Optimization of the N2generation selectivity in aqueous nitrate reduction using internal circulation micro-electrolysis☆
Zhijuan Niu,Sitao Zhang,Yanhe Han, *,Mengmeng Qi
1 Department of Environmental Engineering,Beijing Institute of Petrochemical Technology,Beijing 102617,China
2 Research Center for Eco-Environmental Engineering,Dongguan University of Technology,Dongguan 523808,China
Keywords:Nitrate reduction Internal circulation micro-electrolysis Response surface methodology N2
ABSTRACT The reduction of nitrate using internal circulation micro-electrolysis technology(ICE)was investigated.The effect of the reaction time,initial pH,Fe/C ratio,and aeration rate on the nitrate reduction was investigated using a single factor experiment.Based on the results of the single factor experiment,a response surface methodology(RSM)was applied to optimize the N2generation selectivity.The effects and interactions of three independent variables were estimated using a Box-Behnken design.Using the RSM analysis,a quadratic polynomial model with optimal conditions at pH=8.8,Fe/C=1:1,and an aeration rate of 30 L·min-1 was developed by means of the regression analysis of the experimental data.Using the RSM optimization,the optimal conditions yielded a N2generation selectivity of 72.0%,which is in good agreement with experimental result(73.2%±0.5%)and falls within the 95%confidence interval(IC:66.8%-77.3%)of the model results.This indicates that the model obtained in this study effectively predicts the N2generation selectivity for nitrate reduction by the ICE process,thus providing a theoretical basis for process design.
1.Introduction
Accompanying the rapid development of the petrochemical industry,the concentration of nitrogen in wastewater has risen[1].This nitrogen is ultimately transferred into natural bodies of water,resulting in negative health effects,such as methemoglobinemia and stomach cancer,and causing eutrophication[2].In the European Union,the maximum allowable concentration ofin drinking water is 50 mg·L-1[3,4].In contrast,the limit in China and United States of America is 10 mg·L-1[5],while the World Health Organization sets thelimit as 3 mg·L-1[6].Therefore,the removal of nitrogen from petrochemical wastewater is crucial.Biological nitrogen removal technology is one of the most economical and efficient methods[7].Conventional biological methods of nitrogen removal involve two processes:aerobic nitrification and anaerobic denitrification[8].However,in comparison with chemical nitrification and denitrification,these two processes are slow because the rate of enzymatic denitrification is 30 times lower than that of chemical denitrification[9],which is also carried out in a larger reactor.Meanwhile,biological nitrogen removal technology is not suitable for the treatment of petrochemical wastewater,which has high nitrogen and low carbon contents[10].Therefore,alternative physical and chemical methods have been developed for nitrogen removal,e.g.,reverse osmosis,distillation,ion exchange,electrodialysis,catalysis,and electrochemical methods[11-15].
Iron-carbon micro-electrolysis(IE)is regarded as an effective method for the treatment of industrial wastewater[16-19].It is also effective for removal offrom industrial wastewater that has a low carbon-nitrogen ratio(C/N)[20].However,practically,the IE process has some disadvantages,e.g.,compaction,blockages,and passivation,which reduce the processing efficiency[21,22].Moreover,the discharge and reloading of the iron-carbon mixture not only waste time and financial resources but also interrupt continuous operation when the iron chips are consumed.Therefore,an internal circulation microelectrolysis(ICE)reactor has been developed[21].The ICE reactor can overcome the compaction,blockages,and passivation,which usually occurred during the IE process.But the effect of iron dissolution on the efficiency of the ICE process is unavoidable for a long operation.When there is a significant decrease for the efficiency of the ICE process,the iron-carbon mixture might not need to be discharged and reloaded like the IE process,but a proper raw iron need only be added into the central tube to improve the efficiency.This is another advantage of the ICE reactor.The ICE reactor consists of two sections:the oxidation section in the inner loop tube and the reduction section between the inner loop tube and the outside wall of the ICE reactor[21],which provides favorable conditions for nitrogen removal.can be reduced to several stable soluble(e.g.,)or volatile species(e.g.,N2,NO2)[23].N2is the preferred product because the other products can have direct or indirect effects on human health[24].Therefore,the selective reduction ofto N2is preferred,and this can be achieved by optimizing the operation conditions of the ICE process.
To enhance the N2generation selectivity of thereduction during the ICE process,the response surface methodology(RSM)was applied to optimize the operation parameters.Before carrying out the optimized experiments,single factor experiments were performed to determine the most important operating parameters for the optimized experiments.The results obtained from the optimized experiments provide useful information on the selective denitrification of nitrogencontaining wastewater that has a low C/N ratio.
2.Methodology
2.1.Materials
Iron chips were obtained from our school's metalworking practice factory.These iron chips were first passed through a 10-mesh sieve,and then the undersize chips were sieved by a 16-mesh sieve.The iron chips less than 10-mesh and greater than 16-mesh were selected for this study.The selected iron chips were first treated with a 10%NaOH solution for 2 h to remove the surface grease,then dipped in a 5%hydrochloric acid solution for 30 min to remove any surface oxidation products,and finally rinsed with tap water until the pH was close to neutral.Granular activated carbon(GAC,1-4 mm)was purchased from the Sinopharm Chemical Reagent Beijing Co.,Ltd.In order to keep the similar size for the iron chips and the GAC,the GAC was sieved by the same screening procedure for the iron chips.The screening GAC was cleaned with tap water before use and then soaked in the preparedsolution for 72 h to eliminate any effects of adsorption[21].Thesolution was prepared using NaNO3(Beijing Chemicals,China,analytically pure),and a dilute NaOH solution was used to adjust the pH of the nitrate solution.
2.2.Experimental procedure and methods
The ICE process was carried out according to our previously described method[21].In brief,the ICE reactor consisted of a body and a central tube.The nitrate solution was fed into the ICE reactor through an inlet distributor and discharged from the iron-carbon bed.Air was introduced into the central tube from below.The iron-carbon mixture was sprayed up into the central tube,agitated by the air bubbles,and fell back to the bed.The treated solution was collected from an overflow-weir.Batch experiments were performed to determine the influence of the most important operating parameters,such as the reaction time,initial pH,iron-carbon(Fe/C)ratio,and aeration rate,affecting the reduction of nitrate using ICE technology.Three sample groups were used for all experiments.The samples,obtained after a given interval,were filtered through 0.22μm syringe filters.Then,the concentrations of nitrate and nitrite in the filtrates were determined using ion chromatography(Dionex ICS-1000 system).Ammonia was detected by a UV-vis spectrophotometer(UV-2600,Shimadzu,Japan)at a wavelength of 420 nm using Nessler's reagent spectrophotometry.Thegeneration(G),the N2generation(N2G),and the N2generation selectivity(SN2)were calculated according to Eqs.(1)-(3),respectively.


2.3.RSM experimental design for the optimization of parameters
Box-Behnken designs(BBD)are experimental designs for RSM,devised by George E.P.Box and Donald Behnken in 1960,for the modeling,analysis,and optimization of a response of interest.A quadratic polynomial relationship between the response variable and a group of autonomous test factors was created to optimize the conditions,as shown in Eq.(4).
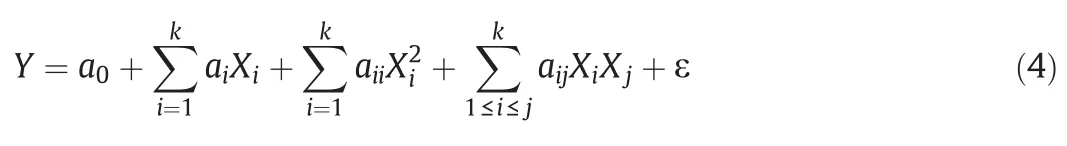
Here,Y is the predicted response(in this study);k is the number of factors;a0,ai,aii,and aijare regression coefficient constants of the developed model for the constant term(the first order terms,quadratic terms,and interaction terms,respectively);and Xiand Xjrepresent the independent variables in the form of coded values,while ε is the experimental residual.
Based on the results of the single-factor experiments,the pH,Fe/C ratio,and aeration rate were selected as three key factors for the RSM experimental design.was selected as the response of interest.Their coded and experimental estimates are listed in Table 1.
3.Results and Discussion
3.1.Effect of the operation time
The effect of operation time on nitrate reduction during the ICE process was investigated at[]0=1500 mg·L-1,Fe/C=1:1,pH=6.5,T=20-25°C,and aeration rate=25 L·min-1.The various nitrogen species in aqueous solution were determined during the denitrification treatment using the ICE process,as shown in Fig.1.As observed,the removal efficiency of nitrate increases rapidly within 60 min,and then is close to 80%after 180 min reaction time.Nitrate can be reduced into nitrite(as shown in Eq.(5)).Hence nitrite was also detected in this study,but its concentration slowly increases(less than 2.5%)in the first stage of the micro-electrolysis,subsequently disappearing.It is likely that nitrite is an unstable intermediate of the nitrate reduced by the ICE treatment process,which is easily reduced into ammonium ion(as shown in Eq.(6))or nitrogen(as shown in Eq.(7))in the solution.Therefore,the concentration of ammonium ions increased with increasing reaction time in the first stage of the micro-electrolysis,which is mainly due to the reduction of nitrite or the direct reduction of nitrate by Fe or H2produced during micro-electrolysis(as shown in Eqs.(8)and(9))[25].Subsequently,the ammonium concentration decreased slightly withincreasing reaction time.This may be due to the adsorption of ammonium ion on the surface of the activated carbon.
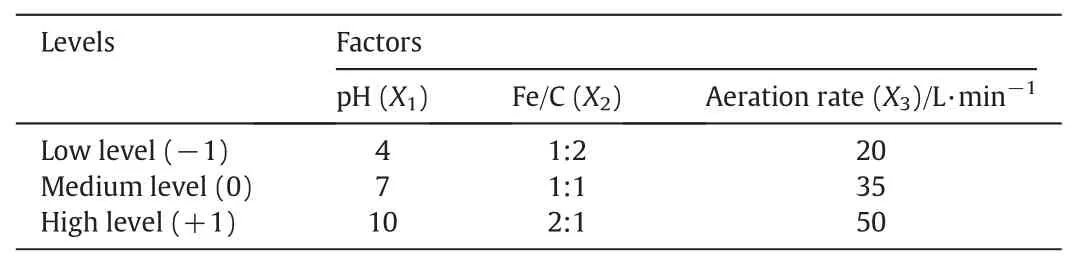
Table 1 Independent variable and levels used for RSM design
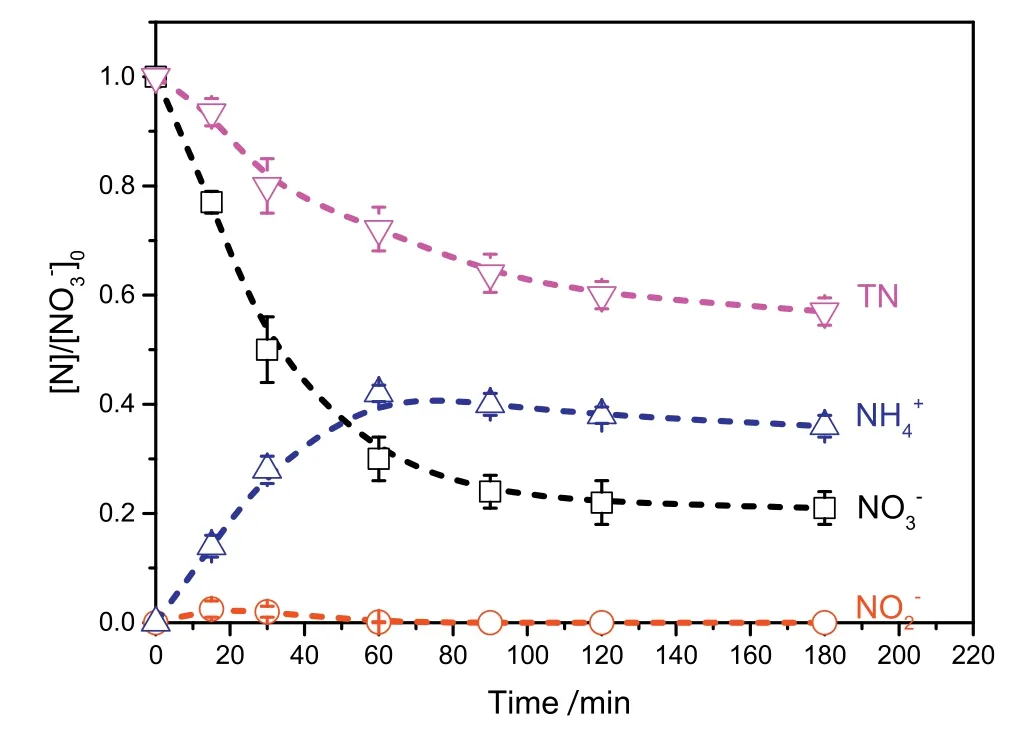
Fig.1.Effect of the operation time on the concentration of nitrogen species(normalized to the initial concentration([]0))during the micro-electrolysis reaction in the innerloop IE reactor at[]0=1500 mg·L-1,Fe/C=1:1,pH=6.5,T=20-25 °C,and aeration rate=25 L·min-1.
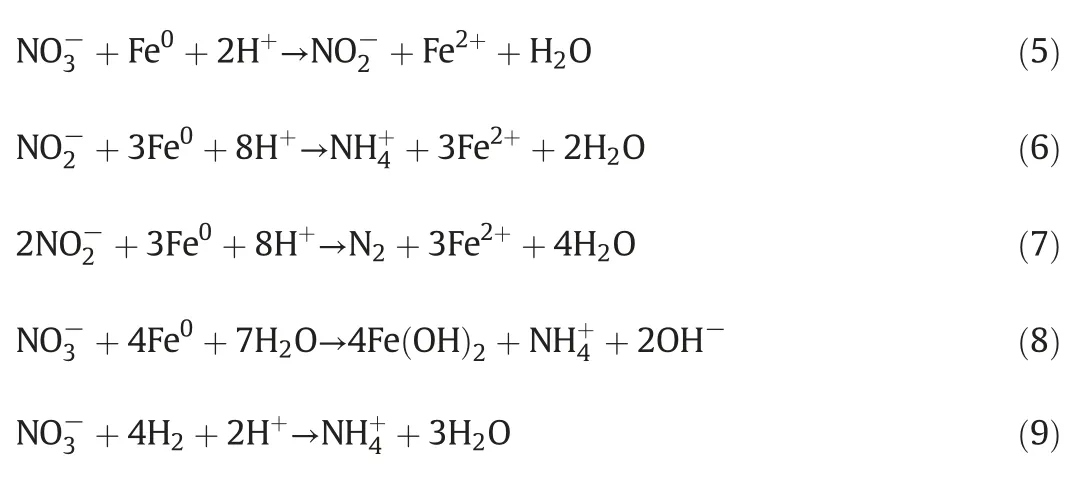
The amount of total nitrogen(TN)for all nitrogen species produced during the ICE micro-electrolysis reduction in solution was detected by total organic carbon(TOC)detectors,and the results are shown in Fig.1.The TN decreased to 57%after 180 min micro-electrolysis treatment,while about 43%of the nitrogen species was undetectable in solution.This discrepancy may arise from the generation of gaseous compounds,such as N2,that are lost from the ICE reactor.Unfortunately,these products have not been identified or analyzed because of a shortage of lab instrumentation.As shown in Fig.1,the concentrations of nitrogen species at 120 min reaction time are similar to those at 180 min.Therefore,the subsequent experiments were carried out for 120 min.
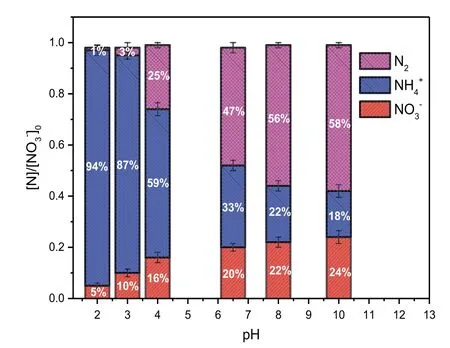
Fig.2.The effect of pH on the concentration of nitrogen species(normalized to its initial concentration([]0))during the micro-electrolysis reaction in the inner-loop IE reactor with[]0=1500 mg·L-1,Fe/C=1:1,reaction time=120 min,T=20-25°C,and aeration rate=25 L·min-1.
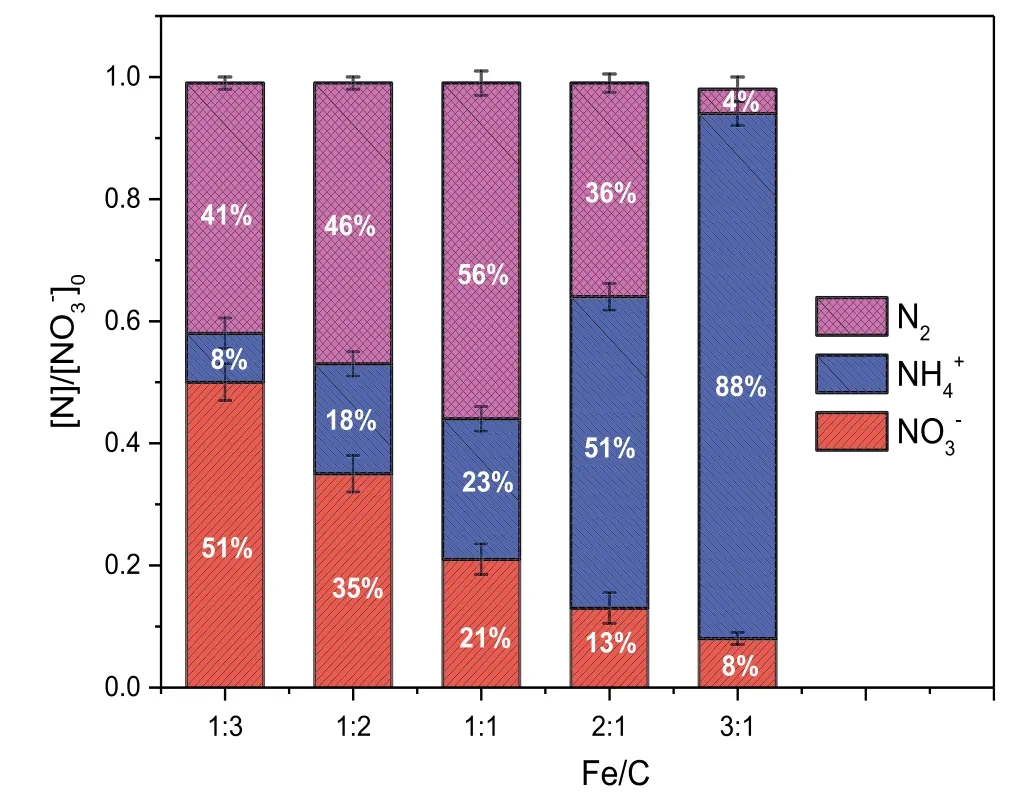
Fig.3.The effect of Fe/C on the amount of nitrogen species(normalized to the initial concentration([]0))during the micro-electrolysis reaction in the inner-loop IE reactor with[]0=1500 mg·L-1,pH=8.0,reaction time=120 min,T=20-25°C,and aeration rate=25 L·min-1.
3.2.Effect of pH
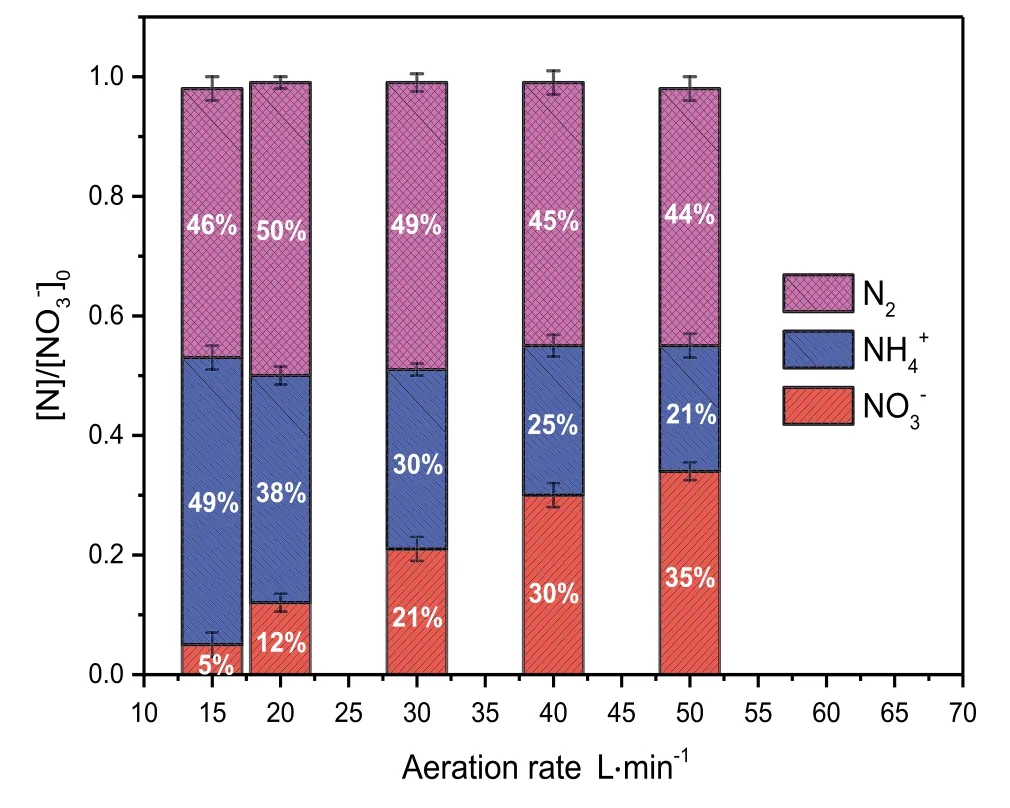
Fig.4.Effect of aeration on the amount of nitrogen species(normalized to the initial concentration([]0))during the micro-electrolysis reaction in the inner-loop IE reactor with[]0=1500 mg·L-1,Fe/C=1:2,pH=8.0,reaction time=120 min,and T=20-25°C.
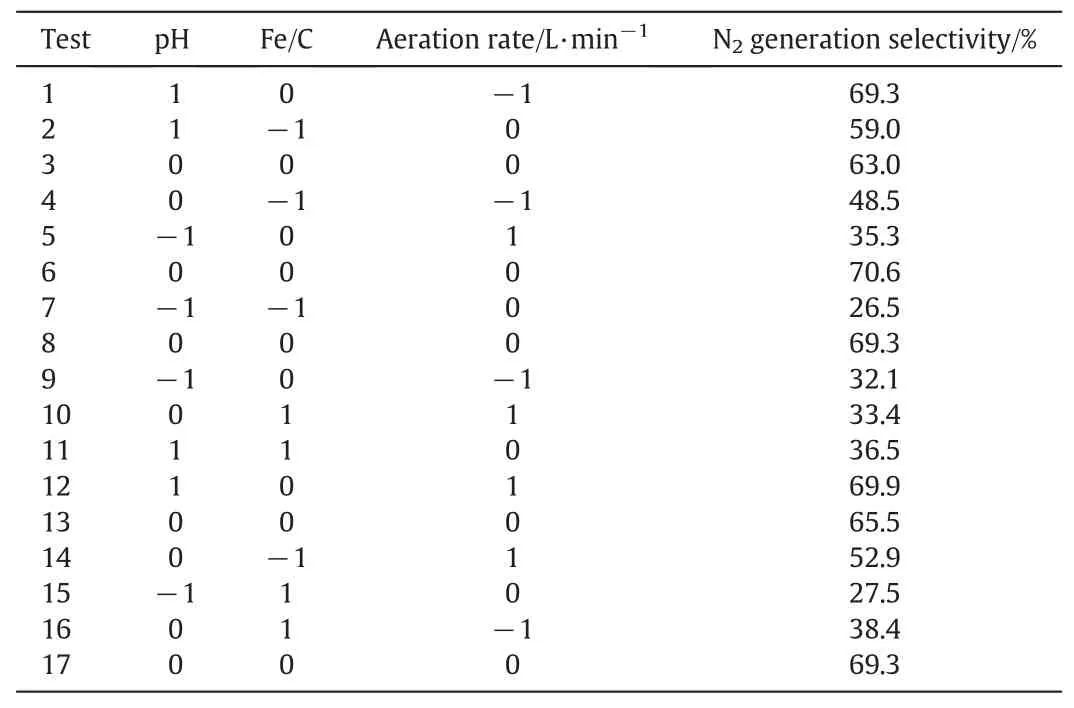
Table 2 The BBD matrix of three variables along with the related experimental response
The pH frequently fluctuates in water and wastewater,and this is a common factor affecting the degradation of pollutants by a given process.Therefore,the effect of pH on the reduction of nitrate during the ICE reaction was investigated,and the results are shown in Fig.2.Because the amount of nitrite was undetectable over the whole pH range after 120 min reaction,the nitrite concentration is not shown in Fig.2.The amount of N2was calculated from the initial concentration ofminus the final concentration ofFig.2 shows thatreduction by ICE micro-electrolysis decreases with increasing initial pH.Theconcentration decreases continuously with increasing initial solution pH.While the N2gas generation shows the reverse tendency;these results are similar to those of Xu et al.[23].The reduction of nitrate into nitrogen gas increased quickly from 1%to 56%when the pH changed from 2 to 8.Then,a slight increase of nitrogen gas generation was observed at pH 10(58%),indicating that the pH has a considerable influence on the selective reduction of nitrate into nitrogen gas by ICE.This can be explained by the greater micro-electrolytic activity on the iron surface under acidic conditions,which results in the formation of more hydrogen for the reduction of nitrate into ammonia during ICE.Under alkaline condition,the generation of hydrogen was restricted,inducing that the reduction reaction of nitrate into ammonia was also restricted.However,nitrate can be reduced into N2under alkaline condition by Eq.(10)[26],inducing the increasing generation of N2.Therefore,the alkaline condition is benefit for the generation of nitrogen.
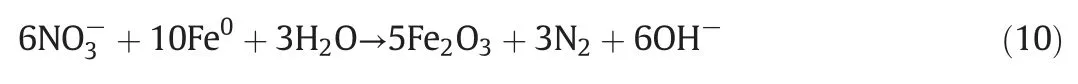
3.3.Effect of the Fe/C ratio
The activity of a galvanic cell containing Fe and carbon is related to the Fe/C ratio,which affects the efficiency of micro-electrolysis for the degradation of pollutants.The effect of the Fe/C ratio on the reduction of nitrate is shown in Fig.3.The reduction rate of nitrate increased from 49%to 91%as the Fe/C ratio increased from 1:3 to 3:1,while the ammonia formation increased from 8% to 88%.Interestingly,the N2gas generation increases as the Fe/C ratio increases from 1:3,reachinga maximum at an Fe/C ratio of 1:1(from 41%to 56%).As the Fe/C ratio increased from 1:1 to 3:1,the generation of N2gas decreased from 56%to 4%.At a low Fe/C ratio,excess activated carbon(AC)may block the contact between iron andresulting in less reduction ofon the iron surface.And the less active sites of iron available for nitrate reduction are provided by decreasing iron chips.In contrast,a high Fe/C ratio bringsinto contact with Fe,which is beneficial for the reduction ofand the generation ofThe nitrate in aqueous solution is reduced to the intermediate product nitrite,finally yielding ammonia or nitrogen after reduction by zero-valent metals[27-29].However,the reduction mechanism of nitrate using a composite zero valent metals and AC is the same as that for zero-valent metals alone,except in two important ways:the amount of removed nitrate increased with the presence of AC as the catalyst support,and the amount of ammonium released differed because of adsorption of ammonium onto the activated carbon.The introduction of AC not only increases the generation of hydrogen that plays a very important role for the reduction of nitrate but also prevents the accumulation of ammonium ions released from the reduction of nitrate by the zero-valent metal by the adsorption action of AC;thus,further treatment is not required for the reduction of nitrate[30].These factors caused the generation of N2gas to increase at first and then decrease with increasing Fe/C ratio.
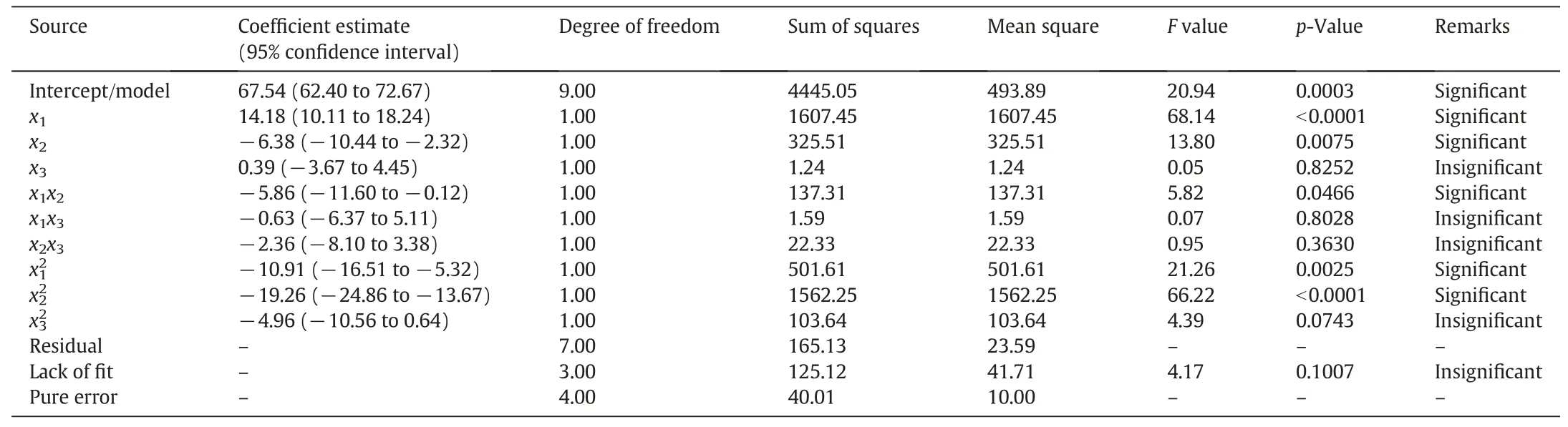
Table 3 The coefficients and ANOVA results of N2generation selectivity with a function of pH,Fe/C,and aeration rate
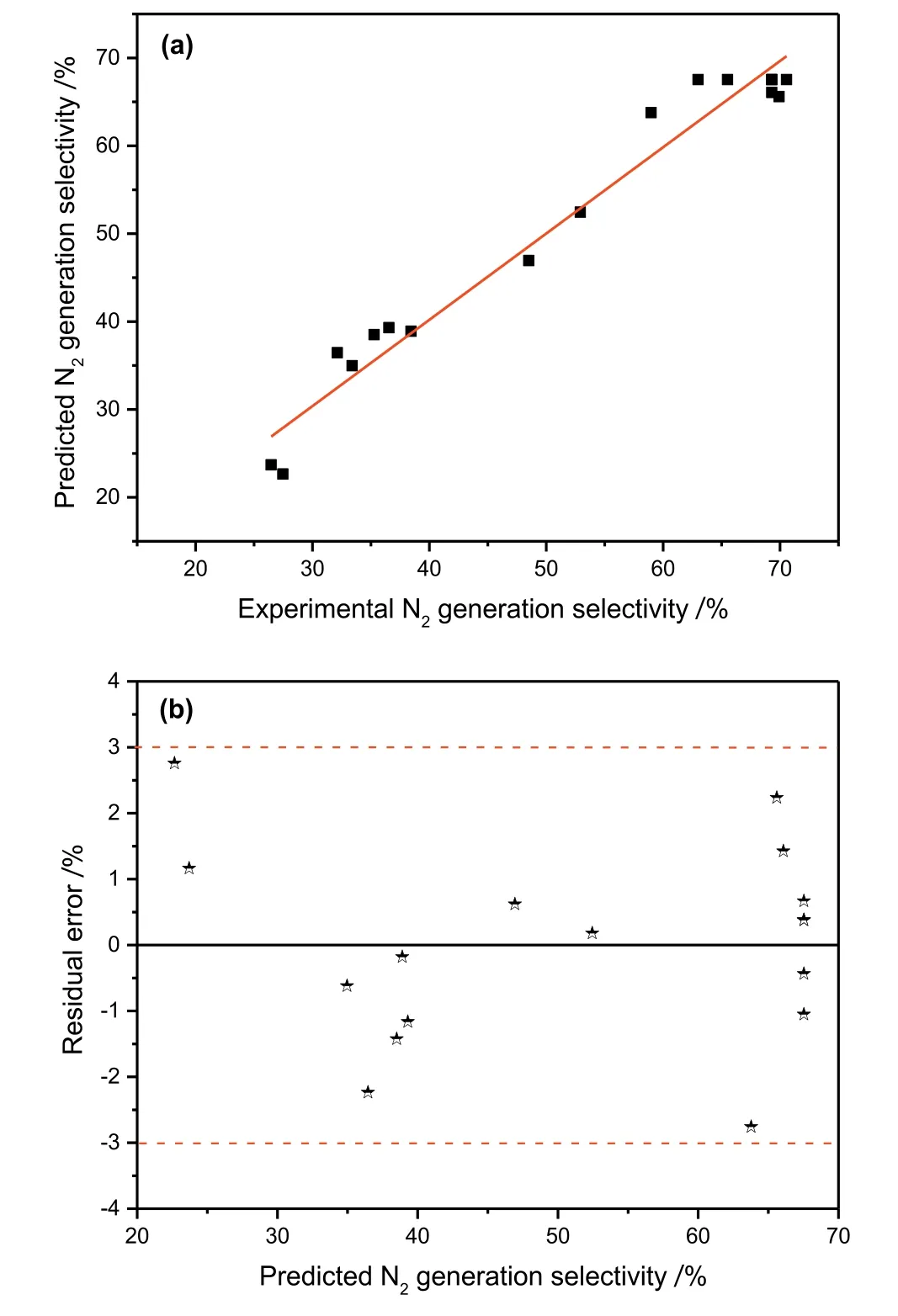
Fig.5.(a)The correlation between the predicted and experimental values of N2generation selectivity.(b)The percent error as a function of predicted N2generation selectivity.
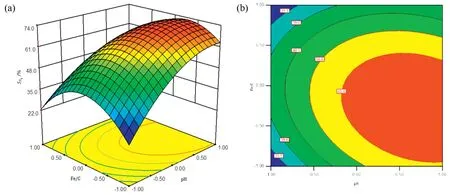
Fig.6.(a)3D response surface and(b)2D contour plots of the as a function of pH and the Fe/C ratio.
3.4.Effect of the aeration rate
As previously reported,to circulate the iron-carbon mixture in the interior of the ICE reactor,the minimum aeration rate should be 12.5 L·min-1.Therefore,in this study,the aeration rate was controlled to between 15 and 50 L·min-1.The results of the reduction of nitrate are shown in Fig.4.The reduction efficiency ofdecreased from 95%to 65%as the aeration rate increased from 15 to 50 L·min-1.In contrast,the generation ofalso decreased from 49%to 21%with increasing aeration rate.Aeration increases the amount of dissolved oxygen,which raises the oxidation and reduction potentials.However,high oxidation and reduction potentials are not useful for the reduction ofand the generation of[31].Fig.4 shows that the generation of N2gas is not significantly with aeration rate;in contrast,the generation ofdecreased gradually with increasing aeration rate.This indicates that aeration has a negligible effect on the generation of N2,while it has a significant effect on the generation of
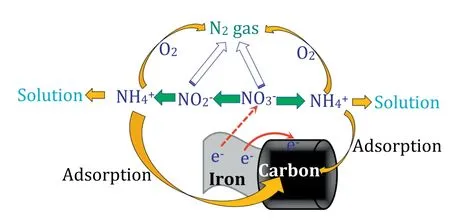
Fig.7.Schematic of the possible mechanism of nitrate by the ICE process.
3.5.Response surface analysis
3.5.1.Development of the regression model equation and analysis
The experimental response results forbased on the BBD matrix are listed in Table 2.The coefficients of the BBD response function were obtained from a quadratic polynomial model correlated with the experimental data.The fitting model and the respective coefficients in terms of coded factors are given by Eq.(11).
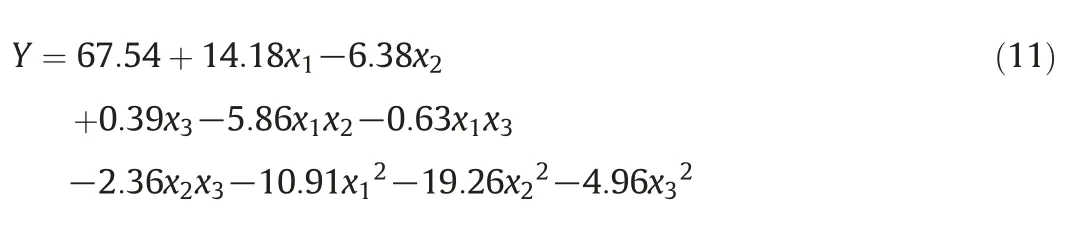
The analysis of variance(ANOVA)shown in Table 3 was carried out with Fisher's statistical test,which can estimate the suitability of the response model and the significance of the effects of the variables.If the probability values(p-values)of the variables in the response are less than 0.05,the variables are statistically significant.According to the ANOVA test,the p-value of the second-order polynomial equation(0.0003)is less than 0.05,indicating that this model can adequately represent the actual relationship between theand the independent variables.While the F-value of the model was 20.94,indicating that the model was statistically significant.The first-order and quadratic term variables,except aeration rate(x3)andwere statistically significant variables forduring the reduction of nitrate because their p-values are relatively low(p<0.05)as shown in Table 3.The interaction terms except x1x2were found to be insignificant,and their p-values are high(p>0.05).This indicates that the only interaction affectingis that between the pH and Fe/C during the reduction of nitrate by ICE.The coefficients of determination(R2and adjusted R2)and the standard error of estimate can be used to evaluate the goodness-of-fit of the regression model.The R2value indicates that the model can explain 96.4%of the variablefor the reduction of nitrate.The value of the adjusted R2(91.8%)is more suitable for comparing models with variable independent variables.The Adeq precision measures the signal-tonoise ratio,and a value greater than 4 is desirable.The ratio of 12.05 in this case indicates an adequate signal for the model,indicating that the regression model equation can predict the required values.The parity and residual plots,which are shown in Fig.5,can be used to verify the accuracy of the model.As shown by Fig.5(a),the predicted and experimental values forare almost identical,suggesting that the regression model can be used to predictAs shown in Fig.5(b),the maximum percent error is less than 3%,indicating that there is a good correlation between the predicted and the experimental values of
3.5.2.Interactive effects of variables on
The response surface plots can be used to assess the interactions between the response value and the independent variables.Fig.6 shows the two-dimensional(2D)contour plots and three-dimensional(3D)response surface plots for the interaction between the pH and the Fe/C ratio,which were obtained from the model-predicted response by changing two variables within the experimental range and keeping the other one at the midpoint of the testing range.Theincreased with increasing initial pH,indicating that pH plays a significant role in the reduction of nitrate by ICE.At low pH,although more hydrogen,which are strongly reducing,is formed,which is beneficial for the reduction of nitrate,the nitrate is more easily reduced toresulting in a decrease inis determined by subtracting the final concentrations of nitrate,ammonia,and nitrite from the initial concentration of nitrate.Therefore,the volatile loss of ammonia at high pH,which is caused by aeration,may result in an increase inHowever,ammonia was not detected in the tail gas adsorption solution of sulfurous acid.This indicated that the basic conditions are conducive to the formation of N2gas,which has also been shown by other researchers[32,33].As the Fe/C ratio increased,theincreased at first and then decreased gradually after reaching a peak value.These results are the same as the results obtained from the single factor experiments.Because the p-value is lower than 0.05,the results in Table 2 suggest that there is an interaction between the pH and Fe/C ratio,which affects
3.5.3.Confirmation and validation
The predicted optimized results obtained from Eq.(8)were verified by additional experiments in triplicate at the optimized levels.The optimalof 72.0%(95%confidence intervals of 66.8%-77.3%)was obtained at a pH of 8.8,Fe/C ratio of 1:1,and an aeration rate of 30 L·min-1at a reaction time of 120 min.Theobtained from the experiment was 73.2%±0.5%,which agrees well with the predicted results.This indicates that the RSM method can be used to optimize the operational conditions of the nitrate reduction in the ICE process.
3.6.Mechanism of nitrate reduction using the ICE process
It is known that the reaction process on the solid surface consists of adsorption,reaction and desorption.According to the literatures,two possible mechanisms were proposed for the nitrate reduction by iron.The first one is direct transfer of electrons from iron,as shown in Eqs.(5)-(7).The second one is indirect electron transfer by the hydrogen atoms generating during the corrosion,as in Eq.(8).The introduction of activated carbon can accelerate the iron reduction because the formation of the microscopic galvanic cells[34],being benefit for the reduction of nitrate.Meanwhile the activated carbon can adsorb the ammonium ions released from the reduction of nitrate.In conclusion,Fig.7 shows the schematic of the possible mechanism of nitrate by the ICE process.These results show that AC plays a very important role during the reduction of nitrate by the ICE process.
4.Conclusions
The effect of operating conditions on nitrate reduction was investigated using ICE technology.The nitrate reduction depends mainly on pH,Fe/C,and aeration rate within a certain reaction time.The optimization of the conditions for the selective reduction of nitrate in aqueous solution was investigated by RSM along with the Box-Behnken design method.The pH,Fe/C,and aeration rate were selected as important influencing factors for the optimization ofduring the reduction of nitrate with the ICE process.The optimal conditions obtained from the predicted model for the nitrate-selective reduction to N2gas were at a pH of 8.8,Fe/C of 1:1,and an aeration rate of 30 L·min-1with theof 72%.The calculated values by the RSM model are in good agreement with the experimental values,indicating that the RSM can be used to optimizefor nitrate reduction using iron-carbon microelectrolysis.The ICE process provides favorable conditions for the reduction of nitrate,especially under the alkaline environments.
 Chinese Journal of Chemical Engineering2019年12期
Chinese Journal of Chemical Engineering2019年12期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
- Preparation and characterization of a novel antibacterial acrylate polymer composite modified with capsaicin☆
- Controlling pore structures of Pd-doped organosilica membranes by calcination atmosphere for gas separation☆
- Relationship between equilibrium potential and radius of lanthanides electrolyzed on the zinc cathode
- Effect of gas composition on nitric oxide removal from simulated flue gas with DBD-NPC method☆
- Solubility and mass transfer of H2,CH4,and their mixtures in vacuum gas oil:An experimental and modeling study☆
