Preparation and characterization of a novel antibacterial acrylate polymer composite modified with capsaicin☆
Jianhui Zhou ,Xiaotong Zhang ,Ying Yan,Jianfeng Hu, *,Hang Wang,Yan Cai,Jinqing Qu
1 State Key Laboratory of Advanced Power Transmission Technology,Global Energy Interconnection Research Institute Co.Ltd.,Beijing 102209,China
2 School of Chemical Engineering,University of Birmingham,Edgbaston,Birmingham B15 2TT,UK
3 School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China
Keywords:Capsaicin Acrylate polymer Solution polymerization Antibacterial
ABSTRACT A novel antibacterial acrylate polymer composite modified with capsaicin was successfully synthesized by a two-step reaction.Capsaicin and acryloyl chloride were firstly esterified,and then applied to solution polymerization with acrylate monomers and styrene.The yield of the esterified products was about 85.3%.The polymer was characterized by Fourier transform infrared spectroscopy(FTIR),gel permeation chromatography(GPC),thermogravimetric analysis(TGA),contact angle(CA)and antibacterial ring tests.The number-average molecular weight(Mn)of the polymer was 27214,based on the capsaicin-acrylate dosage of 6.5 wt%.The TGA revealed a stable thermal property.The contact angles of the polymers films on tinplate increased from 77.5°to 86.2°with the increasing amount of capsaicin-acrylate.The antibacterial tests demonstrated excellent antimicrobial capability of the polymers.
1.Introduction
Marine biofouling is defined as the accumulation of bacteria,microalgae,diatoms and invertebrates on the surface of natural and artificial substrata on seawater[1],which bring about enormous economical losses.For instance,marine biofouling on ships increases frictional resistance and slows down speed,leading to increased fuel consumption,maintenance costs and green-house gases emissions[2,3].Utilizing antifouling coatings to prevent or decrease the growth of fouling organisms,including bacteria is still the most effective method[4,5].Acrylic resin is widely applied as base resin in marine antifouling coating[6-9]for its strong adhesion,great weather-resistance,corrosion resistance and antifouling properties.
Emulsion polymerization and solution polymerization were popularly used in the synthesis of acrylic resins.However,some performances such as poor water resistance,poor adhesion,low temperature brittleness and high temperature viscosity of latex acrylic resins cannot fulfill various demands[10-12].On the contrary,solventborne acrylic resins can overcome the flaw in hardness,water resistance,acid and alkali resistance and stain resistance due to the formation of compact films with the evaporation of solvent[13-16].Hydrophobicity and stain resistance make it possible for solventborne acrylic resins to be applied in marine antifouling coatings.Yet,bad mechanical property,poor solvent resistance and high cost limited their further application[17,18].So the modification of acrylic resins to promote the properties has been paid more attention.
Some additives were added in the polymerization of acrylates to improve the antifouling property of acrylic polymers.Copper,zinc and their oxides were usually grafted to raise the bactericidal and antifouling performance[6,19-22].Furthermore,phosphonium cations were also incorporated in acrylate polymers to prepare biocidal polymeric materials[23].Although,an acrylic resin complexed by tertiary amines had a notified biocidal efficacy[24],it is harmful to the marine environment with the sustained release of biocides for Cu and P being toxic to the marine organism[3].So,seeking an eco-friendly alternative additive is a major challenge.Capsaicin,a pungent vanillylamide alkaloid extracted from chili and pepper,was reported with natural antifungal and antibacterial[25-29].Its chemical structure was showed in Schematic 1.Compared with other antifoulants,capsaicin can not only decrease the attachment of marine microorganism,but also be eco-friendly to biodiversity,environment and human health.Some papers[30-33]covered that the application of capsaicin in coatings by blending or polymerization was effective in antibacterial and antifouling field,which indicated a promising prospect in marine antifouling.Some researchers[30,34]focused on the synthesis of monomers and polymers containing capsaicin analogs such as N-(2-hydroxyl-3-methyl acrylamide-4,6-dimethyl benzyl)acrylamide(HMDA).Nevertheless,there are few reports about the synthesis and characterization of t acrylate monomer that capsaicin reacted with acryloyl chloride.Compared with the direct mixing,chemical modification with capsaicin provides a long-period,and release control strategy.
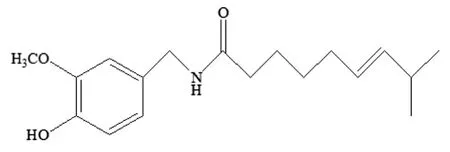
Schematic 1.Chemical structure of capsaicin.
Therefore,the aim of this study is to prepare a novel antibacterial acrylate polymer composite based on capsaicin by solution polymerization.The properties of the polymers such as molecular weight,thermal stability and contact angles are ready to be investigated.
2.Experimental
2.1.Materials
Capsaicin(98%)was purchased from Shaanxi Sciphar Natural Products Co.,Ltd.(China).Acryloyl chloride(AC,≥96%)was purchased from Aladdin.Triethylamine(TEA,≥99.0%),the catalyst of esterification,and methyl methacrylate(MMA,≥98.0%)were purchased from Shanghai Lingfeng Chemical Reagent Co.,Ltd.(China).Acrylic acid(AA,≥99.5%)and butyl acrylate(BA,≥99.0%)were purchased from Tianjin Fuchen Chemical Reagent Co.,Ltd.(China).Styrene(St,≥98.0%)and n-butyl acetate(99.0%)were purchased from Tianjin Damao Chemical Reagent Co.,Ltd.(China).Dichloromethane(≥99.5%),the solvent of esterification,was obtained from Guangdong Guanghua Chemical Reagent Co.,Ltd.(China).Sodium hydrogen carbonate(≥99.5%)and 2,2′-azobis(isobutyronitrile)(AIBN,≥99.0%)were purchased from Tianjin Kermel Chemical Reagent Co.,Ltd.(China),and AIBN was used as the initiator of polymerization.DMSO-d6(99.9%,Cambridge Isotope Laboratories,Inc.)was used as the solvent of NMR.Deionized water was used in all experiments in this work.All the reagents were used without further purification.
2.2.Reaction mechanism
The reaction mechanism was exhibited in Schematic 2.First,esterification between the acid chloride bond of acryloyl chloride and the phenolic hydroxyl group of capsaicin was carried out.The unsaturated bond in acryloyl chloride was thereby introduced into the macromolecule monomer.The obtained sample was then polymerized with acrylic acid,butyl acrylate,methyl methacrylate and styrene in n-butyl acetate.
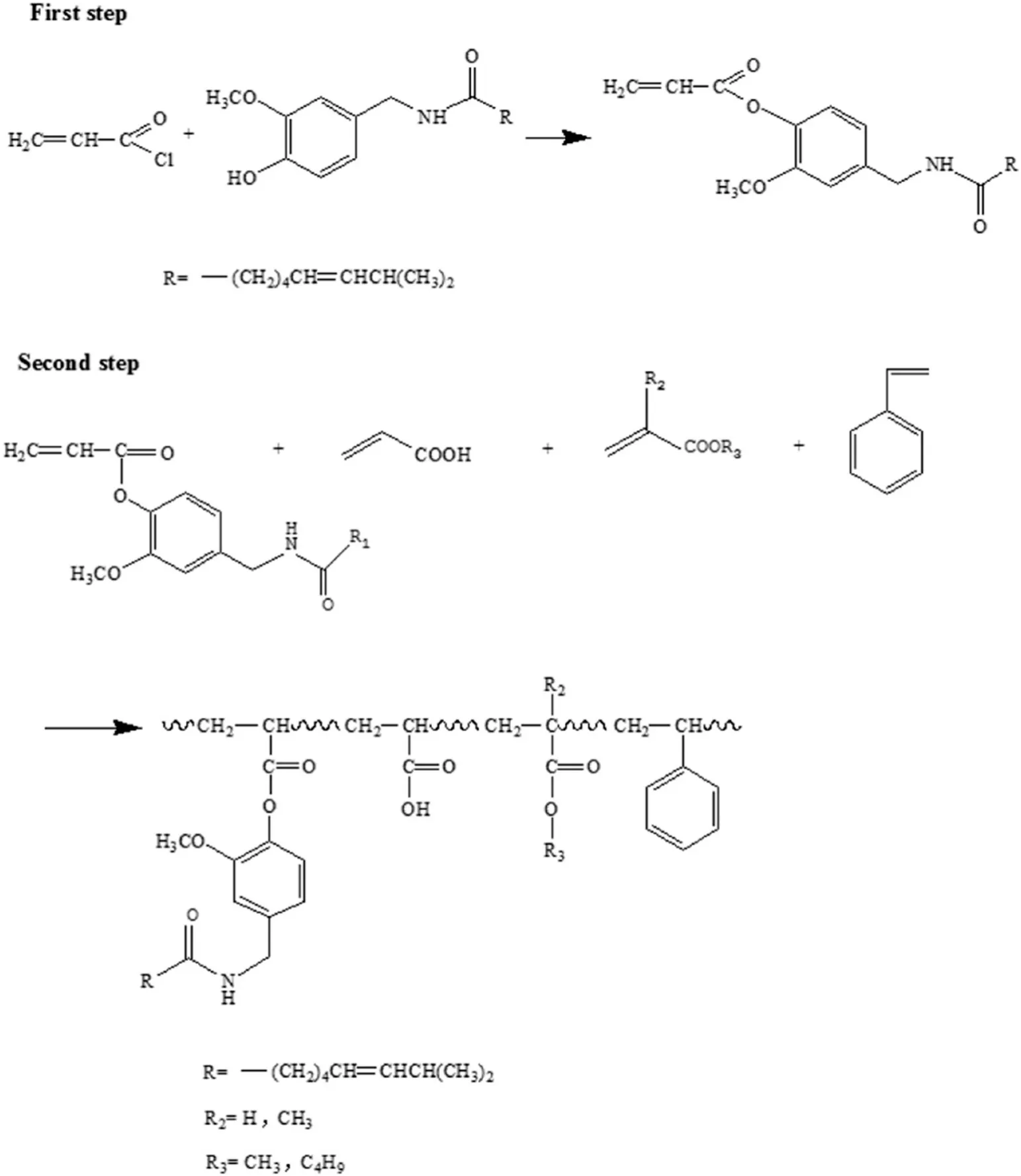
Schematic 2.Reaction equations for acrylate polymer composite modified with capsaicin.
2.3.Preparation of acrylate polymer
The acrylate polymer based on capsaicin was prepared by a two-step reaction,involving esterification and solution polymerization.
Esterification was carried out between capsaicin and AC.The solution of capsaicin(5 g,0.016 mol)and TEA(3.73 g,1.5 eq of AC)resolved in 30 ml CH2Cl2was put in a 250-ml two-necked round-bottomed flask equipped with a stirrer.The solution of AC(2.23 g,1.5 eq of capsaicin)resolved in 5 ml CH2Cl2was added dropwise at 2-4 °C.The mixture was stirred from 2 to 4°C to room temperature and reacted about 24 h.
After reaction,the mixture was filtered and washed by saturated aqueous of NaHCO3and deionized H2O.The solvent was removed by rotary evaporation to furnish a crude mixture.Further purification was carried out by column chromatography and using hexane and ethyl acetate(the volume ratio was 1:1)as eluents.
The synthesis was carried out in a four-necked round-bottomed flask equipped with a reflux condenser,stirrer,thermometer and peristaltic pump.A part of n-butyl acetate(30 g,as the solvent for the polymerization)was added in the flask and heated to 70 °C.3.5 g of capsaicinacrylate,2 g of AA,20 g of BA,25 g of MMA,3 g of St(the contents were displayed in Table 1)and 0.35 g of AIBN were dissolved in n-butyl acetate(20 g)and then gradually added into the system for 3 h by a peristaltic pump.After the feeding,the mixture was stirred for further 1 h.Then,solution of AIBN(0.15 g)in n-butyl acetate(5 g)was supplemented in the flask to react homogeneously.Finally,the polymerization was maintained for 1 h and then cooled down to room temperature.
2.4.Characterization
The product of esterification reaction was purified by column chromatography.The yield was calculated as follows

where Y(%),mW(g)and mT(g)respectively represented the yield,weight of weighing and theory of the capsaicin-acrylate in Eq.(1).
The chemical structure of capsaicin-acrylate monomer and polymer were determined by Fourier transform infrared spectroscopy(FTIR)on a Tensor 27 spectrophotometer(BRUKER,Germany)with samples in KBr pellets.All the spectra were scanned from 4000 to 400 cm-1and measured at a resolution of 4 cm-1.32 scans were performed for each spectrum.
1H-NMR and13C-NMR spectra(40 MHz for1H-NMR and 100 MHz for13C-NMR)were recorded on an AVANCE III 400 MHz spectrometer(BRUKER,Switzerland)using deuterated dimethyl sulfoxide(DMSO-d6)as solvent and tetramethylsilane(TMS)as an internal standard.
ESI-HRMS(high resolution mass spectrometer)spectra were obtained on a maxis impact mass spectrometer(BRUKER,Germany).
The elemental analysis about the capsaicin-acrylate monomer was carried out by the elemental analyzer Vario EL cube(ELEMENTAR,Germany).C,H,N and S of the monomer were detected.
Molecular weight and molecular weight distribution of the polymers were determined by gel permeation chromatography(GPC)using Waters Co(USA),equipped with a refractive index detector(Waters 2414),an injector(Waters 717),an HPLC pump(Waters 515)and three Styragel columns(particle size:10 μm).Tetrahydrofuran(THF)was used as an eluant at a flow rate of 1 ml·min-1.And the concentration of the sample was 20 mg·ml-1.
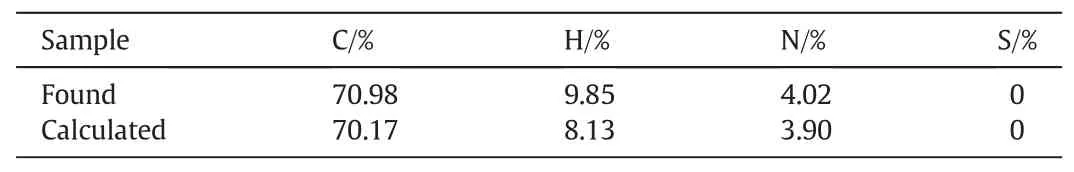
Table 1 The elemental analysis of capsaicin-acrylate
Thermogravimetric analysis(TGA)was conducted on a TA Instruments SDT Q600(TA Inc.,USA).The polymers were determined under nitrogen and heated from 25 to 600°C at a heating rate of 10°C·min-1.
The solution of polymers was coated on the surface of tinplate using an applicator of 60 μm.After drying,the contact angle of water droplets on the films was measured by JC2000C1 Powereach 1(Shanghai Zhongchen)at 25 °C.10 μl of water droplets was used and average values were obtained from at least 4 times of measurements on different regions of the same sample.
The antibacterial ring tests were carried out in vitro to determine the bacteriostatic activity against Staphylococcus aureus(S.aureus)of the polymers.The bacteria were inoculated in sterilized culture medium.The sterilized filter papers with polymer solutions were put in the Petri dishes.The diameters of antibacterial rings were measured after cultivating for 24 h at(37±1)°C.
3.Results and Discussion
3.1.The characterization of capsaicin-acrylate monomer
3.1.1.Elemental analysis
Some white or faint yellow powder was obtained.The morphology of capsaicin-acrylate was displayed in Fig.1.The yield of capsaicin-acrylate monomer was about 85.3%,calculated by the amount of capsaicin.
The Elemental analysis of capsaicin-acrylate was listed in Table 1.The discrepancy between the found and calculated may be because the raw material capsaicin is not pure.
3.1.2.FTIR analysis

Fig.1.Photograph of capsaicin-acrylate.
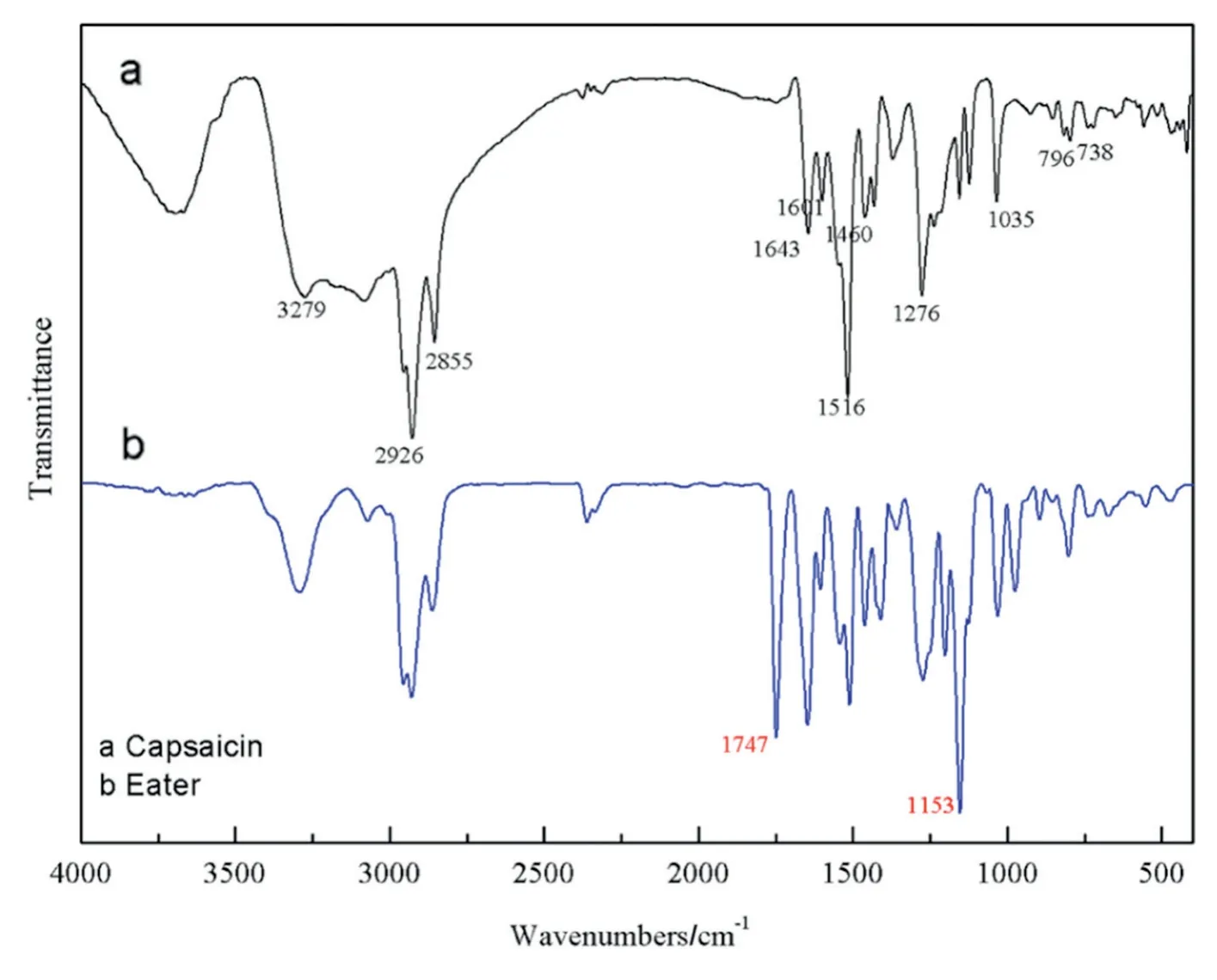
Fig.2.FTIR spectra of capsaicin(a)and capsaicin-acrylate(b).
FTIR spectra of capsaicin(a)and capsaicin-acrylate(b)are shown in Fig.2,respectively.In Fig.2(a),the presence of an aromatic C--C stretching band at 1643,1601,1516 and 1460 cm-1[34,35]and aromatic C--H bending band at 796 and 738 cm-1confirms the presence of aromatic ring of capsaicin[5].The peaks at 2926 and 2855 cm-1correspond to aliphatic C--H stretching of CH2and CH3[36,37],correspondingly.The peaks at 1035 and 1276 cm-1can be assigned to the C--O--C[38]stretching of methoxy group in aromatic ring.The CO stretching vibration at 1645 cm-1overlaps with aromatic CC stretching[34].And the peaks at 3279 cm-1can be ascribed to N--H[39]stretching in the group of NHCO.All of the above stretching and bending peaks indicate the chemical structure of capsaicin.Compared with capsaicin,the spectrum of capsaicin-acrylate(Fig.2(b))shows the strong characteristic stretching vibration of CO at 1747 cm-1,as well as the characteristic stretching vibration of C--O--C at 1153 cm-1[40,41].This can be attributed to the generation of ester bond.Other peaks of spectrum(b)almost are same with(a).The characteristic peaks of the product coincide with the peaks of acryloyl chloride and capsaicin.That means,the product was composed of the two reactants.The results implied the occurrence of esterification between capsaicin and acryloyl chloride.
3.1.3.NMR analysis
The1H-NMR spectrum of capsaicin-acrylate is shown in Fig.3.The spectrum in Fig.3 can be summarized as follows:1H-NMR δ:8.31(--NHCO--,capsaicin),7.05-6.82(aromatic ring protons,capsaicin),6.52-6.48(CH2CH--,AC),6.42-6.36(CH2CH--,AC),6.13-6.10(--CHCH--CH(CH3)2,capsaicin),5.36-5.33(--CH2--CHCH--,capsaicin),4.27-4.26(--CH2--NH--,capsaicin),3.74(CH3O--Ar,capsaicin),2.17-1.24(--CH2--,capsaicin),0.94-0.83(CH3--,capsaicin)[42-45].The1H-NMR spectra of solvent and water were at 2.5 and 3.33,respectively.The chemical shifts of capsaicin-acrylate are from the groups of monomers of acryloyl chloride and capsaicin.The results of1H-NMR spectra also supported the report that esterification happened between capsaicin and acryloyl chloride.
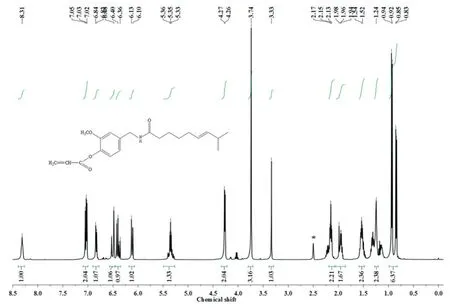
Fig.3.1H-NMR spectrum of capsaicin-acrylate.
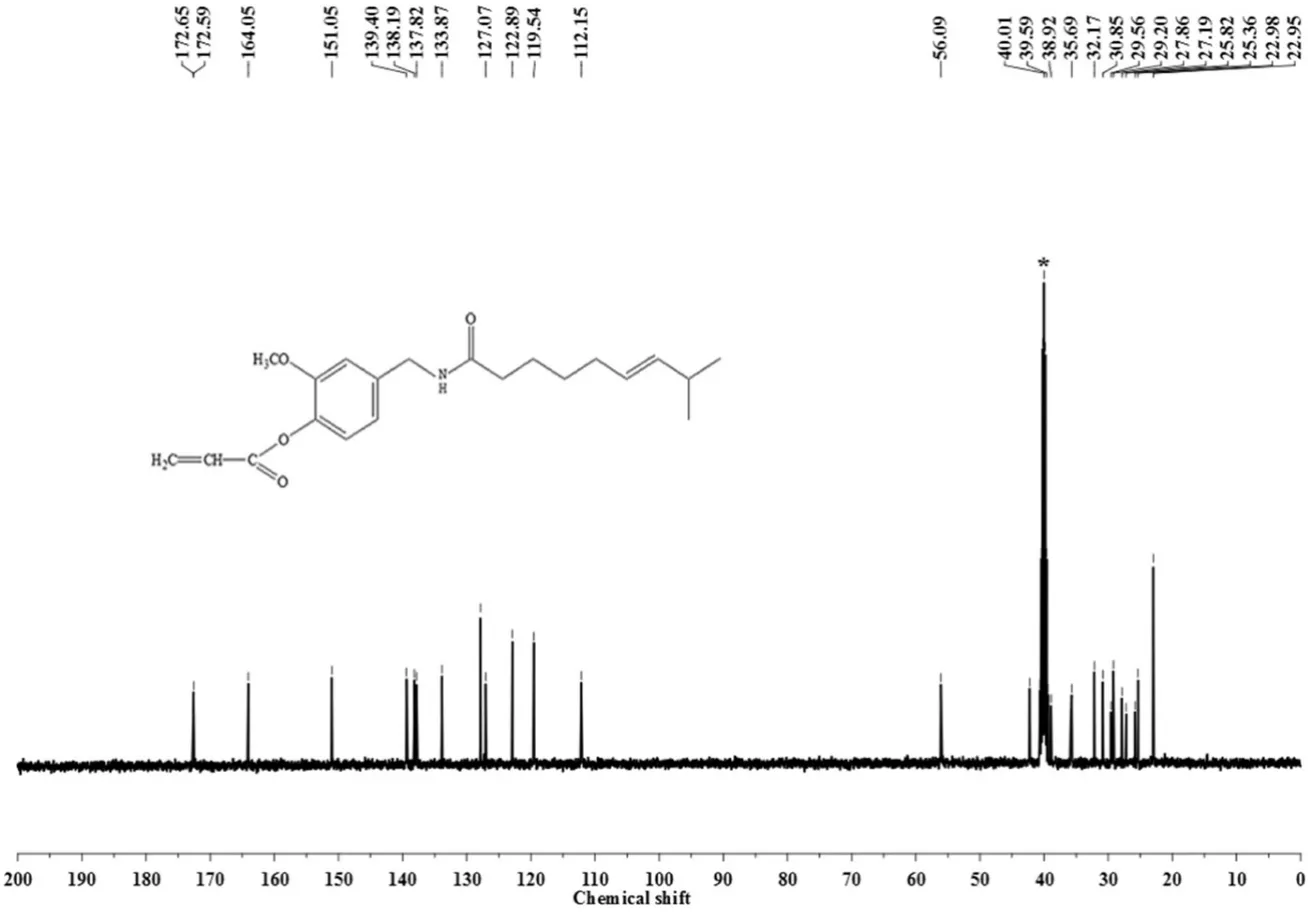
Fig.4.13C-NMR spectrum of capsaicin-acrylate.
Fig.4 shows the13C-NMR spectrum of capsaicin-acrylate.13C-NMR δ:172.65-172.59(--CONH--),164.05(--COO--),151.05(aromatic C adjacent to--OCH3),139.40(aromatic C adjacent to--CH2NH--),138.19-137.82(aromatic C adjacent to--COO--),133.87(CH2CH--COO--from AC and--CHCH--CH(CH3)2from capsaicin),122.89-112.15(the other three aromatic C without adjacent to others),56.09(CH3O--),42.25(--CH2--NH--),40.63-39.38(DMSO-d6),38.92-22.95(--CH,--CH2,--CH3)[42,43].The obvious peak of ester group at 164.05 revealed the compound synthesized was ether.The results further confirmed the esterification reaction.
3.1.4.HRMS analysis
The high resolution mass spectrometry results of capsaicin(a)and the ester(b)were shown in Fig.5.The molecular weight of capsaicin measured is 316.1887 and the theoretical(C18H27NO3)is 305.1991.The discordance may be caused by the impurity.The HRMS of capsaicin-acrylate shows m/z=370.1998([M+H]+),which coincided with the measured results of capsaicin.The molecular weight of synthetic substance is the sum of acryloyl chloride and capsaicin except the molecular weight of Cl and H.In brief,the HRMS further confirmed the successful synthesis of acryloyl chloride and capsaicin.
3.2.Characterization of polymer composite synthesized by capsaicin-acrylate monomer
3.2.1.FTIR analysis
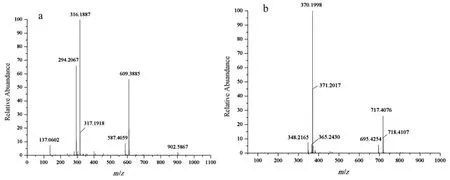
Fig.5.HRMS of capsaicin-acrylate.(a):Capsaicin,(b):capsaicin-acrylate.
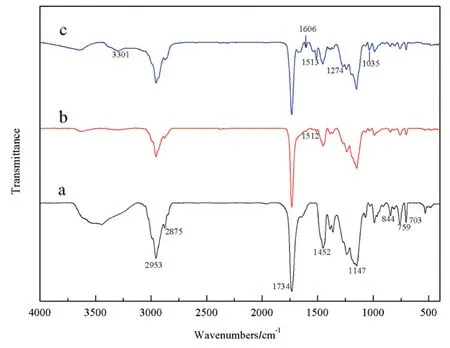
Fig.6.FTIR spectra of the polymer(a:capsaicin-acrylate at 0 wt.%,b:capsaicin-acrylate at 6.5 wt.%,c:capsaicin-acrylate at 12.3 wt.%).
The FTIR spectra of the polymer compounded by capsaicinacrylate,AA,BA,MMA and St are shown in Fig.6.Fig.6(a),(b),(c)represents the spectra of the polymer without capsaicinacrylate,the polymer based on capsaicin-acrylate at 6.5 wt% and the polymer based on capsaicin-acrylate at 12.3 wt%,separately.There are bands at 2953 cm-1and 2875 cm-1in all spectra due to C--H stretching vibration of CH2and CH3[36,37],respectively.The presence of the stretching vibration of CO at the peak of 1734 cm-1and stretching vibration of C--O at the peak of 1147 cm-1associated with carbonyl ester groups is also noted[40,41].The ester groups exist in BA,MMA and capsaicin-acrylate.The aromatic C--C stretching at the peak of 1452 cm-1[34,35]and aromatic C--H bending band at 844,759 and 703 cm-1[5]confirm the existence of aromatic ring in the polymers which may be from St or capsaicin-acrylate.The inexistence of CC stretching vibration at 1680-1640 cm-1confirms the occurrence of polymerization.Compared with the polymer control(a),the peaks(N--H stretching at the peak of 3301 cm-1,aromatic CC stretching at 1601 and 1513 cm-1,C--O--C stretching of methoxy group in aromatic ring at 1274 and 1035 cm-1[37])attributed to capsaicin-acrylate are more distinct with the increase of capsaicin-acrylate monomer.
3.2.2.NMR analysis of the polymers
The NMR spectra of polymer are shown in Fig.7.In the1H-NMR spectrum(a),the signals at δ=3.04,1.87 and 0.94 correspond to protons in the main chain of the polymer.The aromatic protons from St and nonivamide-acrylate unit are respectively at δ=7.17 and 6.92.And the rest shifts of protons are all from branch chain.For example,δ=8.24(NHCO--),3.84(--OCH3),4.39(--CH2--NH--),and--CH2--at 2.26,2.04,1.53,and 1.34 are from nonivamide-acrylate.And the peaks at δ=4.04 and 3.65 are due to--CH from BA and MMA,separately.
In the13C-NMR spectrum(b),δ:170.50-177.71(CO from ester bond or--CONH--);126.40-128.45(aromatic C);30.34-44.38(C--H in main chain of polymer);20.22-22.51,13.38 and 51.48(--CH3);16.66-19.06,25.77 and 64.20(--CH2--).
The results of NMR indicate that all the monomers participated in reaction and the polymers was as expected.
3.2.3.Molecular weight analysis
Fig.8 shows the molecular weight and the molecular weight distribution of polymers.The high molecular weight demonstrates the occurrence of polymerization.In Fig.8,the polymers prepared in the presence of capsaicin-acrylate exhibited a molecular weight distribution spreading to a higher molecular weight,compared with that prepared without capsaicin-acrylate.The large increase of molecular weight states that capsaicin-acrylate was involved in the polymerization.In Fig.8(b)and(c),the number-average molecular weight of polymer increased from 27214 to 28861 with the capsaicin-acrylate increasing from 6.5 wt%to 12.3 wt%.The small increase of molecular weight could be owing to the low concentrations or the incomplete consumption of initiator[46,47].The polydispersity index(PDI=Mw/Mn)was about 1.7,indicating an ideal range of molecular weight distributions[5,48].
3.2.4.Thermal properties
The thermal properties of polymers were determined by thermogravimetric analysis,TGA.In Fig.9,the polymers are stable with negligible mass loss before 300°C,and then start thermal degradation rapidly at 320°C[41].After 440°C,the mass loss of polymers is terminal and most of the polymers have been burnt out.This decomposition temperature is typical of acrylic polymers[49].The polymers have a good thermostability for they almost do not decompose under 320°C.
3.2.5.Contact angles
In Fig.10,the contact angles of the polymers based on 0 wt%,6.5 wt% and 12.3 wt% of capsaicin-acrylate are 77.5°,81.6° and 86.2°,individually.The obvious incremental trend with the increase of capsaicin-acrylate indicated that the addition of capsaicin-acrylate contributes to the water repellence of acrylic polymers.The contact angles exceeded 70°,which was indicative of good water repellence[12].It can be speculated that the capsaicin-acrylate is attributed to the hydrophobicity of polymers,which may be because capsaicin is a hydrophobic monomer and cannot dissolve in water.The polymers synthesized based on capsaicin have potential application prospect in hydrophobic materials.
3.2.6.Antibacterial ring results
The microbial activity of the polymers against S.aureus was investigated by the disc diffusion method.Each experiment was performed in triplicate and the result was displayed in Fig.11.The average values of the diameter of antibacterial rings were exhibited in Table 2(the diameter of the filter paper is 5 mm).The antimicrobial activity is directly proportional to the diameter of antibacterial rings.Compared with the n-butyl acetate without tested compounds,the polymers present significant antibacterial activity,especially the polymer based on capsaicin-acrylate at 6.5 wt%.But the antibacterial activity didn't increase respectively,when the capsaicin-acrylate increased.It may be because the range of circle is the limit of capsaicin-acrylate diffusion.The polymer prepared without capsaicin-acrylate also performs antibacterial activity from Fig.11.
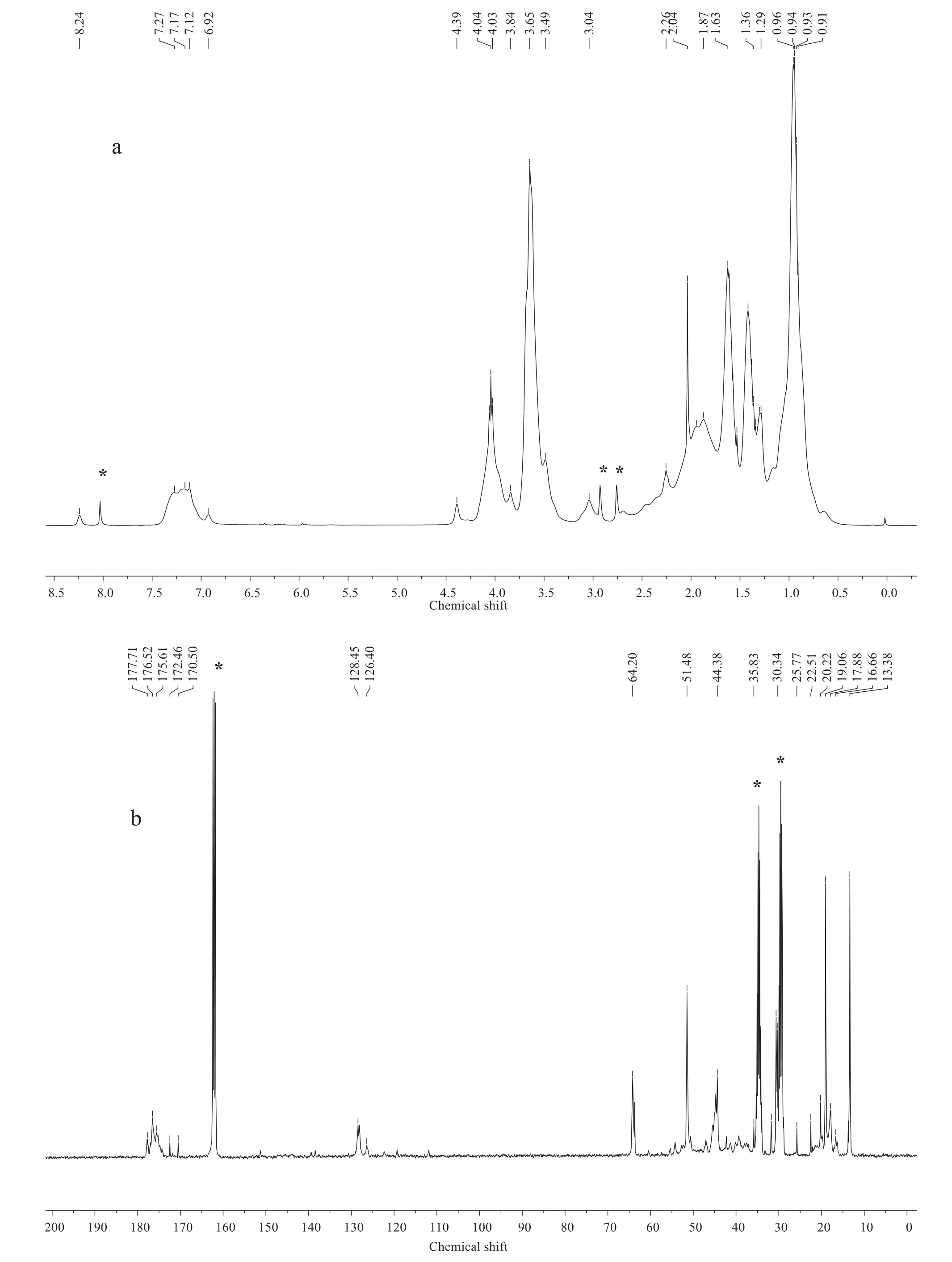
Fig.7.1H-NMR(a)and13C-NMR(b)spectra of polymer.
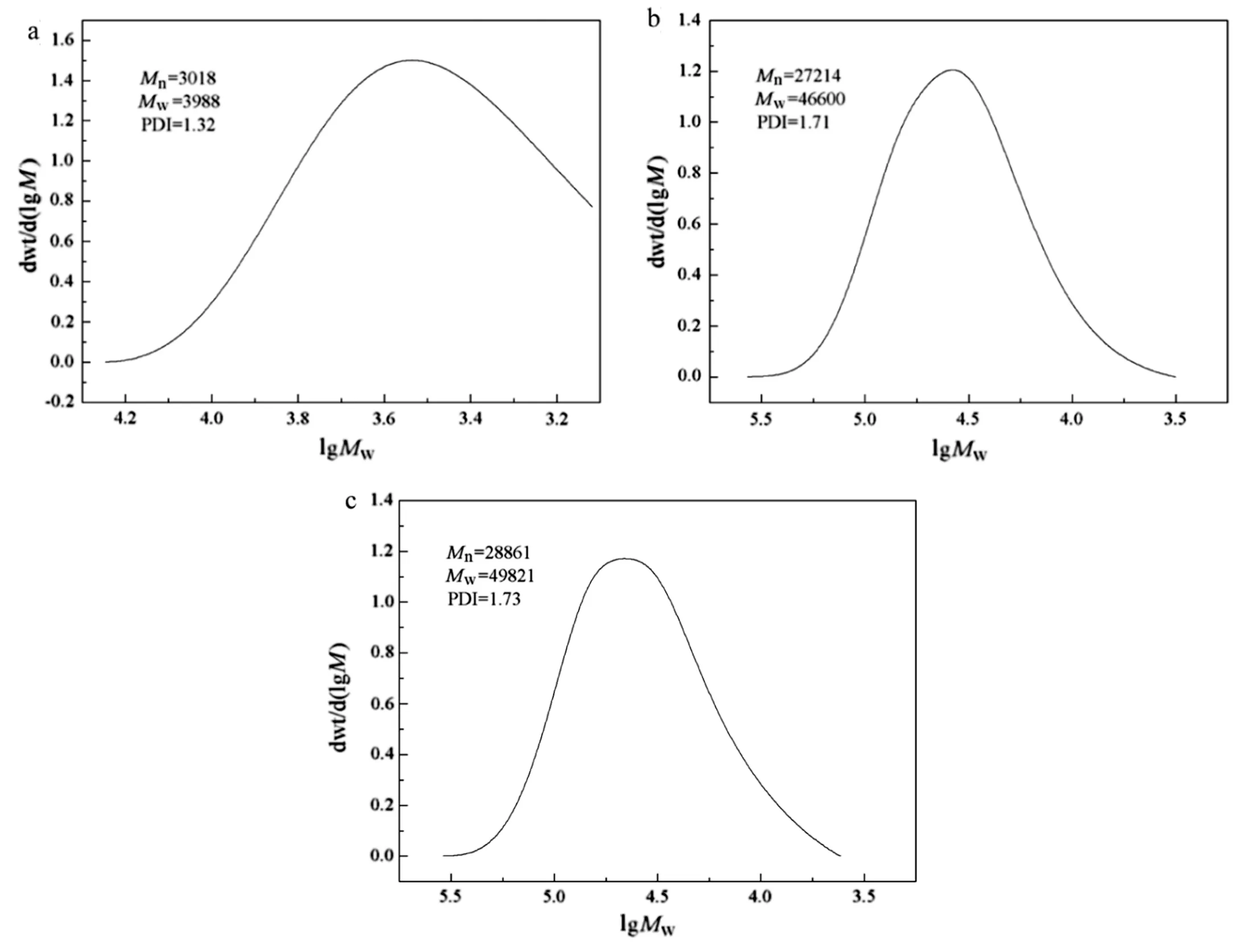
Fig.8.GPC chromatogram of polymers(a:capsaicin-acrylate at 0 wt%,b:capsaicin-acrylate at 6.5 wt%,c:capsaicin-acrylate at 12.3 wt%).
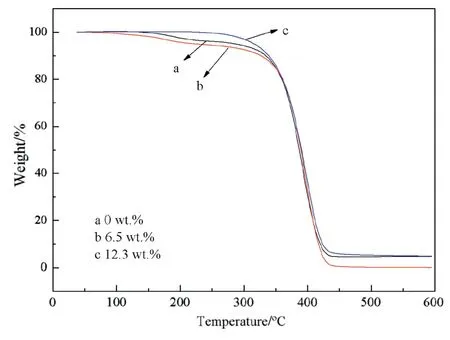
Fig.9.The TGA of polymers(a:capsaicin-acrylate at 0 wt%,b:capsaicin-acrylate at 6.5 wt%,c:capsaicin-acrylate at 12.3 wt%).
4.Conclusions
A novel antibacterial acrylate polymer composite based on capsaicin was prepared by a two-step reaction,involving esterification and solution polymerization.The yield of the esterification reached 85.3%.The strong characteristic stretching vibration of ester bond at 1747 cm-1of FTIR spectra,the chemical shift of C--H from AC at 6.52-6.36 of1H-NMR spectrum and the chemical shift of C from ester bond at 164.05 of13C-NMR supported the synthesis of capsaicin-acrylate.The results of MS and EA were also in accordance with anticipation.The Mnof the polymer prepared by capsaicinacrylate at 6.5 wt%was 27214 and an appropriate molecular weight distribution was obtained by GPC.The thermal properties of the polymers were stable.The contact angles increased from 77.5° to 86.2° with the increasing amount of capsaicin-acrylate.The polymers based on capsaicin-acrylate present great antibacterial activity.The coatings based on the capsaicin-acrylate polymers painted on the surface of ships and other marine equipment can be effective to prevent the attachment and breed of marine organism.Also,capsaicin is extracted from plants and is friendly to marine ecosystem.All the results suggest that the obtained composites have immense potential in the field of marine antifouling.
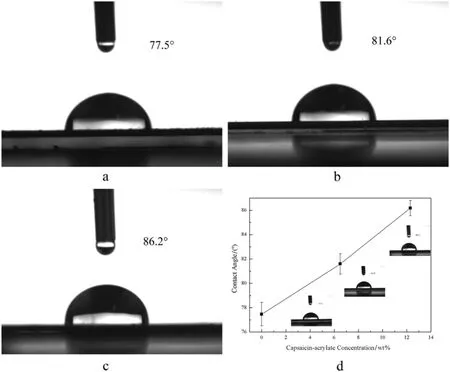
Fig.10.The contact angles on the polymer films(a:capsaicin-acrylate at 0 wt%,b:capsaicin-acrylate at 6.5 wt%,c:capsaicin-acrylate at 12.3 wt%).
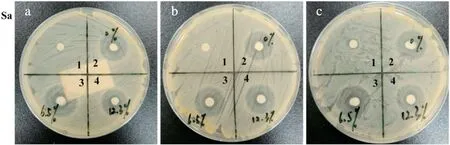
Fig.11.Antibacterial activity against S.aureus of the polymers(1:n-butyl acetate without tested compounds,2:capsaicin-acrylate at 0 wt%,3:capsaicin-acrylate at 6.5 wt%,4:capsaicinacrylate at 12.3 wt%).

Table 2 The diameter of antibacterial rings
 Chinese Journal of Chemical Engineering2019年12期
Chinese Journal of Chemical Engineering2019年12期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
- Controlling pore structures of Pd-doped organosilica membranes by calcination atmosphere for gas separation☆
- Relationship between equilibrium potential and radius of lanthanides electrolyzed on the zinc cathode
- Effect of gas composition on nitric oxide removal from simulated flue gas with DBD-NPC method☆
- Optimization of the N2generation selectivity in aqueous nitrate reduction using internal circulation micro-electrolysis☆
- Solubility and mass transfer of H2,CH4,and their mixtures in vacuum gas oil:An experimental and modeling study☆
