Combined Use of Co-immobilized Lysozyme and Lipase and Chemical Inhibitors in Circulating Cooling Water System
Jiang Guofei; Chen Xiaorui; Lu Xin; Liu Fang; Wang Yongqiang; Zhao Chaocheng; Sun Juan
(College of Chemical Engineering, China University of Petroleum, Qingdao 266580;State Key Laboratory of Petroleum Pollution Control, Beijing 102206)
Abstract: In order to improve the stability and corrosion inhibition performance of bioenzyme, lipase and lsozyme were co-immobilized on the mesoporous molecular sieve MCM-41 by the adsorption method. Then the immobilized enzymes were combined with amino trimethylene phosphonic acid and polyaspartic acid to prevent corrosion caused by circulating cooling water. The weight-loss method and electrochemical techniques were used to evaluate the performance of composite inhibitors. The co-immobilized lysozyme and lipase achieved good inhibition effects. After they were combined with amino trimethylene phosphonic acid and polyaspartic acid, the corrosion inhibition properties were further improved. The inhibition efficiency was promoted to 94.4%. During the corrosion inhibition process, the immobilized enzymes played an important role. The addition of corrosion inhibitor could inhibit the anodic dissolution and cathodic hydrogen evolution process of carbon steel at the same time. The adsorption of co-immobilized lysozyme and lipase composite inhibitor on the steel surface was a joint action involving physical adsorption and chemical adsorption.
Key words: carbon steel; electrochemical impedance spectroscopy; potentiodynamic polarization; co-immobilized lysozyme and lipase
1 Introduction
It is an effective way to conserve water resources by reusing refinery wastewater in the circulating cooling water system after the advanced treatment. Many researchers have focused on it[1-2]. However, the water quality of refinery wastewater is more complicated than that of fresh water, which can easily cause corrosion problems[3]. In the process of metal corrosion, chemical or electrochemical reactions occur on the interface of metals, which can lead to surface degradation. In order to delay corrosion, more chemical corrosion inhibitors are applied in practical f ield. Corrosion inhibitors with remarkable effect are organic compounds containing π bonds, heteroatoms (P, S, N, and O), and inorganic compounds[4-7]. However, excessive use of these chemical inhibitors is questioned recently because of their negative effects on the environment[8]. Therefore, it is essential to research environmentally friendly inhibitors for corrosion inhibition.
Anti-corrosion system favors the use of inhibitors with low or no environmental impact. Accordingly, the research has been carried out for finding novel, effective and non-toxic corrosion inhibitors[9]. In pursuit of natural resources having non-toxic properties or having slight impacts on the aquatic environment, enzymes as ideal candidates for corrosion inhibitors have been attracting great attention. In our previous research, lysozyme and laccase were conf irmed to have corrosion inhibition performance in the cooling water system[10-12]. Subsequently, the lipase was also conf irmed to have corrosion inhibition performance[13]. Qiao, et al.[14]studied the synergistic effect of lysozyme and VitB1 on corrosion inhibition of carbon steel in 0.5 mol/L H2SO4solution with the help of potentiodynamic polarization and EIS methods. However, compared with chemical corrosion inhibitors, the stability of enzymes is susceptible to environmental factors such as temperature, pH, etc. Therefore, it is necessary to use a proper method to improve the stability and corrosion inhibition performance of enzymes.
In the 1 970 s, the immobilized enzyme technique was proposed[15]. The immobilized enzyme, which is f ixed to an insoluble carrier, still has catalytic effect, while its stability is also improved. It can be recycled and reused[16-17]. There are several techniques for immobilizing enzymes on solid carriers. The immobilization of enzymes mainly includes the chemical and physical mechanisms[18]. The methods of enzyme immobilization cover: the enzyme attachment to carriers by adsorption process, the covalent bonds between enzyme and carriers, the cross-linking between enzyme and carriers, and capture of enzyme molecules in porous hollow f ibers[19-20].
After enzyme is immobilized, the thermal stability and acid-base stability of immobilized enzyme are improved[13]. However, it is important to note that the corrosion inhibition efficiency of immobilized enzyme is slightly lower than that of chemical corrosion inhibitor. Therefore, in order to inherit the environmentally friendly property of immobilized enzyme and enhance the inhibition performance of chemical inhibitors, the immobilized enzyme and chemical inhibitors can be used in combination to achieve good inhibition effects. There are few reports on the combined use of immobilized enzymes and chemical inhibitors.
In this research, lipase and lysozyme were co-immobilized on the mesoporous molecular sieve MCM-41 by the adsorption method. Then the co-immobilized lysozyme and lipase were combined with chemical inhibitors (amino trimethylene phosphonic acid (ATMP) and polyaspartic acid (PASP)) for improving corrosion inhibition effects associated with the circulating cooling water. The weight-loss method, the electrochemical impedance spectroscopy (EIS), and the potentiodynamic polarization techniques were used to evaluate the performance of composite inhibitors. The adsorption behaviour of composite inhibitors on carbon steel surface was also investigated.
2 Experimental
2.1 Inhibitors and enzymes
ATMP, PASP, lysozyme (>40 000 units/mg protein) and lipase (>150 000 units/mg protein) were used in this research with their molecular structures displayed in Figure 1. The stability of lysozyme and lipase was described in detail in our previous study[13].

Figure 1 Chemical molecular structures of ATMP, lipase, PASP and lysozyme (a-ATMP; b-lipase; c-PASP; d-lysozyme)
2.2 Lipase and lysozyme co-immobilization
According to our previous research, the selected co-immobilization method covered the sequential immobilization of lysozyme and then lipase. 3 g of MCM-41 mesoporous silica particles were added into 100 mL of Na2HPO4-KH2PO4buffer solution (50 mmol/L with a pH value of 6.5) containing 0.8 g/L of lysozyme. The mixture was subjected to immobilization for 10 h at 30 °C. The resulting solid particles were f iltered and then added into 100 mL of Na2HPO4-KH2PO4buffer solution (25 mmol/L with a pH value of 8.0) containing 1.5 g/L of lipase. The mixture was subjected to immobilization again for 8 h at 30 °C. After f iltration, the resulting powder was washed with distilled water to remove free enzyme. After being dried at 30 °C for 6 h, the co-immobilized sample was obtained.
2.3 Optimization of immobilized enzymes in combination with chemical inhibitors
The optimum schemes of co-immobilized lysozyme and lipase in combination with chemical inhibitors were investigated by orthogonal experiments using different concentration of ATMP and PASP.
2.4 Weight-loss method for corrosion inhibition measurements
The weight-loss method was performed to determine the inhibition efficiency of corrosion inhibitors. The standard carbon steel coupons (50 mm×25 mm×2 mm) were used as corrosion objects with a composition covering 0.16 % of C, 0.30% of Si, 0.53% of Mn, <0.035% of P, <0.04% of S and balanced Fe. The inhibitors were added into the circulating cooling water, which was taken from a petrochemical enterprise. The cooling water contained 82 mg/L of Ca2+, 38 mg/L of Mg2+, 303 mg/L of Cl-, 260 mg/L of, 393 mg/L of total hardness, and 350 mg/L of total alkalinity with a pH value of 8.9 and a conductivity of 2 821 μS/cm. The coupons were immersed in the cooling water that contained inhibitors for 72 h at 40 °C under a revolution rate of 80 r/min. After the immersion process was completed, the corroded coupons were soaked in a mixed solution for 5―10 min to remove the corrosion products. The mixed solution composed of 500 mL of hydrochloric acid, 20 g of hexamethylenetetramine and 1 L of distilled water. Finally, the coupons were washed with deionized water, and then were dried and weighed again to obtain their final weight. The test was repeated three times. The corrosion rate (CR) and inhibition efficiency (η) are calculated by Eqs. (1) and (2):
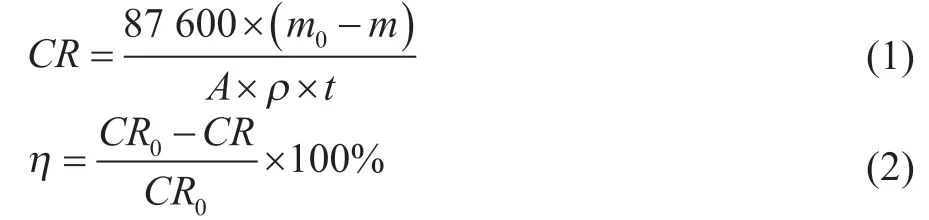
in whichm0is the original weight of coupon before test, g;mis the coupon weight after removal of the corrosion product, g;Ais the coupon surface area, cm2;ρis the coupon density, g/cm3;tis the test time, h;CR0is the corrosion rate in the absence of inhibitors, mm/a.
2.5 Electrochemical measurements
The electrochemical measurements were carried out in a conventional three-electrode glass cell. A saturated calomel electrode (SCE) served as the reference electrode. A platinum foil with a diameter of 0.5 mm and a length of 35 mm was used as the counter electrode. The carbon steel specimen with an exposed surface area of 1 cm2functioned as the working electrode. All potentials quoted were referred to the SCE. Experiments were carried out under stagnant aerated conditions at 30±1 °C, and the working electrode was immersed in the test solution for 1 h. Electrochemical impedance spectroscopy (EIS) tests were performed at open circuit potential (Eocp) over a frequency range from 100 kHz to 0.01 Hz with an amplitude of 10 mV peak-to-peak using AC signals at the open circuit potential. The polarization studies were carried out by sweeping the potential between -1 000 mV and 0 mV with respect to the steady-state potential at a scan rate of 0.001 V/s.
3 Results and Discussion
3.1 Characterization of co-immobilized lysozyme and lipase
Figure 2 presents the IR spectra of MCM-41 and coimmobilized lysozyme and lipase. Compared with the curve a, the absorbance at the curve b increases significantly at the wavenumbers of 3 450 cm-1and 1 180 cm-1. There are the amino absorption peaks at the wavenumbers of 3 300—3 500 cm-1and 1 180—1 360 cm-1, indicating that the amine compound is immobilized on MCM-41. The absorbance at 1 650—1 690 cm-1also proves that the amide is immobilized on MCM-41. The data in Figure 2 show that the nitrogen content of MCM-41 increases significantly after immobilization, and lipase and lysozyme are immobilized successfully on MCM-41.
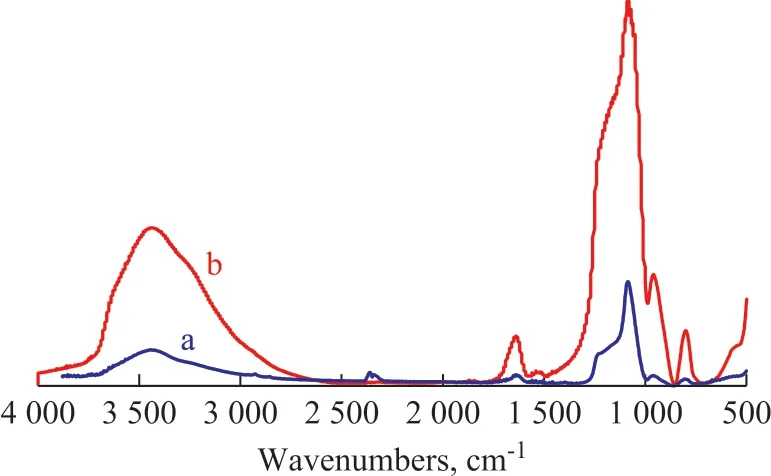
Figure 2 IR spectra of MCM-41 and co-immobilized lysozyme and lipase
In Figure 3a, the particle diameter of MCM-41 is about 0.5 μm. At the same magnification of 15 000 times, a large number of fine particles are found on the MCM-41 surface with the diameter equating to around 0.1-0.2 μm (Figure 3b). Combined with the analysis of Figure 2, these fine particles are enzyme molecules.

Figure 3 SEM images of MCM-41 and co-immobilized lysozyme and lipase
3.2 Inhibition performance with the addition of immobilized enzymes
Figure 4 shows the morphology images of coupons after 72 h of immersion in the cooling water in the absence and the presence of immobilized enzymes. In Figure 4b, remarkable improvement and fewer pits on coupon surface are observed. Promotion of the surface morphology may possibly be ascribed to the formation of co-immobilized lysozyme and lipase protective film on the coupon surface, resulting in corrosion inhibition.
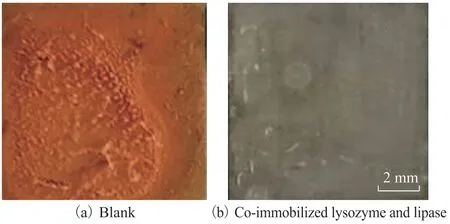
Figure 4 Carbon steel coupons in the absence and the presence of immobilized enzymes
3.3 Corrosion Inhibition performance of co-immobilized lysozyme and lipase in combination with chemical inhibitors
Table 1 reveals the corrosion inhibition efficiency (η) according to the experimental scheme. The inhibition efficiency (η) is obtained by Eq. (2).
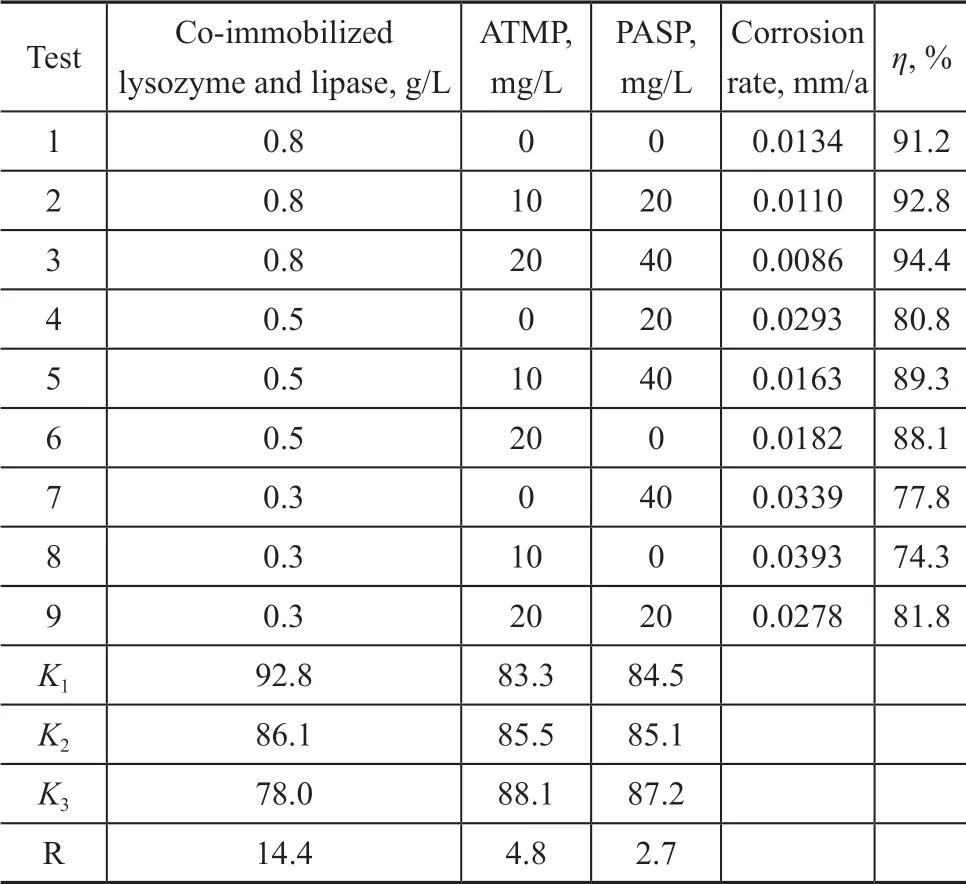
Table 1 Inhibition results of co-immobilized lysozyme and lipase in combination with ATMP and PASP
The maximum value of corrosion inhibition efficiency (94.4%) is obtained in Test 2, in which the concentration of co-immobilized lysozyme and lipase, ATMP and PASP is 0.8 g/L, 20 mg/L, and 40 mg/L, respectively. This inhibition efficiency is greater than that achieved by three inhibitors used alone. In the experiments, the coimmobilized lysozyme and lipase plays a decisive role on the corrosion inhibition process. Its range (14.4) is obviously lager than that of ATMP and PASP. Touir, et al.[21]found that with the increase of the corrosion inhibitor concentration, the adsorption of the corrosion inhibitor on the metal surface can be observed. As a result, these inhibitors can obviously alter the corrosion behavior, possibly owing to the formation of a uniform resistance layer, resulting in a greater reduction in corrosion.
3.4 Inhibition performance of co-immobilized lysozyme and lipase in combination with chemical inhibitors by electrochemical measurements
Based on the results shown in Table 1, the composite inhibitors with larger inhibition efficiency are selected for subsequent electrochemical tests.
In Figure 5, as time goes on,Eocpmoves towards the negative direction, which may possibly be caused by water molecules or inhibitor molecules on carbon steel surface. Compared with the blank test, the addition of coimmobilized lysozyme and lipase composite inhibitors can result in the increase ofEocp. The reason may be that the co-immobilized lysozyme and lipase composite inhibitors can affect the metal anode reaction[22].
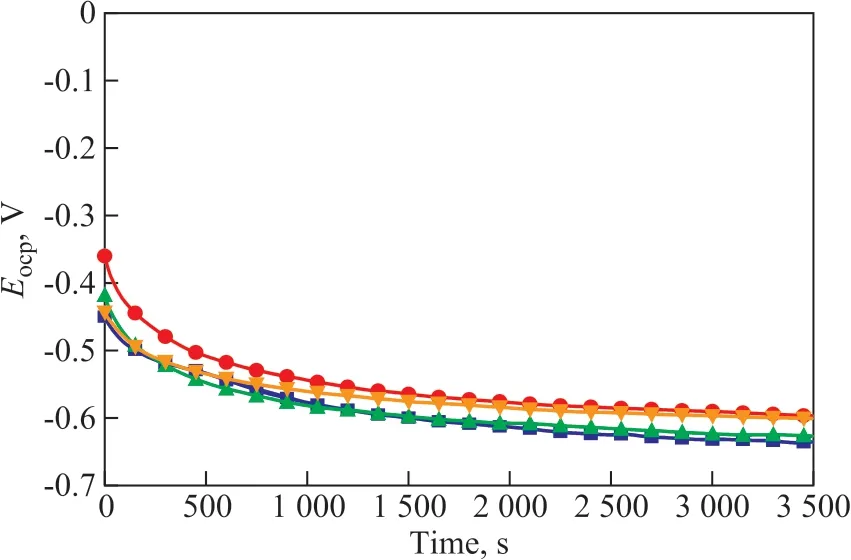
Figure 5 Change curve of Eocp with time
As shown in Figure 6, Nyquist plots of carbon steel in the blank only have a compressed capacitive arc and the corresponding Bode curve only has a time constant. This indicates that the corrosion of carbon steel in the blank cooling water is mainly controlled by the charge transfer process. After the addition of co-immobilized lysozyme and lipase composite inhibitors into the cooling water, the increase of capacitance arc diameter of Nyquist curve reveals that the inhibitor molecules are adsorbed on the surface of carbon steel to form a protective film. The formation of protective f ilm can reduce the active surface area of carbon steel and improve the corrosion resistance of carbon steel[9]. As shown in Figure 6b, for an ideal capacitor, the slope of the linear region is -1. However, the slope of this experiment is -0.80 to -0.88, which is obtained mainly due to the non-ideal interface structure from the OHP (outer Helmholtz plane) and diffusion layer, so that the equivalent circuit must be designed with a constant phase element instead of an ideal capacitor. A simple Randles circuit is used to fit the data, and the equivalent circuit is shown in Figure 7. The corresponding impedance of CPE (constant phase element) is obtained by Eq. (3)[23].
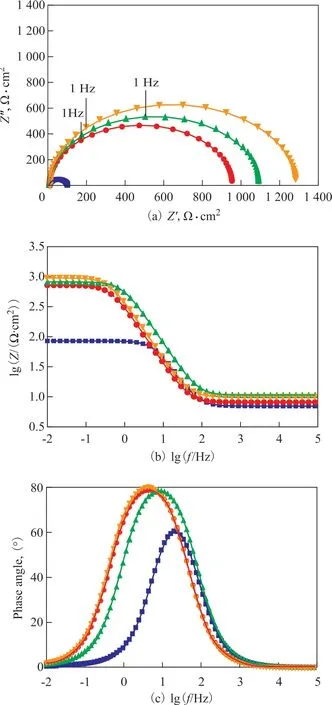
Figure 6 Nyquist (a), lgf–lgZ (b), and lgf–Phase angle (c) plots for carbon steel in the absence and in the presence of co- immobilized lysozyme and lipase in combination with ATMP and PASP
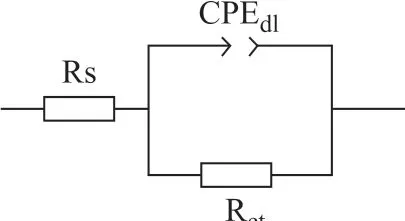
Figure 7 Equivalent circuit used to f it the impedance spectra in the absence and in the presence of investigated inhibitors

whereZCPEis the impedance of CPE, Ω·cm2;Qis the proportion factor of CPE, μSn/(Ω·cm2);ωis angular frequency(ω= 2πf);jis imaginary unit(j2= -1); andαis a change factor between 0 and 1.
TheQis proportional to the active area of the corrosion, but it does not represent the interface capacitance or the double layer capacitance (Cdl). Based on the fitting values ofQandRct, the correspondingCdlvalue can be obtained by Eq. (4)[24]. As shown in Table 2, the inhibition efficiency (η) is calculated from Eq. (5).
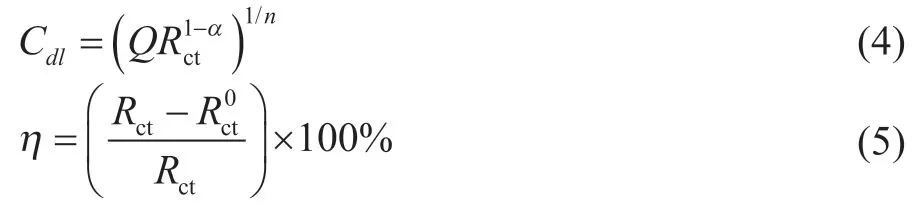
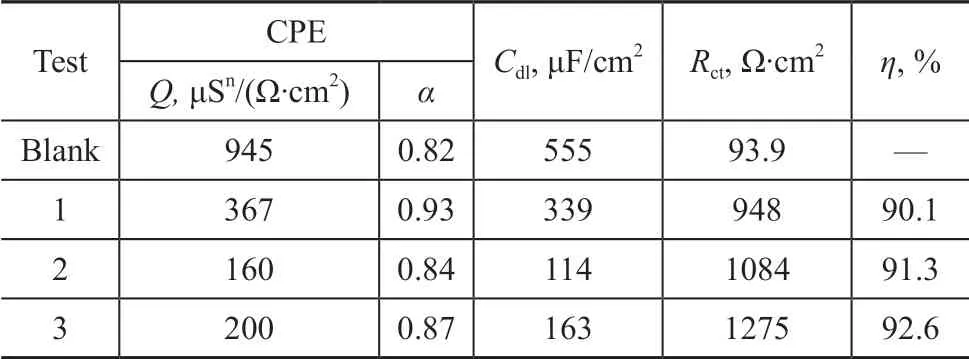
Table 2 Fitting parameters of EIS for carbon steel in the cooling water in the absence and in the presence of co-immobilized lysozyme and lipase in combination with ATMP and PASP
As shown in Table 2, after the addition of co-immobilized lysozyme and lipase composite inhibitors into circulating cooling water, the diameter of capacitive arc increases obviously. This indicates that the addition of inhibitor changes the dynamic process of corrosion on the surface of carbon steel. On the other hand, the shape of Nyquist curve is not changed, which indicates that the inhibition mechanism of the composite inhibitor does not change with the change in inhibitor composition. After the addition of corrosion inhibitor, the charge transfer resistanceRctincreases, and the value ofQandCdldecreases. This indicates that the co-immobilized lysozyme and lipase composite inhibitors are adsorbed on the surface of carbon steel and the formed adsorption f ilm can effectively inhibit the charge-transfer on the surface of carbon steel.
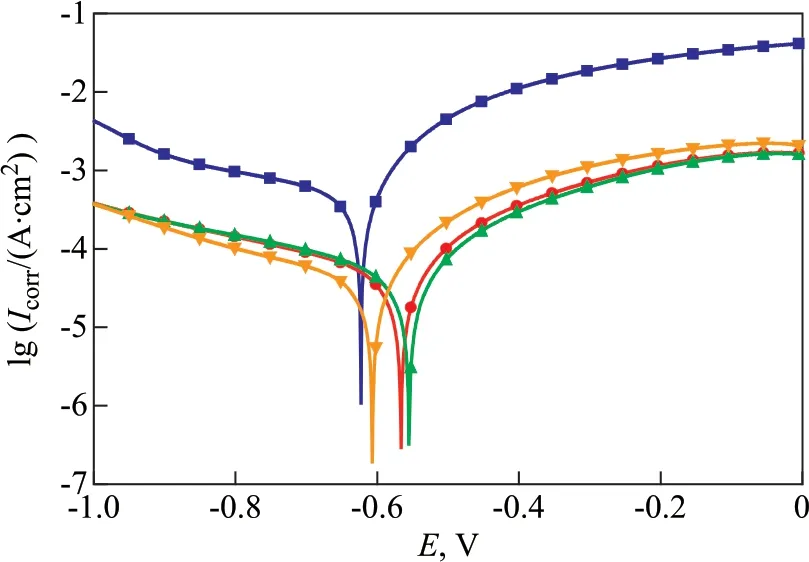
Figure 8 Polarization curves of carbon steel in the cooling water in the absence and the presence of co-immobilized lysozyme and lipase in combination with ATMP and PASP
Figure 8 shows the potentiodynamic polarization curves of carbon steel in the absence and the presence of co-immobilized lysozyme and lipase composite inhibitors. The relevant fitting parameters, such as the self-corrosion potentialEcorr, the corrosion current densityIcorr, the cathode Tafel slopeβc, the anode Tafel slopeβa, and the inhibition efficiencyη, are listed in Table 3. The inhibition efficiencyηis calculated by Eq. (6).

in whichis the corrosion current density of carbon steel in the blank test; andIcorris the corrosion current density of carbon steel in the presence of inhibitors.
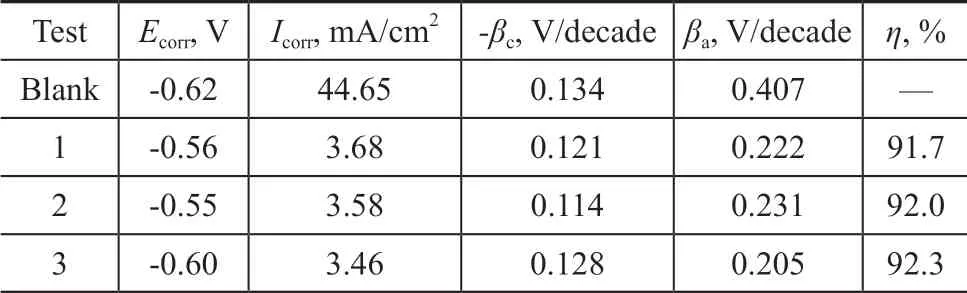
Table 3 Fitting parameters of polarization curve of carbon steel in the cooling water in the absence and in the presence of co-immobilized lysozyme and lipase in combination with ATMP and PASP
As shown in Figure 8, the addition of inhibitors simultaneously inhibits the anodic dissolution and the cathodic hydrogen evolution process of carbon steel. After the addition of inhibitors, there is no overlap segment between the polarization curves of corrosion inhibitors and the blank. It shows that the corrosion inhibitor shows a very slight desorption on the surface of carbon steel and the inhibitor can maintain its performance for a long time. The polarization curves of inhibitors and the blank have no overlap, confirming that the adsorption performance of inhibitors on the surface of carbon steel is better. In Table 3, after the addition of inhibitors into circulating cooling water,Ecorrhas a shift toward the anodic directly in comparison with the blank. In three tests, the maximum shift ofEcorris 70 mV, which is less than the criterion of the standard 85 mV[25-26]. So, these three inhibitors can be classified as the mixed-type inhibitors with predominant control of anodic reaction.
3.5 Study on adsorption
The inhibition effect of inhibitor on metal is achieved by the adsorption film formed on the metal/solution interface. The absorption of inhibitor molecules on the metal surface is achieved by replacing the adsorbed water molecules on the interface. In order to describe the process of replacing water molecules with corrosion inhibitor molecules, the adsorption isotherm can be fitted by Eq. (7)[27].

In whichCinhis the concentration of inhibitor, mol/L;θis the surface coverage, which is represented in this study by using the corrosion inhibition efficiency obtained via the weight-loss method; andKadsis the adsorption equilibrium constant.
The co-immobilized lysozyme and lipase composite inhibitor is used as the inhibitor in this part of experiments. As shown in Eq. (7), there is a linear relationship betweenCinhandCinh/θ.The relationship curve betweenCinhandCinh/θis displayed in Figure 9, with the related parameters listed in Table 4.
As shown in Figure 9 and Table 4, the correlation coefficient is 0.999 7, which proves that the adsorption behavior of corrosion inhibitor on carbon steel surface can be well described by the Langmuir model. Based on Eq. (8), the adsorbed Gibbs free energy can be calculated, and ΔGadsis equal to -28.58 kJ/mol.
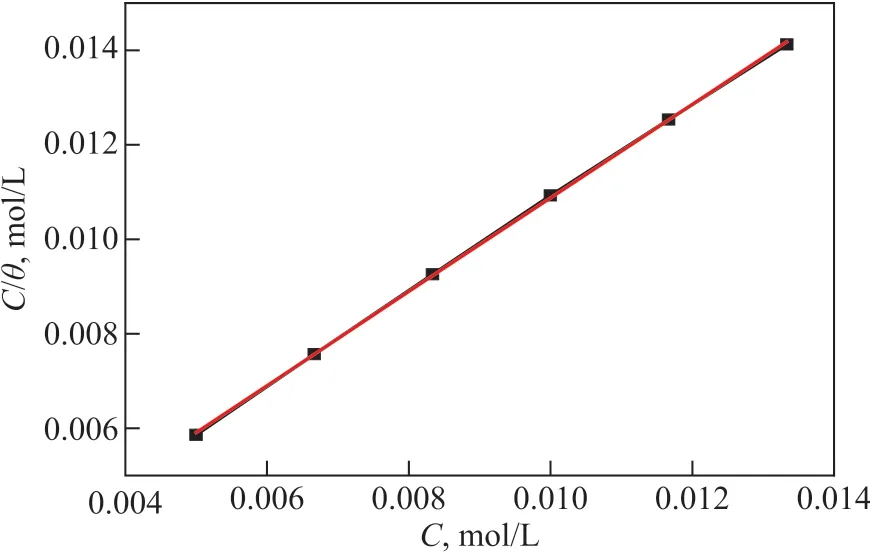
Figure 9 Adsorption isotherm of co-immobilized lysozyme and lipase composite inhibitor on carbon steel surface
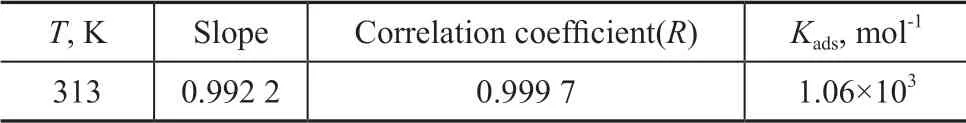
Table 4 Thermodynamic parameters of co-immobilized lysozyme and lipase composite inhibitor on carbon steel
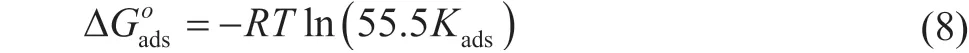
The value of ΔGadsis negative, which indicates that the adsorption of co-immobilized lysozyme and lipase composite inhibitor on the surface of carbon steel is spontaneous. When the absolute value of ΔGadsis less than 20 kJ/mol, it denotes a kind of physical adsorption realized by electrostatic interaction between the inhibitor molecules and device surface. When the absolute value of ΔGadsis more than 40 kJ/mol, it is a chemical adsorption of covalent bonds by charge sharing or transfer[28]. However, the value of ΔGadsis -28.58 kJ/mol during this experiment. It proves that the adsorption of coimmobilized lysozyme and lipase composite inhibitor on the surface of carbon steel is a joint action involving physical adsorption and chemical adsorption.
4 Conclusions
1) Characterization of immobilized enzyme reflects that enzyme is successfully immobilized on the mesoporous molecular sieve MCM-41. The co-immobilized lysozyme and lipase demonstrate good inhibition performance. The inhibition efficiency is 91.2%.
2) By OAD, the optimal composite inhibitors are composed of 0.8 g/L of co-immobilized lysozyme and lipase +20 mg/L of ATMP+40 mg/L of PASP. The corrosion inhibition efficiency reaches 94.4%.
3) The co-immobilized lysozyme and lipase composite inhibitor is adsorbed on the surface of carbon steel and the formed adsorption f ilm can effectively inhibit the chargetransfer on the surface of carbon steel. The addition of inhibitors can simultaneously inhibit the anodic dissolution and cathodic hydrogen evolution process of carbon steel.
4) The adsorption of co-immobilized lysozyme and lipase composite inhibitors on the surface of carbon steel is spontaneous coupled with a joint action involving physical adsorption and chemical adsorption.
Acknowledgement:This research was f inancially supported by Shandong Natural Science Foundation (ZR201702140013).
- 中國煉油與石油化工的其它文章
- Boosted Biodegradability and Tribological Properties of Mineral Base Oil by Methyl Diethanolamine Fatty Acid Esters
- Extractive Distillation of Methyl Acetate-Methanol Azeotrope Using [DMIM]DMP as Solvent
- Shortcut Method of Design and Energy-Saving Analysis of Sargent Dividing Wall Column
- Exploring the Degradation Potential of Halomonas Bacteria from Oil-contaminated Marine Environment
- Phenol Wastewater Degradation by Electrocatalytic Oxidation with RuO2-IrO2-SnO2/Ti Anodes in the High Gravity Field
- Evaluation of Powdered Activated Carbon Treatment Process in Petrochemical Wastewater Purification

