, and cooled down to room temperature. The above procedure of brushing, drying and pyrolysis was repeated until the concentration of surface active material on the titanium substrate was increased to 15 g/cm2.
2.2 Experimental setup and phenol wastewater degradation
MCCE-RB was used to degrade phenol wastewater. As shown in Figure 1, the concentric cylindrical cathodes and anodes are respectively connected with a cathode plate and an anode plate, which are arranged alternately to form the main structure of the MCCE-RB. The concentric cylinder cathodes and cathode plate are relatively static to the setup shell, while the concentric cylinder anodes and anode plate are connected with the rotating shaft and can be driven by the control system to form different high gravity f ields. The strength of the high gravity f ield is measured by the high gravity factor (β) which refers to the ratio of centrifugal acceleration and gravitational acceleration at any point in the high gravity f ield.
The degradation process of phenol wastewater is realized as follows. Firstly, the DC-regulated power supply is turned on to supply the total current for the system, and the wastewater in the storage tank is pressurized in the MCCE-RB via a pump and is measured by a f lowmeter. Secondly, the wastewater flows continuously from the center to the periphery of the MCCE-RB through the alternate cylindrical anodes and cathodes in an S-shape mode in the gravity f ield formed by rotating anodes and anode plate. Then, the wastewater contacts with the electrodes to be degraded continuously. In this case, the wastewater is subjected to shear force strongly, and the electrodes surface is washed by the wastewater, while the concentration polarization is reduced, and the mass transfer is enhanced. Finally, the degraded wastewater can be directly discharged from the outlet via the valve or can be discharged into the wastewater storage tank for recycling.
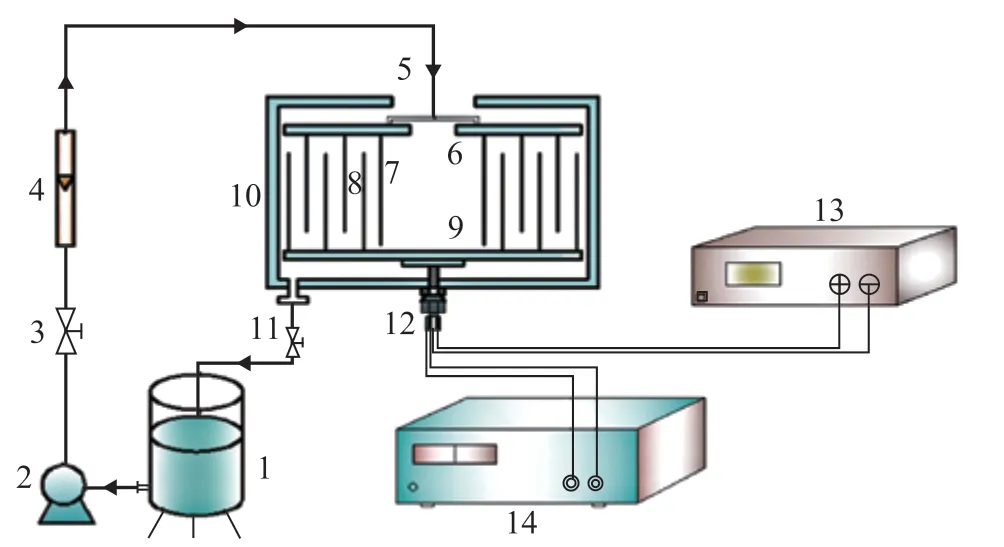
Figure 1 Flow diagram of phenol wastewater degradation
2.3 Apparatus and analytical methods
The concentration of phenol and intermediates was measured by HPLC (UltiMate 3000, Thermo Fisher, USA.). A C18reversed-phase column (Accucore XL C18, 250 mm×4.6 mm, 4 μm) was operated at 25 °C. The mobile phase was methanol/water (with a volume ratio of 60/40) and the flowrate was 1.0 mL/min. The detection wavelength was 270 nm and the injection volume was 20 μm[13].
The concentration of TOC was detected by a TOC tester (1030W, OI, USA) and the concentration of COD was monitored by a multispectral COD analyzer (5B-3A, Lianhua, China). The phenol or TOC or COD removal efficiency (η) is defined as:

in whichc0andctare the phenol or TOC or COD concentration in solution before and after electrocatalytic oxidation at a timet(mg/L), respectively.
3 Results and Discussion
3.1 Effect of current density
The effect of different current density on the efficiency of phenol removal was studied under conditions covering a high gravity factorβof 20, an inlet liquid circulation flowrate of 48L/h, a sodium chloride concentration of 8.5 g/L, and an initial pH value of 6.5. The initial concentration of phenol was 500 mg/L, and the same amount was used in other experiments. As shown in Figure 2, with an increasing current density, the efficiency of phenol removal showed a sharp increase and then changed slightly. The current density has an influence on the phenol removal efficiency, because it is related to the electron transfer process and the generation of ·OH and ClO-in the electrocatalytic oxidation system. The higher current density indicates that more electrons are transferred in the system to generate ·OH and ClO-easily, which have strong oxidizing capability. So the reaction rate and the phenol removal efficiency increased. But when the current density was too large, the overpotential would increase, which could cause not only the increase of energy consumption, but also the aggravation of oxygen evolution. When the current density was 35 mA/cm2and the reaction time was 100 min, the phenol removal efficiency reached 99.7%. When the current density was 40 mA/cm2and 50 mA/cm2under the same conditions, the phenol removal efficiency reached 99.79% and 100%, respectively. Therefore, the optimal current density was determined as 35 mA/cm2.
3.2 Effect of inlet liquid circulating f lowrate
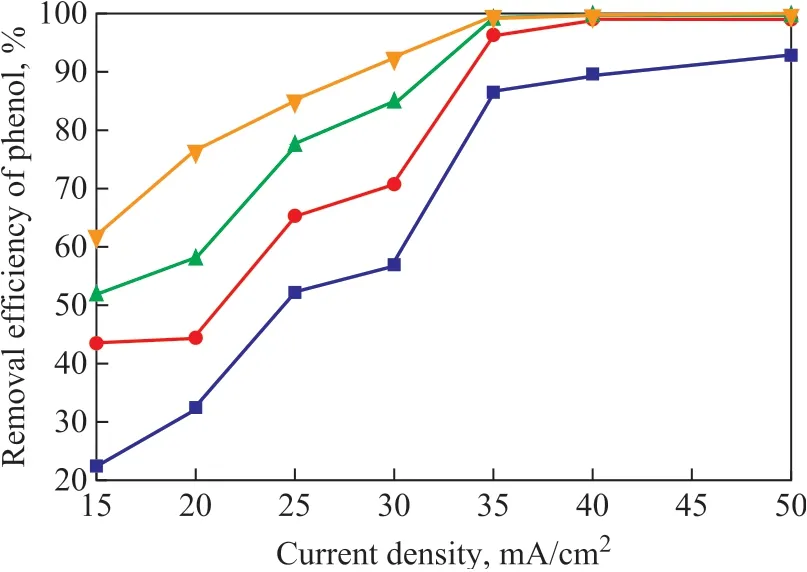
Figure 2 Effect of current density on phenol removal efficiency
The effect of different inlet liquid circulation flowrate on the phenol removal efficiency under conditions covering a high gravity factorβof 20, a current density of 35 mA/cm2, a sodium chloride concentration of 8.5 g/L, and an initial pH value of 6.5 is shown in Figure 3. The results showed that the efficiency for removal of phenol increased obviously and then decreased with the increase of inlet liquid circulation flowrate. The inlet liquid circulating f lowrate has an influence on the phenol removal efficiency, because it is related to the transport energy consumption and liquid hold up in the system, which can affect the phenol removal efficiency. When the inlet liquid circulation f lowrate was small, the liquid hold up in the MCCE-RB was less. It means that the electrodes are not completely used to degrade phenol and the utilization of ·OH and ClO-is low. So the phenol removal efficiency was low. However, a too large inlet liquid circulation f lowrate would lead to faster wastewater regeneration rate on the electrodes and shorter contact time between the organic pollutants in wastewater and the electrodes. A lot of organic pollutants in the wastewater were replaced by the new wastewater before reacting on the electrodes or with active particles. So the phenol could not react in time with the ·OH and ClO-on the electrode surface and the phenol removal efficiency decreased significantly. When the liquid circulation flowrate was 48 L/h, the phenol removal efficiency was the highest. Therefore, the optimal current density was specified at 48 L/h.
3.3 Effect of high gravity factor β
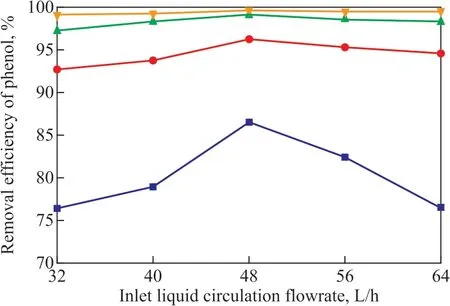
Figure 3 Effect of inlet liquid circulation f lowrate on phenol removal efficiency
Figure 4 plots the effect of high gravity factor on the phenol removal efficiency under conditions covering a current density of 35 mA/cm2, an inlet liquid circulation flowrate of 48 L/h, a sodium chloride concentration of 8.5 g/L, and an initial pH value of 6.5. The results showed that the phenol removal efficiency increased obviously at f irst and then decreased with the increase of the high gravity factorβ. When the high gravity factorβwas small or in the normal gravity field, the mass transfer efficiency was low, and the phenol and other organic pollutants could not spread to the electrode surface for electrocatalytic oxidation in time, resulting in the concentration polarization. At the same time, the bubbles generated from electrocatalytic reaction could not be separated from the electrodes surface in time, and thus the contact area between the wastewater and the electrodes would decrease. When the high gravity factorβreached a large level, the whirlpool would be formed in the wastewater, which could lead to a fast wastewater renovation rate and a decrease in the amount of wastewater contacting with the surface of electrodes. Therefore, the phenol removal efficiency decreased.
However, when the high gravity factorβwas selected properly, it not only could promote the regeneration rate of the wastewater on the electrode surface, but also could increase the buoyancy of the bubbles and accelerate the phase slip rate, then effectively promote the separation of the bubbles and improve the efficiency of phenol removal. According to Figure 4, under the condition of the same electrocatalytic time, the efficiency of phenol removal was improved by 10.4% in the high gravity f ield than that in the normal gravity field. It showed that the high gravity f ield could enhance the mass transfer. When the high gravity factorβwas 20, the efficiency of phenol removal was relatively higher. Therefore, the optimum high gravity factorβwas specified at 20.
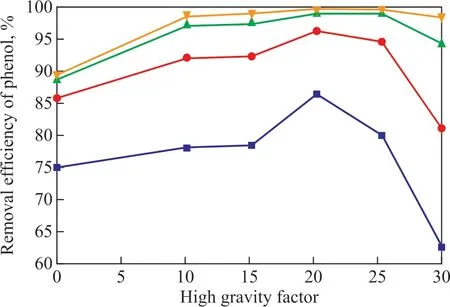
Figure 4 Effect of high gravity factor on phenol removal efficiency
3.4 Effect of sodium chloride concentration
Sodium sulfate and sodium chloride are used as electrolytes generally. If sodium sulfate is used as the electrolyte, the amount of active substance produced in the solution is limited and the energy consumption is high. So sodium chloride was used as the electrolyte to generate active chlorine which could enhance the indirect oxidation and reduce energy consumption[14].
Figure 5 shows the effect of sodium chloride concentration on the phenol removal efficiency under conditions covering a high-gravity factorβof 20, a current density of 35 mA/cm2, an inlet liquid circulation flowrate of 48 L/h, and an initial pH value of 6.5. The results showed that the phenol removal efficiency increased at first and then decreased with the increase of sodium chloride concentration. When the sodium chloride concentration increased, the conductivity of the system increased, while the electrons transfer rate accelerated and the amount of ClO-increased, so the phenol removal efficiency increased. When the sodium chloride concentration exceeded a certain value, some ions could not participate in the reaction quickly and were attached to the electrode surface, which could lead to the steric hindrance effect and would hinder the formation of ·OH, resulting in the decrease of phenol removal efficiency. When the sodium chloride concentration was 8.5 g/L and the reaction time was 100 min, the phenol removal efficiency reached a highest value. Therefore, the appropriate sodium chloride concentration was set at 8.5 g/L.

Figure 5 Effect of sodium chloride concentration on phenol removal efficiency
3.5 Effect of initial pH value
Figure 6 shows the effect of initial pH value on the phenol removal efficiency under conditions covering a high gravity factorβof 20, a current density of 35 mA/cm2, an inlet liquid circulation flowrate of 48 L/h, and a sodium chloride concentration of 8.5 g/L. The electrocatalytic oxidation of phenol wastewater mainly includes two processes[15], among which one is the direct oxidation process that occurs on the anode, and the other one is the indirect oxidation process that occurs between the phenol and the active substances produced on the anode. Under the acidic conditions, the direct oxidation played a leading role, and the oxygen evolution potential was high while it could not occur easily, so more phenol species were oxidized directly on the anode. Under the alkaline condition the indirect oxidation played a major role, while the oxygen evolution potential was low and could occur easily, so that few phenol species were oxidized directly on the anode. Obviously the acidic condition was benef icial to the degradation of phenol[9]. When the initial pH value was 6.5 and the reaction time was 100 min, the phenol removal efficiency could reach a highest value. Therefore, the appropriate initial pH value was set at 6.5.
3.6 Change in phenol and TOC removal efficiencies and COD reduction with time
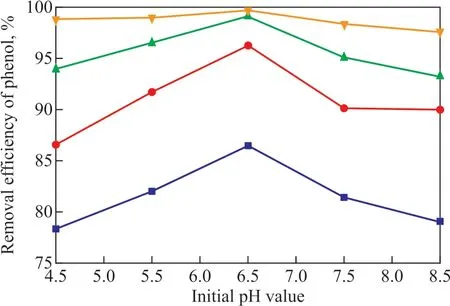
Figure 6 Effect of initial pH value on phenol removal efficiency
Under the optimal operating conditions covering a current density of 35 mA/cm2, an inlet liquid circulation f lowrate of 48 g/L, a high gravity factorβof 20, a sodium chloride concentration of 8.5 g/L, and an initial pH value 6.5, the change in efficiencies for removal of phenol, TOC and COD with the time was investigated. As shown in Figure 7, the changing tendency increased at first and then changed slightly. From the perspective of kinetics[16], this could occur because the ClO-, ·OH and other active substances were sufficient to degrade the phenol and intermediates quickly at the initial stage of reaction. With the extension of the reaction time, the amount of ClO-, OH and other active substances in the system decreased, while the amount of intermediates increased. The active substances are not enough to degrade the phenol and more intermediates, so the changes tended to be insignificant.
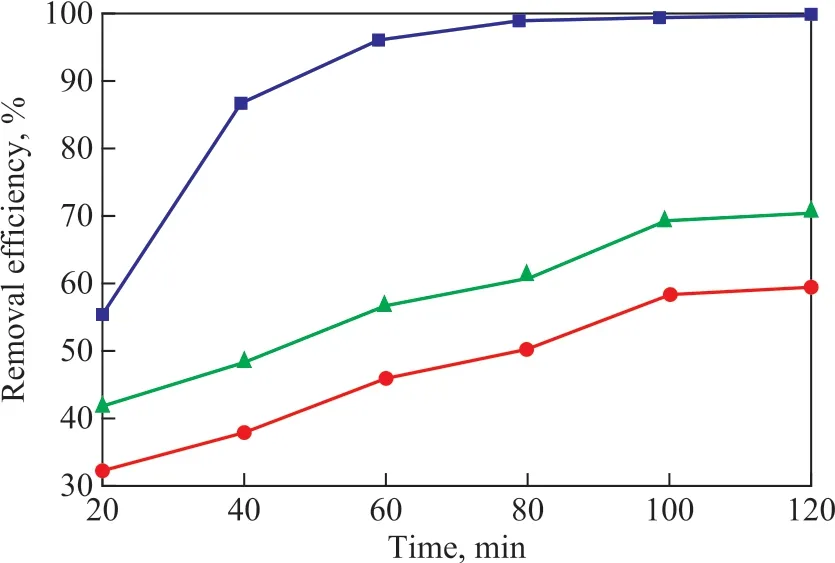
Figure 7 The change in efficiencies for removal of phenol, TOC and COD with the time
3.7 Phenol degradation pathway
In order to study the pathway for degradation of phenol on RuO2-IrO2-SnO2/Ti anode in the high gravity f ield, the HPLC chromatograms of phenol degradation at different times were monitored under the optimal operating conditions (Figure 8). According to the retention time of standard material, possible degradation intermediates were obtained (Table 1).
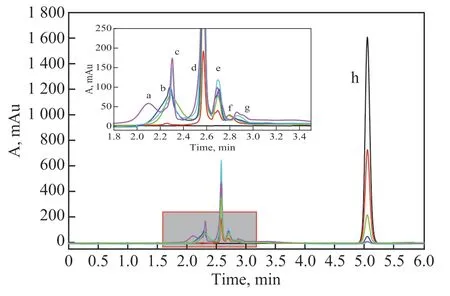
Figure 8 HPLC chromatograms of phenol degradation at different times in the high gravity f ield
With the prolonging of reaction time, oxalic acid (a), fumaric acid (b), malonic acid (c), maleic acid (d), succinic acid (e), hydroquinone (f), and p-benzoquinone (g) were detected. The phenol degradation pathway was proposed, as illustrated in Figure 9. In the f irst stage, the hydroxyl radicals attacked the benzene ring of phenol, and hydroquinone andp-benzoquinone were generated. In the second stage, the benzene ring structure was destroyed and the ring was opened to produce maleic acid and fumaric acid, which were further oxidized to produce succinic acid, malonic acid, and oxalic acid. Finally, these compounds were decomposed into CO2and H2O.
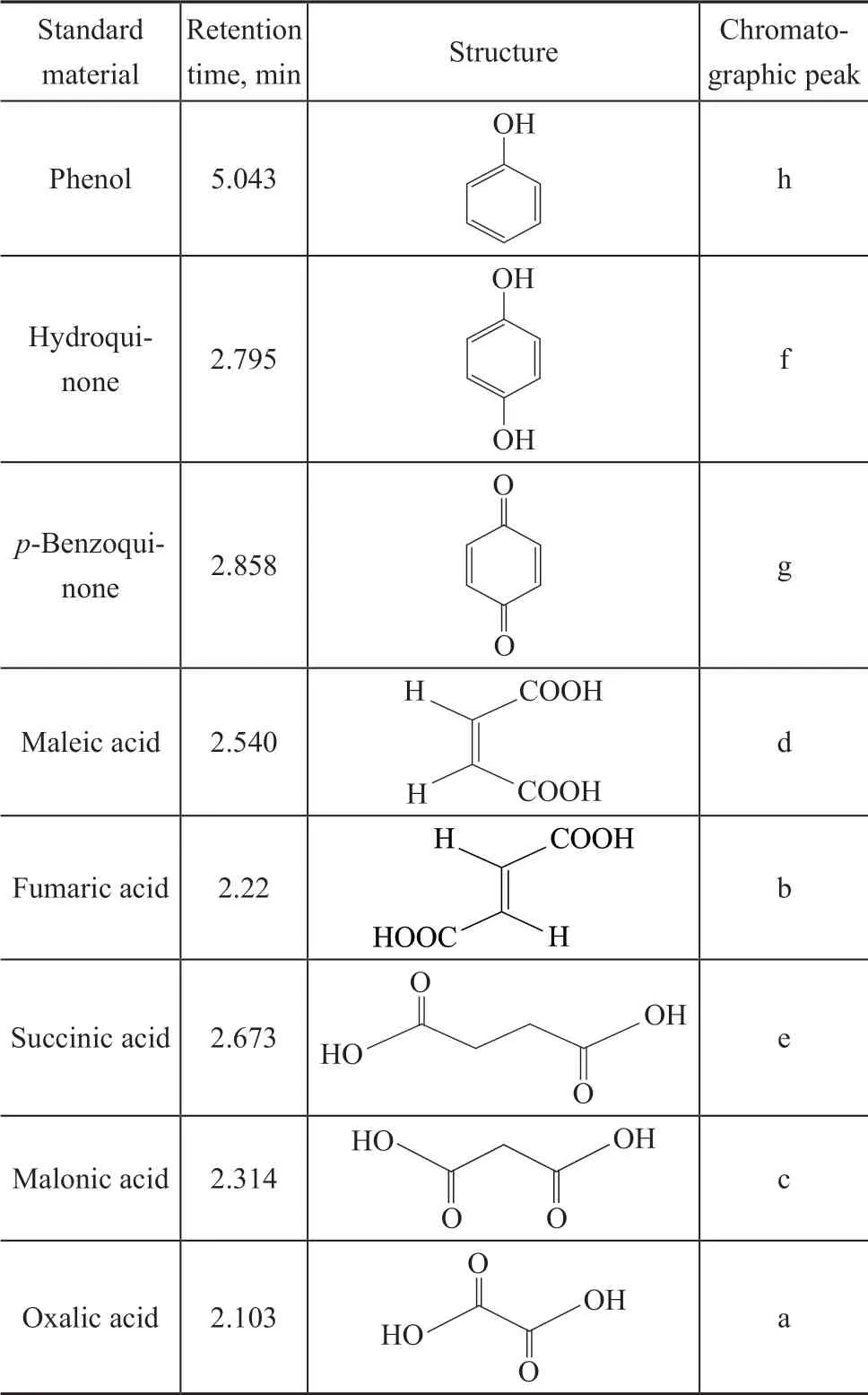
Table 1 Retention time of standard materials
4 Conclusions
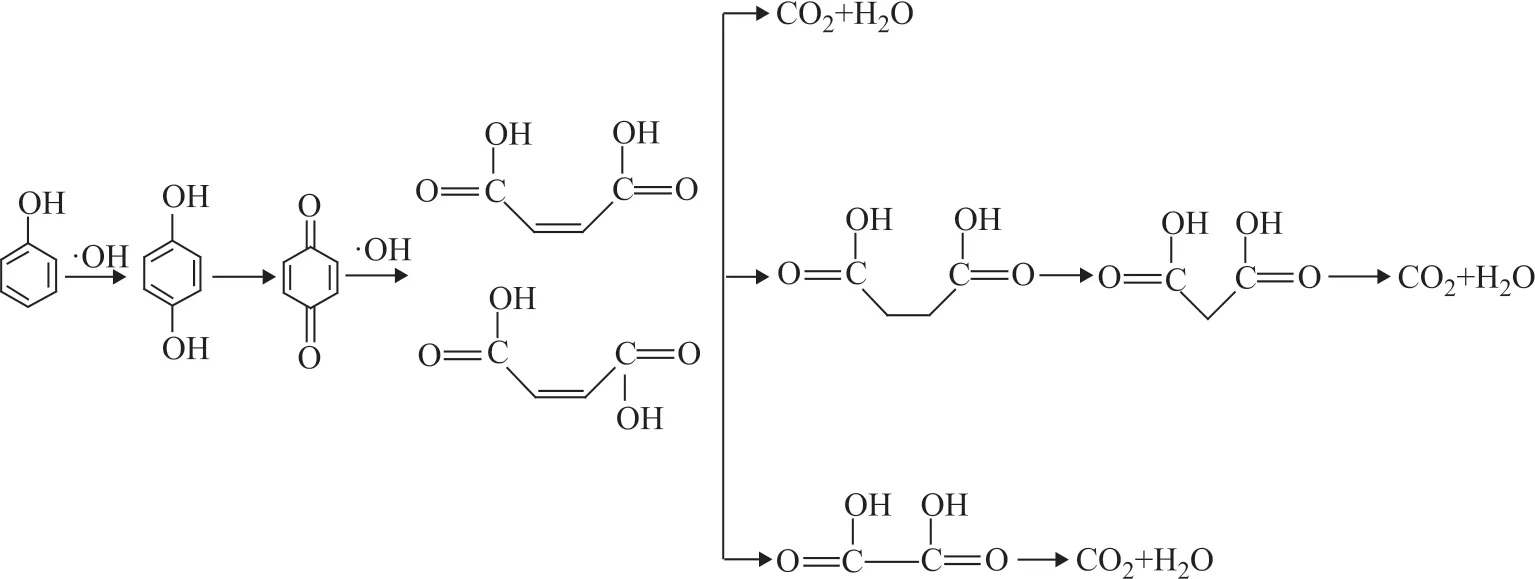
Figure 9 Degradation pathway of phenol on RuO2-IrO2-SnO2/Ti anode in the high gravity f ield
A novel high gravity electrocatalytic reactor, MCCE-RB, with the self-made RuO2-IrO2-SnO2/Ti anodes was used for electrocatalytic degradation of phenol wastewater. The optimal operating conditions covered: a current density of 35 mA/cm2, an inlet liquid circulation f lowrate of 48 L/h, a high gravity factor of 20, a sodium chloride concentration of 8.5 g/L, an initial pH value of 6.5, and a reaction time of 100 min, resulting in a phenol removal efficiency of 99.7%, which was increased by 10.4% than that achieved in the normal gravity field. The results indicated that the high gravity field enhanced the mass transfer and improved the electrocatalytic degradation of phenol. Under the optimal operating conditions, the TOC removal efficiency increased and COD decreased at f irst and then changed slightly.
The phenol degradation pathway on the RuO2-IrO2-SnO2/Ti anode in the high gravity f ield was proposed on the basis of HPLC analysis. In the f irst stage, the hydroxyl radicals attacked the benzene ring of phenol, and hydroquinone and p-benzoquinone were generated. In the second stage, the benzene ring of phenol was opened to produce maleic acid and fumaric acid, which were further oxidized to produce succinic acid, malonic acid, and oxalic acid. Finally, these compounds were decomposed into CO2and H2O.
Acknowledgement:The study was f inancially supported by the Nature Science Foundation of China (Grant No.U1610106) and the Nature Science Foundation of China (Grant No.21703208).
- 中國煉油與石油化工的其它文章
- Boosted Biodegradability and Tribological Properties of Mineral Base Oil by Methyl Diethanolamine Fatty Acid Esters
- Extractive Distillation of Methyl Acetate-Methanol Azeotrope Using [DMIM]DMP as Solvent
- Shortcut Method of Design and Energy-Saving Analysis of Sargent Dividing Wall Column
- Exploring the Degradation Potential of Halomonas Bacteria from Oil-contaminated Marine Environment
- Combined Use of Co-immobilized Lysozyme and Lipase and Chemical Inhibitors in Circulating Cooling Water System
- Evaluation of Powdered Activated Carbon Treatment Process in Petrochemical Wastewater Purification

