Effect of gas composition on nitric oxide removal from simulated flue gas with DBD-NPC method☆
Lan Yang,Xiang Zhang,Qing Kan,Binran Zhao,Xiaoxun Ma*
School of Chemical Engineering,Northwest University,Xi'an 710069,China Chemical Engineering Research Center of the Ministry of Education(MOE)for Advanced Use Technology of Shanbei Energy,Xi'an 710069,China Shaanxi Research Center of Engineering Technology for Clean Coal Conversion,Xi'an 710069,China International Scientific and Technological Cooperation Base of the Ministry of Science and Technology(MOST)for Clean Utilization of Hydrocarbon Resources,Xi'an 710069,China Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi,Xi'an 710069,China
Keywords:Dielectric barrier discharge(DBD)Corona discharge Nitric oxide Alcohols Flue gas
ABSTRACT A new method for nitric oxide(NO)removal was developed by combining dielectric barrier discharge(DBD)and negative pulse corona(NPC).The effects of gas composition(O2,CO2,and H2O)on NO removal were investigated with this method,and the effect of alcohols(methanol and ethanol)addition on NO removal was also investigated as well as the reaction mechanisms to enhance the NO removal efficiency.The experimental results showed that O2,CO2,and H2O had obvious inhibition effects on NO removal,and the negative effects were in the following order:O2>CO2>H2O.The addition of methanol or ethanol in the reaction system could mitigate the negative effects of O2,CO2,and H2O on NO removal,and also eliminated the production of NO2.The positive effect of alcohols addition with DBD-NPC denitration method was also validated in the simulated flue gas,in which the NOx(NO,NO2)was mainly converted into N2.
1.Introduction
Burning of fossil fuels produces NOX(NO and NO2),which is one of the main factors to cause various problems and threat the environment and human health[1].During the fuels incineration processes,NO with the small chemical activity constitutes 90%-95%of the NOXemission from fluegas.Thus,aneffective NOremoval method ismeaningful and necessaryforfossilfuelsburningwithNOXemission.
Conventional techniques for NO abatement from flue gas are hard to satisfy the increasing requirement of stringent regulation and low-cost treatment of NO removal,including selective catalytic reduction(SCR),selective non-catalytic reduction(SNCR)and flue gas recirculation(FGR)[2],due to the major drawbacks of ammonia leakage,waste disposal,high operation temperature or catalysts dependence,and high costs[3,4].
Non-thermal plasma(NTP)technology is one of the most promising candidates for NOXremoval as it can activate molecules and realize many reactions that are difficult to be carried out at room temperature[5,6],especially for the corona discharges and dielectric barrier discharge(DBD)methods[7,8].Corona discharges can generate a high concentration of radicals with high energy,but due to the limited corona area,it is difficult to get a stable and uniform discharge under high voltage corona discharge in a large space[7,9].For the negative pulse corona(NPC),the discharge can be generated more easily than that with positive pulse corona,the number of electrons is greater[10],and with a lower N2O production efficiency[11].DBD plasma provides a more stable and homogeneous discharge than corona discharge that improves the discharge intensity and the range of corona discharge[7,12-16],but the energy efficiency of DBD is low,thus high NO removal efficiency can only be achieved when a high power supply is reached[14].
NTP and SCR catalysis methods typically use reducing agents such as ammonia(NH3-SCR)[17]or hydrocarbons(HC-SCR,CH4,C2H2,C2H4,C3H6,C3H8etc.)[18-21],the removal efficiencies with these methods are mediocre,the effective temperature is high and narrow,and catalyst-based SCR method is necessary.For example,Wang et al.reported that the NO removal increased from 17.3%to 66.8%with catalysts of Ag/Al2O3and the addition of ethanol under the assistance of DBD and SCR[22].Therefore,it is necessary and meaningful to develop a simple and low-cost method without the catalyst,exhaust absorption,and external heating or pressure to optimize the NO removal efficiency for denitration technology development.
DBD coupled NPC was used in this study to provide more radicals and electrons for the NO removal reaction to neutralize the disadvantages of DBD,and also increase the electric field intensity and discharge range of NPC.The combined technology could obtain a more continuous current,high concentration of radicals and homogeneous plasma discharge.
Most studies mainly focused primarily on the NO removal efficiency of the method and other additional characteristics to emphasis the high denitration rate,but the study with the actual coal-fired power plant is little.Considering the flue gas typically contains 2%-15% O2,2%-12%H2O vapor and 10%-12% CO2[23,24],the objectives of this new denitration method with DBD-NPC were to investigate the effects of gas composition(O2,CO2,H2O)on NO removal;to study the effect of alcohols(methanol or ethanol)addition on NO removal as well as its reaction mechanisms;to verify the effect of alcohols in the simulated flue gas when O2,CO2,and H2O co-existed.
2.Experimental
2.1.Experimental equipment and methods
The schematic of the experimental setup used is shown in Fig.1.It consisted primarily of the gas feeding,a negative high-voltage pulse discharge device,a quartz tubular reactor(20 mm i.d.×320 mm length×2 mm thickness),and analytical systems.The gas mixtures were prepared with mass flow controllers(D07-7 and CS200D,Beijing Sevenstar Huachuang,China)and flowed through a glass buffer bottle,then introduced into the plasma reactor via a gas distributor made of a sand core chip(25-50 μm porosity)fitted at the reactor inlet.The concentration of the output gases was continuously analyzed and recorded by a measurement system in which a flue gas analyzer(Vario Plus,MRU,Germany)was used to monitor NO,NO2,CO2,CO,and a thermostar gas Mass Spectrometer(MS)(GSD 320,Pfeiffer Vacuum,Germany)was used to monitor N2,O2,H2,CO2,and N2O.
Pure N2was first flowed through the reaction pipeline to remove the residual O2in the system until the flue gas analyzer(with a 0.1%accuracy)indicated that the reaction pipeline was free of O2.Then,the simulated flue gas was passed through the reactor to the analyzer until a stabilized concentration was obtained from the flue gas analyzer.The discharge experiment was started by turning on the discharge power supply.The steam of ethanol or methanol was introduced into the flue gas by flowing the gas through a flask containing ethanol/methanol.The concentration of vaporized H2O,methanol or ethanol was varied by changing the temperature of the water bath in which a bottle containing water or alcohols(methanol or ethanol)was immersed.Unless specifically noted otherwise,the O2concentration was less than 0.1%and balanced by N2.While each experiment was sustained for 20 min,the Vario Plus on-line flue gas analyzer took samples and determined gas composition at an 8-second interval.The applied current used in this study was the input watt current of the power supply,which is clear and direct to reflect the energy input state.Ranges of variables studied were 0-8%for O2concentration,0-12%for CO2concentration,0-10 vol%for H2O concentration,300-1100 ppm for NO concentration,400-1000 ml·min-1for gas flow rate.In some experiments,methanol and ethanol were added at two volume fraction 0.05%(0.31 ml·min-1)and 0.5%(3.1 ml·min-1).All experiments were conducted at room temperature(~298 K)and atmospheric pressure.
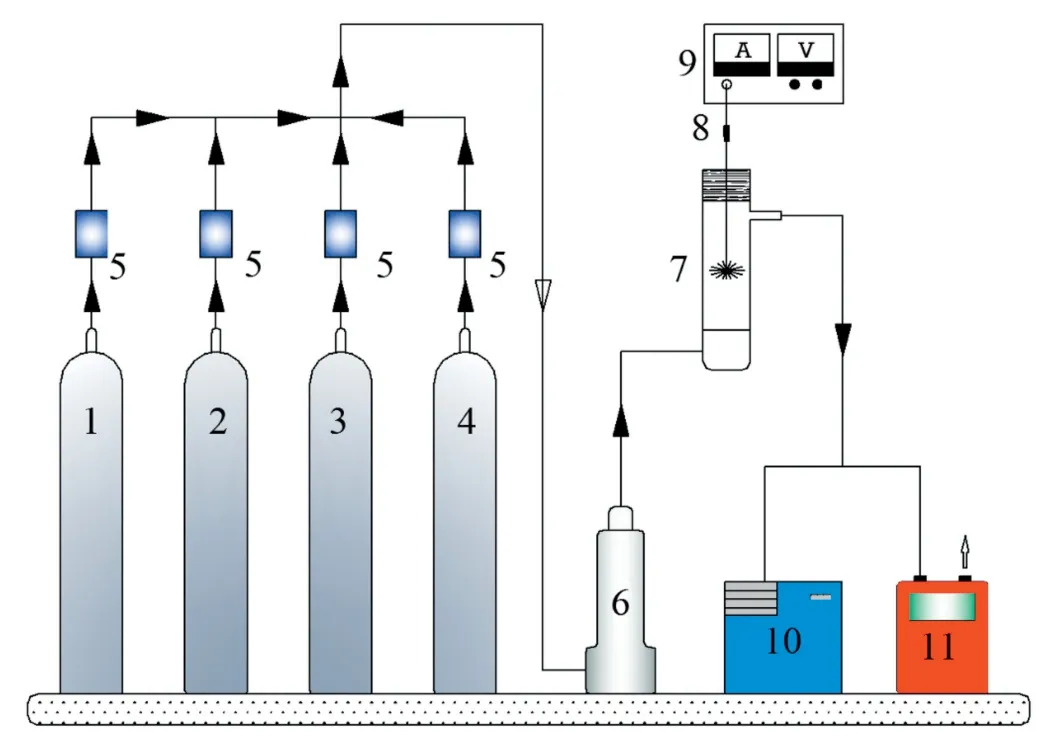
Fig.1.Schematic diagram of the experimental setup.(1-4)N2,O2,CO2,and NO gas cylinders,(5)Mass flow controllers,(6)Buffer,(7)Reactor,(8)Half-wave rectifier unit,(9)High voltage power supply,(10)Mass Spectrometry,(11)Flue gas analyzer.
2.2.Reagents
The reagents used in this study were methanol(99.5%)and ethanol(99.7%)from Tianjin Zhiyuan Chemical Co.China.Gas cylinders of N2(99.999%),NO(99.5%),O2(99.9%)and CO2(99.9%)were provided by Dalian Date Standard Gas Co.China.The deionized water(with a resistivity of≤18.2 MΩ at 293 K and reducing the micro-particles≤1/ml and heavy metals≤0.1 ppb)was purified by the UPR-II system(UPHW-IV-90T,CCUDP,Chinese).
2.3.Discharge system
The discharge power was provided by a power supply(SJ-2000E,Jiafu,China)with an output voltage of 15 kV and a variable frequency of 0-15 kHz.A half-wave silicon rectifier unit was constructed to connect the power supply and generate a stable negative impulse.The electrode comprised needles,a quartz tube and a copper clip(40 mm width×2 mm thickness),which,respectively,worked as the cathode,the dielectric barrier,and the anode.Thirty-eight silver-plated steel discharge needles(spherical probe head,35 mm in length,0.5 mm in diameter)were placed in the center of the reactor and connected to the negative pulse power supply.
2.4.Data processing
The removal efficiency of NO(ηNO),and the yields of NO2(ηNO2)and CO(ηCO)were defined as follows:
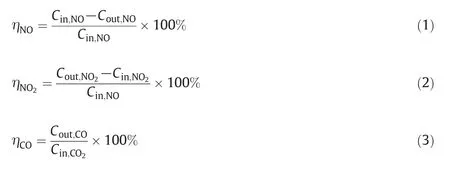
where Cinand Coutare the steady-state concentrations of inlet and outlet gas streams,respectively;the units are parts per million(ppm).
3.Results and Discussion
3.1.Effect of O2
Flue gas typically contains 2%-15%O2.It can be observed in Fig.2a that the NO removal efficiency decreased with O2concentration in the inlet stream.When the O2concentration approached zero,the NO removal efficiency reached its highest point of 89%at 6 A.The effect of O2can be attributed to the following two points:1)at a low O2concentration(0-3%),the moderate amount of O species present facilitates the NO removal as the discharge current increases[9,11],as described with Eqs.(4)-(6);

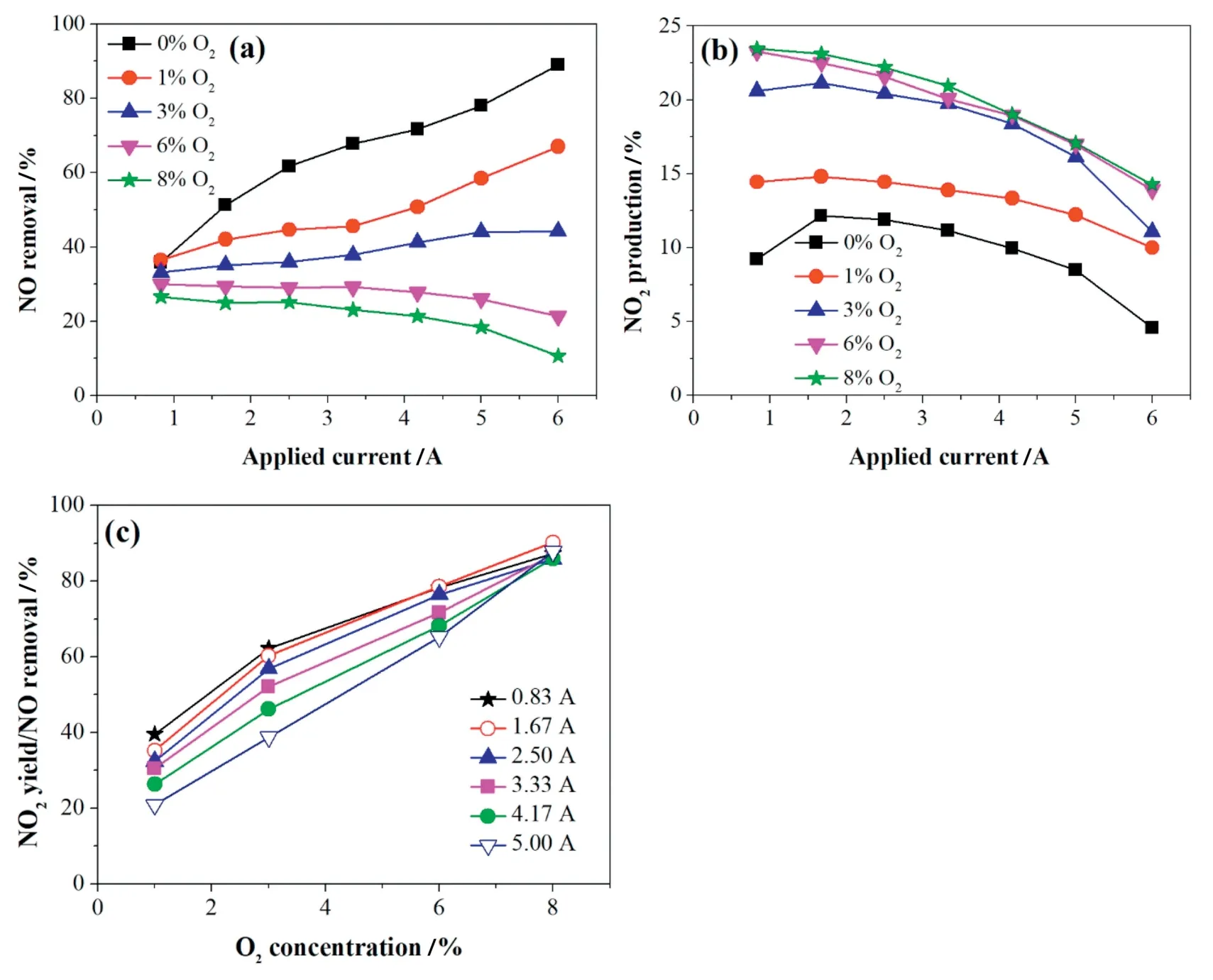
Fig.2.Effect of O2concentration on(a)NO removal,(b)NO2production rate and(c)NO2yield/NO removal.The gas flow and initial NO concentration were 900 ml·min-1 and 500 ppm,respectively.

and 2)O2is a product of NO decomposition reaction.Higher O2concentration could accelerate the reverse reactions of Eqs.(7)-(10)to produce more NO and consume more N species and electrons.Therefore,the NO removal efficiency decreased with O2concentration and showed a downward trend with the current at high oxygen levels(6%and 8%).
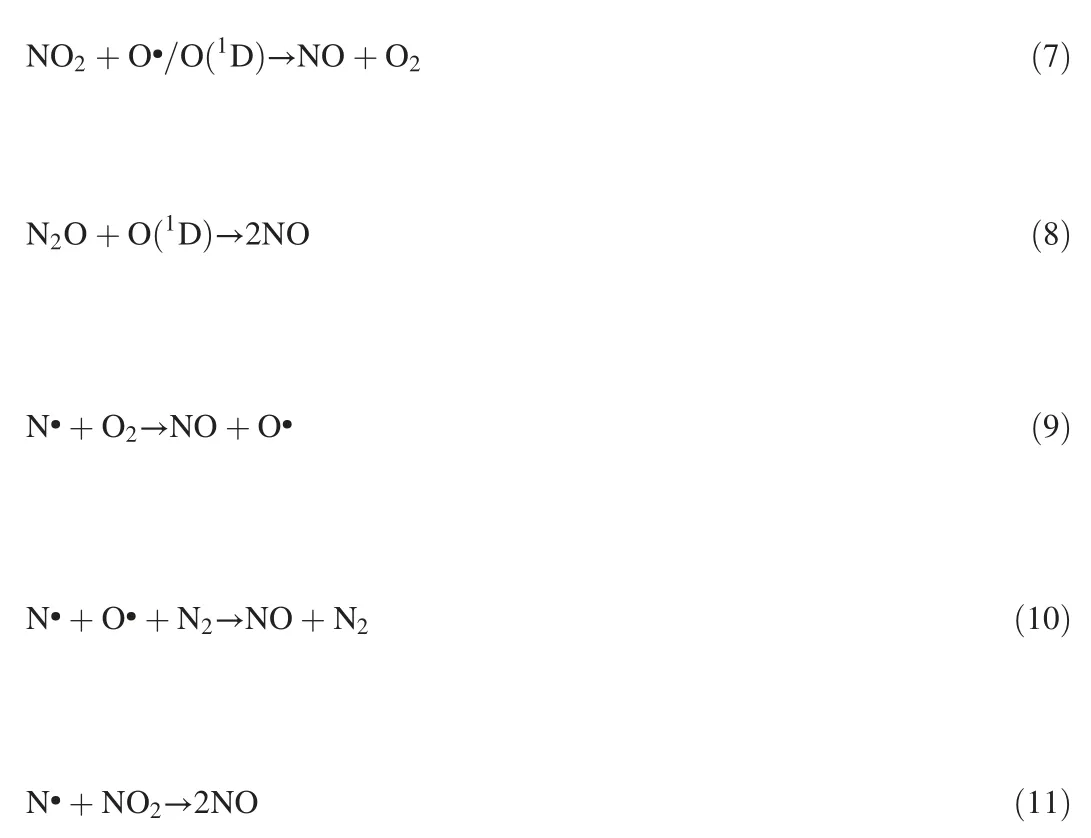
Fig.2b demonstrates the influence of different O2concentrations on NO2production,the NO2production efficiency increased with the O2concentration,due to the increasing number of O species.It also can be known that the NO2production efficiency decreased as applied current increased due to the NO2decomposition.
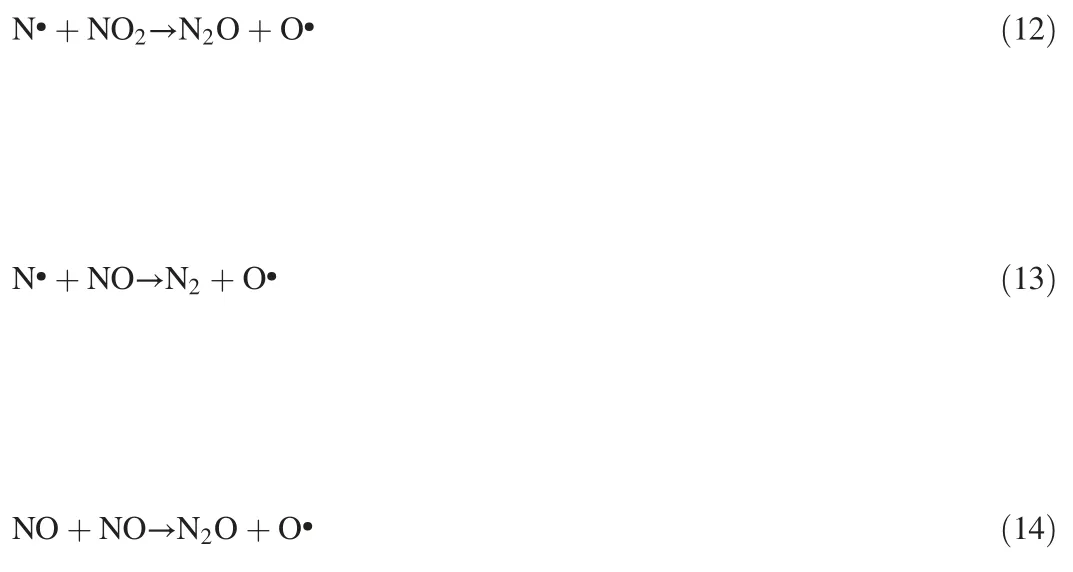
Fig.2c displays that the contribution of the NO2yield to NO removal of different currents increased almost linearly with the O2concentration and implies that a higher quantity of NO could be oxidized to NO2(Eqs(4)-(6))rather than reduced to N2with the addition of more O2.Moreover,more O species may inhibit N2and N2O production through Eqs.(12)-(14).Therefore,the existence of O2can not only reduce NO removal efficiency but also promote NO2production,namely,reducing NOXremoval efficiency.The paths of NO removal can be attributed to the competition between oxidation prevailing under oxygen-rich conditions and reduction prevailing under oxygen-poor conditions[19].
3.2.Effect of CO2
As shown in Fig.3a,NO removal efficiency decreased as the CO2concentration increased,due to the competitive relationship between CO2and NO for electrons and N species,as shown in Eqs(15)-(16)[25].Besides,the existence of CO2could weaken the current due to the electronegativity of CO2,then reducing the concentration of electrons and radicals.

Fig.3b shows that the CO production rate increased with the applied current,and the CO production rate continuously decreased along with the CO2concentration,that decreased from 3.6%to 1.8%with 1%and 12%CO2added,respectively,at 6 A.However,in Fig.3c,the total production amount of CO greatly increased with the increase of CO2concentration at each current,this may be due to the fact that the increasing CO production efficiency is lower than that of the CO2concentration.
Fig.4 indicates that a small amount of NO and NO2can be produced from the reactions between CO2and N2,as shown in Reaction(16).The produced quantities of NO and NO2increased with the applied current as shown in Eqs.(5),(11),(16)-(17)[25].Therefore,the presence of CO2in the reaction system not only reduced NO removal efficiency but also promoted CO,NO2production.

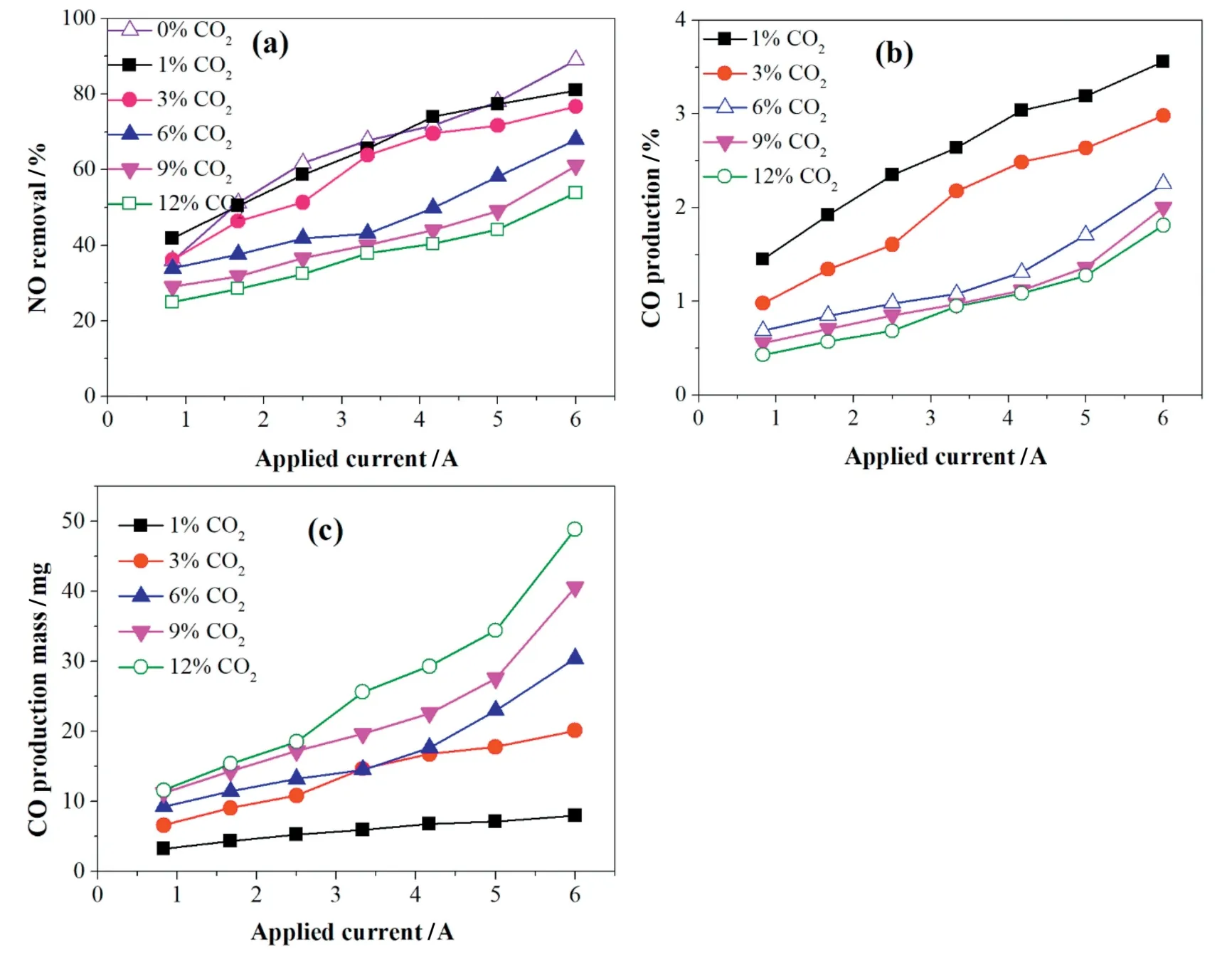
Fig.3.Effect of CO2concentration on(a)NO removal,(b)CO production,and(c)CO production mass with the gas flow and initial NO concentration of 900 ml·min-1 and 500 ppm,respectively.
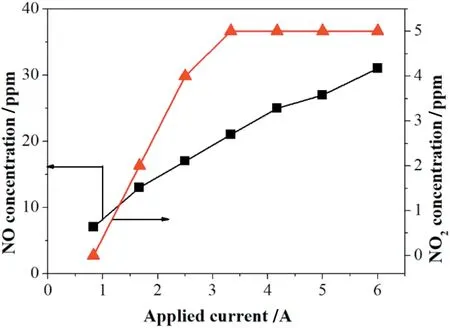
Fig.4.The generation of NO and NO2from CO2and N2at different applied currents with the gas flow and CO2concentration of 1000 ml·min-1 and 12%,respectively.
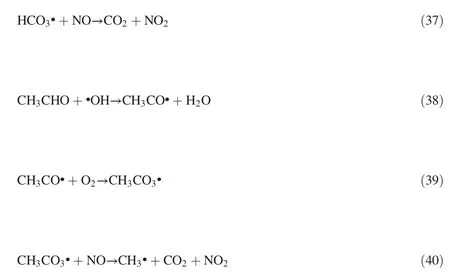
3.3.Effect of H2O
In thermal power plants and diesel exhaust,H2O unavoidably exists in the flue gas,and it was very important to evaluate the effect of H2O on NO removal for possible industrial application.As shown in Fig.5,the NO removal efficiency decreased slightly as the water vapor concentration increased.
The effect of H2O can be attributed to the following two points:1)·OH and HO2·radicals can be produced from water vapor according to the Eqs(18)-(20),which are capable of oxidizing NO,NO2,HNO2to form HNO2and HNO3according to Eqs.(21)-(23)[22,26,27]or the direct reactions between H2O molecules and NOXmolecules(Eq.(24))[23].
Fig.5.Effect of H2O addition on NO removal with the gas flow and initial NO concentration of 625 ml·min-1 and 500 ppm at 1.67 A respectively.
2)Simultaneously,the moist flue gas also has a suppressing effect to the discharge that water molecules can easily absorb electrons in the corona and turn into slow negative ions,which reduce the electrons production,discharge current and the ions migration rate.Then,electron energy cannot reach the bond dissociation energy of the reactant that decreases the concentrations of the active species[19,20,23,28].The produced·H,HN·,·OH radicals from H2O can also lead to the production of NO and NO2by consuming electrons and N species(Eqs.(25)-(29))[27].Besides,the produced·OH radicals from H2O can also deplete ozone as Eq.(30)[19].Here,the existence of H2O vapor may not only reduce NO removal efficiency but also promote NO2production.
The related equations are as follows:

3.4.Effects of O2,CO2,H2O addition on NO removal
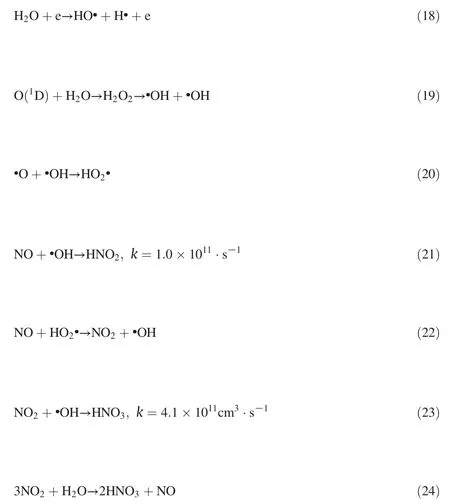
The volumetric ratios of the O2,H2O vapor,and CO2in the flue gas from coal-fired power plant boilers are generally 3%,10%,and 10%-12%,respectively[23].As mentioned before,the three factors all noticeably inhibit the NO removal.The effect of 3% O2,10%CO2,10%H2O separately adding either on NO has been compared.It can be seen from Fig.6 that although the added quantity of CO2and H2O are much higher than the quantity of 3% O2added(3%),the negative effect of the O2is greater than that of CO2and H2O respectively on NO removal.So the negative effects of O2,CO2,and H2O on NO removal were listed respectively in descending order.
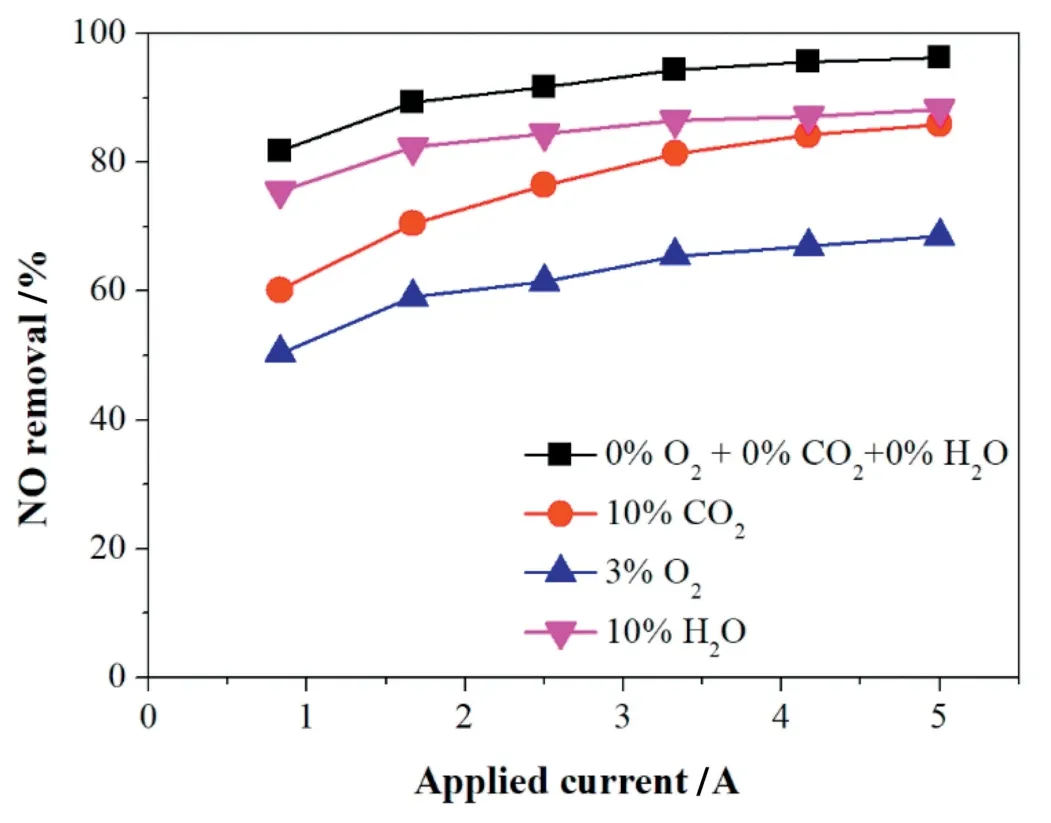
Fig.6.Compare the effects of O2,CO2,H2O on NO removal at different applied currents with the gas flow and initial NO concentration of 625 ml·min-1 and 500 ppm,respectively.
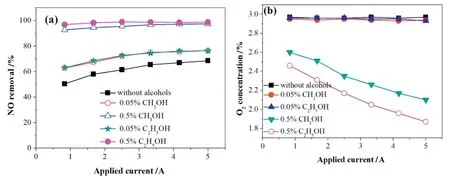
Fig.7.Effects of ethanol/methanol on(a)NO removal,(b)O2concentration.The gas flow,initial NO and O2concentration were 625 ml·min-1,500 ppm and 3%,respectively.
3.5.Effect of ethanol/methanol addition on NO removal
3.5.1.Effect of ethanol/methanol addition on NO removal efficiency
Considering the negative effects of O2,CO2,H2O on NO removal,the O2,CO2,and H2O should be isolated or consumed in the reaction.Methanol and ethanol were chosen in this study as they are convenient to deliver and low cost.As seen in Fig.7a,the NO removal efficiency was significantly improved by the addition of methanol or ethanol.With 0.5%volume fraction of alcohols added,the NO removal efficiency increased by over 40%to 92.7%and 96.9%at 0.83 A for methanol and ethanol respectively,and then reached 97.37%and 98.84%as the applied current increased to 5 A.Moreover,the amount of alcohols added also greatly influenced the NO removal efficiency,the NO removal efficiency increased by over 20%in comparison between the reaction with 0.5%and 0.05% alcohol addition at all applied currents.It is notable that NO2was not detected in the reaction products by the on-line flue gas analyzer that indicated that the addition of alcohols may have altered the reaction path of NO removal process.Adding alcohols could be a replacement of the commonly used water or alkaline solution-based methods for NO2absorption[30].
The positive effect of alcohols addition on NO removal can be attributed to the following reasons:1)NO oxidation promotion process:H2,CH3CHO,CO,CH4,C2H6,C3H8,and C4H10were the dominant products of ethanol decomposition,H2,HCHO,CO,CH4,C2H6,and C3H8were the dominant products of methanol decomposition[31],the accompanied free radicals of CH3·,CH2OH·,CH3O·,·CH2,CH3CO·,CH·,and OH·[31,32]could form various alkyl,alkoxy radicals,and molecules by reaction with O2or O species,the radicals produced above may oxidize NO and CO into NO2and CO2(Eqs.(31)-(40)).These radicals could be further formed from the produced hydrocarbons during the NO oxidation promotion process[19].The participation of O2in the reaction can be verified by Fig.7b.It can be seen that the O2consumption increased with the increase of applied current and the amount of alcohols.Besides,ethanol addition consumes more O2than methanol due to that the ethanol can generate more free radicals.

Fig.8.MS profile of NO conversion with methanol(a)or ethanol(b)addition.The applied current,gas flow,initial NO,alcohols,and O2concentration were 2.5 A,1000 ml·min-1,1500 ppm,0.5%,and 3%respectively.
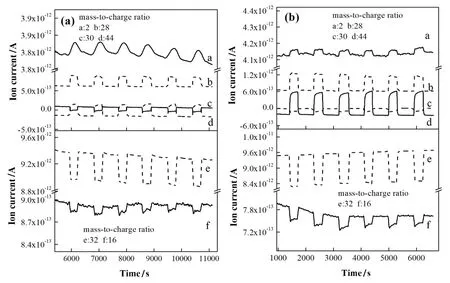
Fig.9.MS profile of NO conversion in N2base of(a)0.5%methanol addition,(b)0.5%ethanol addition.The gas flow,applied current,initial NO and O2concentration were 625 ml·min-1,2.5 A,500 ppm and 3%,respectively.The mass charge ratios(m/e)of N2/CO,O2,NO/NO2,H2,N2O/CO2,and O species are 28,32,30,2,44 and 16,respectively.The discharge was repeated once for 5 min after a 10 min interval.
2)Simultaneously,NOXcan also be converted to N2with CH3OH/C2H5OH as reductant,as Eqs.(41)-(44)indicated that CH3OH/C2H5OH oxidized to CO2and H2O.In this experiment,the reduction effect of CH3OH/C2H5OH on NO removal was obvious as the non-existence of NO2in the products.The similar result could be achieved from the ethanol-SCR-DBD system with a high temperature of 255°C[22].
Overall,the combined DBD-NPC method with methanol/ethanol addition is superior to the previous methanol/ethanol-SCR[29,33-36],ethanol-SCR-DBD methods[22],methanol-positive corona discharge[37]and methanol direct oxidation method under high temperature and pressure[38]for its higher NO removal efficiency with simple reaction system and without external heating,pressure,catalysts and CO,NO2production.
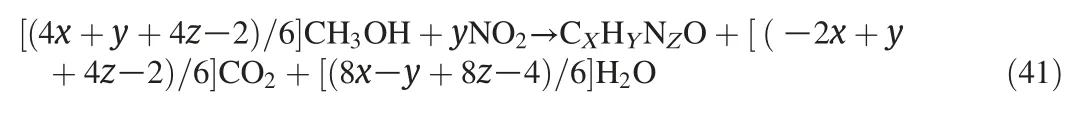
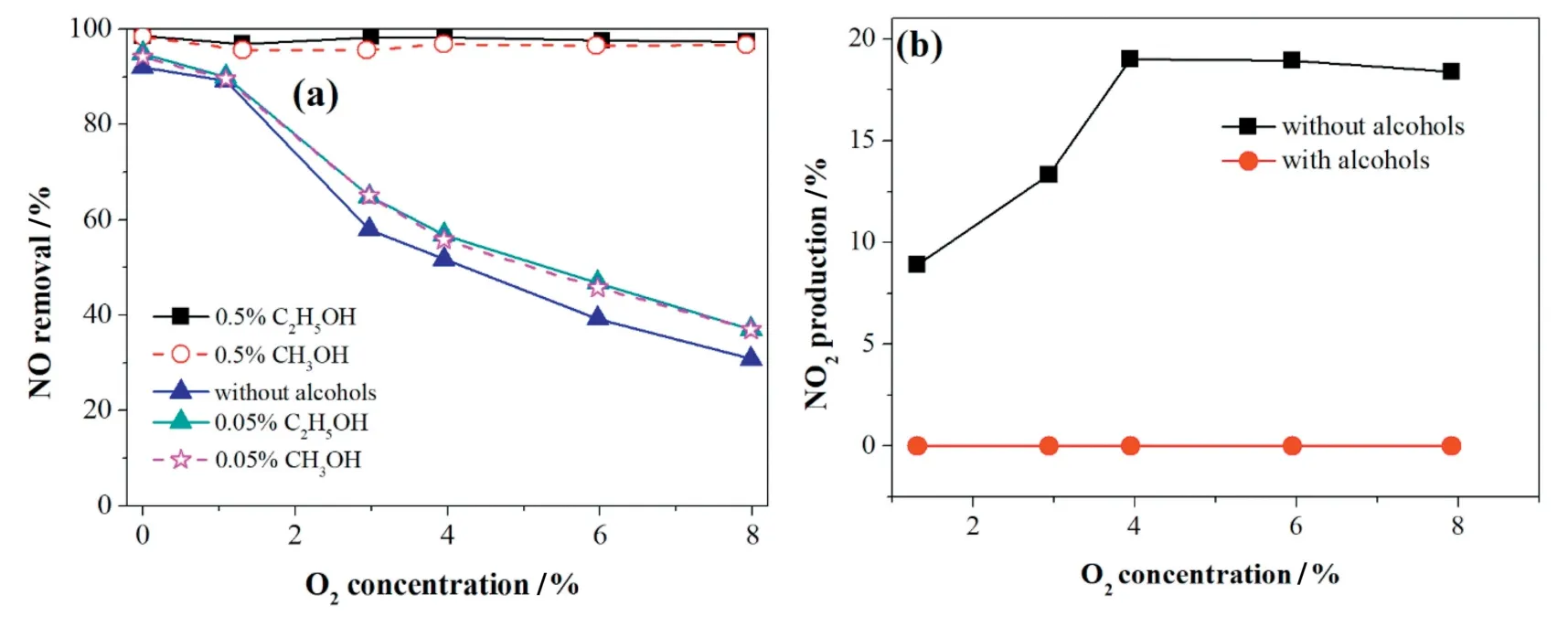
Fig.10.Effect of O2concentration on(a)NO removal and(b)NO2production with the addition of ethanol/methanol.The gas flow,initial NO concentration,and applied current were 625 ml·min-1,500 ppm and 1.67 A,respectively.
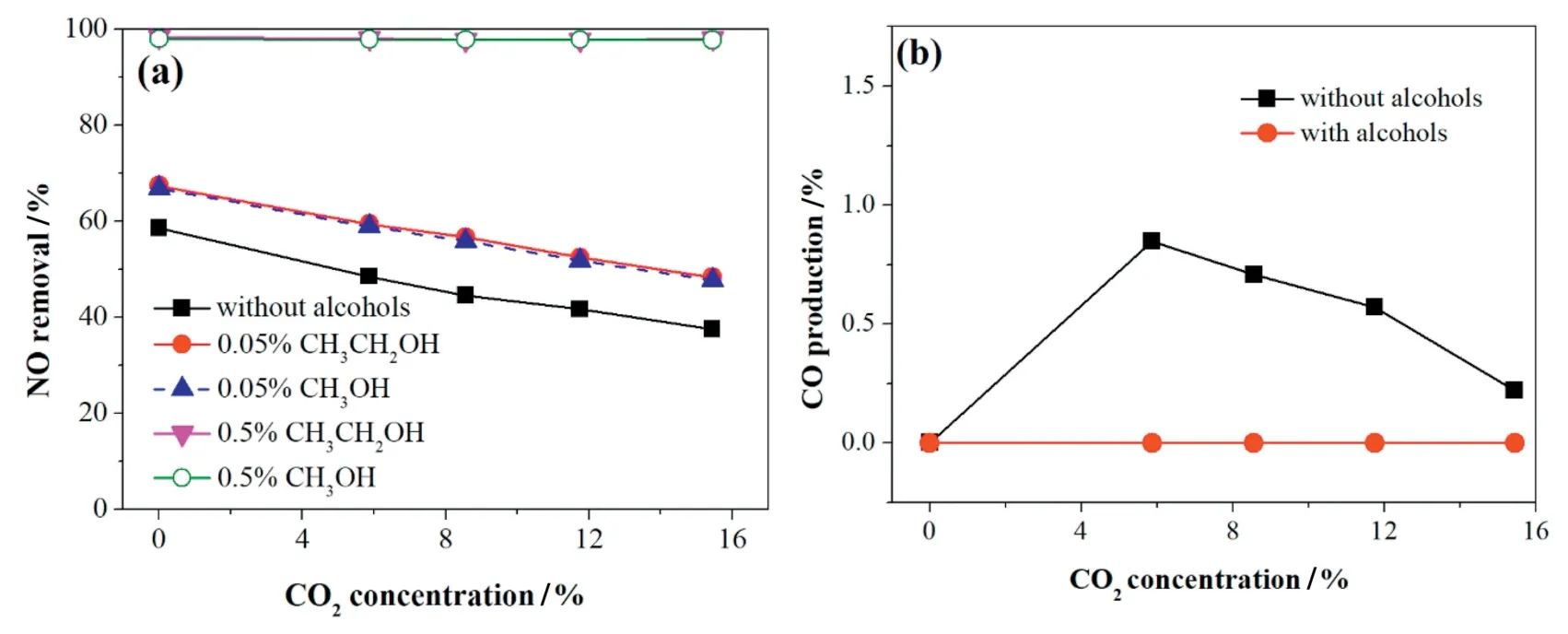
Fig.11.Effect of CO2concentration on(a)NO removal and(b)CO production with the addition of alcohols.The gas flow,applied current,initial NO and O2concentration were 625 ml·min-1,1.67A,500 ppm,and 3%,respectively.
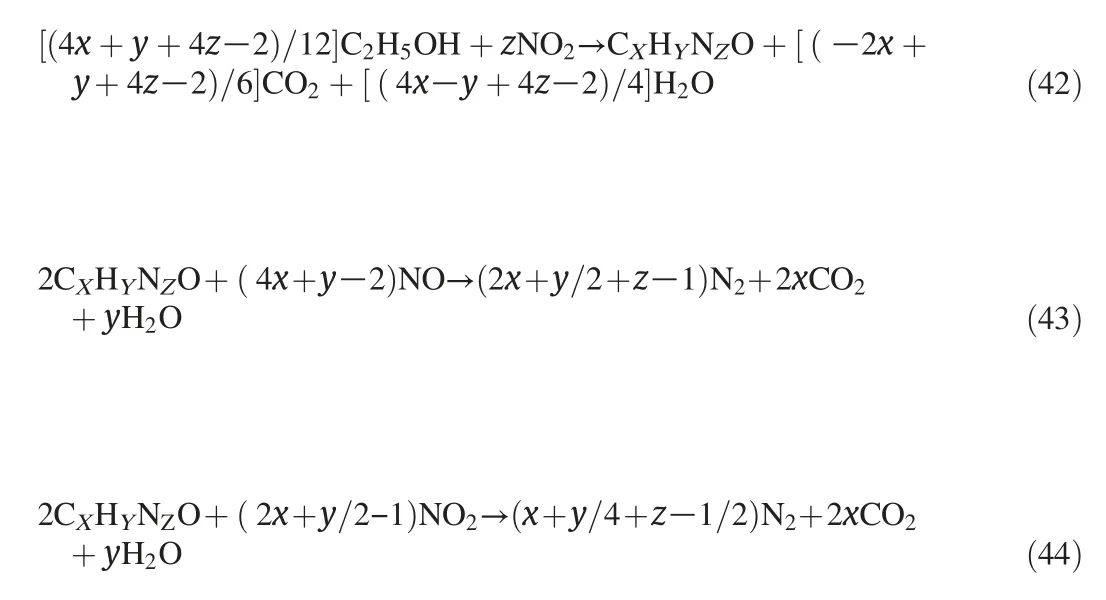
3.5.2.Analysis of the reaction paths
With the MS scanning,the products from the reaction of methanol or ethanol assisted DBD-NPC for NO removal were shown in Fig.8.The main components after the reaction were N2,O2,H2,H2O,CH4,C3H4,and CO2,in which CH4and C3H4have the positive effect on the NO removal[18,37].
Fig.9 shows the mass spectrum(MS)profile of NO conversion with intermittent discharge in N2atmosphere.The two variation trends of materials with methanol or ethanol addition were similar that the ion-current intensity(proportional to concentration)of NO/NO2,O2,and O decreased while those of N2/CO,N2O/CO2,and H2increased during the discharge process.Moreover,CO and NO2were not detected in the outlet stream,but CO2can be detected,suggesting that the CO may be completely oxidized by the free radicals.The amount of N2O can be ignored due to the fact that the alcohol addition is unfavorable for N2O production due to the insufficient amount of NO and O2.Thus,with the alcohols added,the NO primarily converted into N2.This observation is consistent with the proposed mechanisms in Section 3.5.1.
3.5.3.Effect of O2concentration with alcohols added on NO removal
It can be known from Section 3.5.1 that alcohols can not only consume O2but also accelerate the reduction of NOxto N2.To obtain further validation of the effect of alcohols,various O2concentrations were studied with and without alcohols.
In Fig.10a,it is clear that the NO removal efficiency without alcohols was inhibited by the increase of O2concentration.However,with the addition of 0.5%volume fraction of alcohols,the NO removal efficiency reached over 97.0%compared to that of 30.9%for the reaction without alcohols with 8%O2existence.Therefore,the alcohols overcame the negative effect of O2and greatly enhanced the NO removal efficiency.It is notable that NO2was not found in the reaction products from Fig.10b,so the strong effect of alcohols could also be used as a replacement of the commonly used NO2absorbed procedure with water or alkaline solution[30].
3.5.4.Effect of CO2concentration with alcohols added on NO removal
Fig.11 illustrates the effect of CO2concentration on NO removal with the addition of alcohols.The NO removal efficiency without alcohols was inhibited by the increase of CO2concentration.It is clear that the alcohols addition(methanol or ethanol)reduced the negative effect of CO2on NO removal.The NO removal efficiency was close to 100%when adding 0.5% volume fraction of alcohols even with 15% CO2,whereas the NO removal efficiency without alcohols was only 37.6%,due to that the alcohols addition could not only produce free radicals and intermediates that reacted with NO,but also accelerated the reduction of NOxto N2.Furthermore,it is notable from the flue gas that NO2and CO were still not found in the gaseous products with the increase of CO2concentration.
3.5.5.Effect of H2O addition with alcohols added on NO removal
As shown in Fig.12,when adding 0.5%alcohols(methanol or ethanol),the negative effect of 10%H2O on NO removal was almost eliminated and the NO removal efficiency increased by over 50%to closely 100%.This also because of the effect of free radicals and intermediates produced from the alcohols.
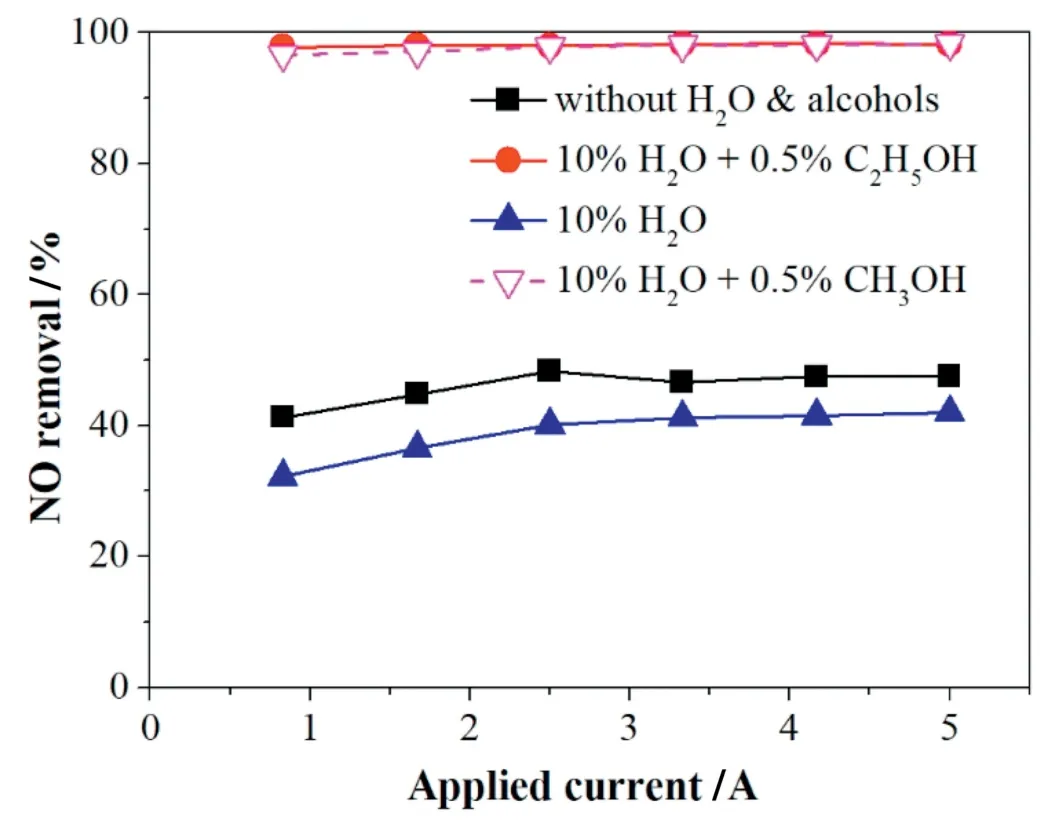
Fig.12.Effect of H2O on NO removal with the addition of alcohols.The initial NO,O2,CO2concentration,and gas flow were 500 ppm,3%,10%and 625 ml·min-1,respectively.
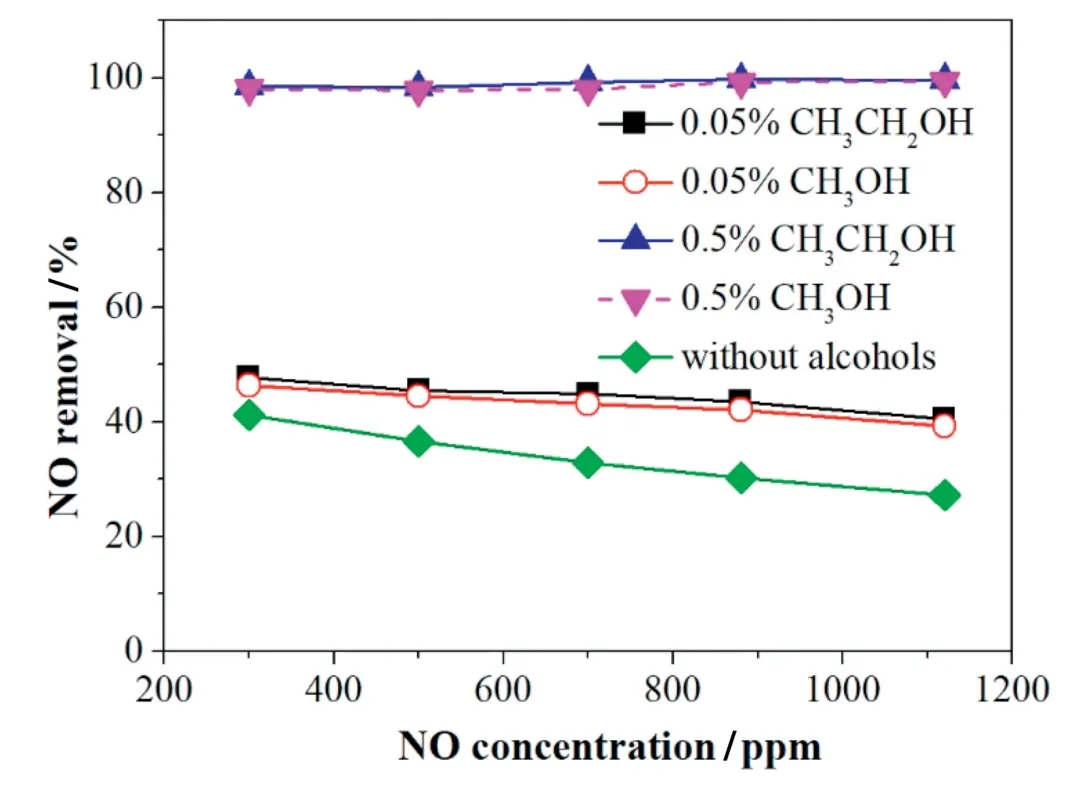
Fig.13.Effect of initial NO concentration on NO removal with the addition of alcohols.The gas flow,H2O,O2,CO2concentration,and applied current were 625 ml·min-1,10%,3%,10%,and 1.67 A,respectively.
Moreover,under the condition of 10%H2O,10%CO2and 3%O2,the addition of 0.5 vol% alcohols enhanced the NO removal efficiency to closely 100%.
3.5.6.Effect of alcohols in the simulated flue gas
To verify the effect of alcohols in the simulated flue gas,the experiment was conducted when O2,CO2,and H2O co-existed.
3.5.6.1.Effect of initial NO concentration with alcohols added on NO Removal.The effect of the initial NO concentration on NO removal was investigated.As shown in Fig.13,a higher NO concentration decreased the removal efficiency without adding alcohols,and similar results can be found in other reports[39].This may because of more NO molecules in the inlet,less reactive species and high-energy electrons would be available for a NO molecule.In contrast,the addition of alcohols(methanol or ethanol)reduced the negative effect of high NO concentration on NO removal.With the addition of 0.5%alcohols,the NO removal efficiencies were close to 100%with the increase of NO concentration,due to that alcohols addition is equivalent to the increase of the radicals and intermediates quantity that produced from alcohols during the discharge.
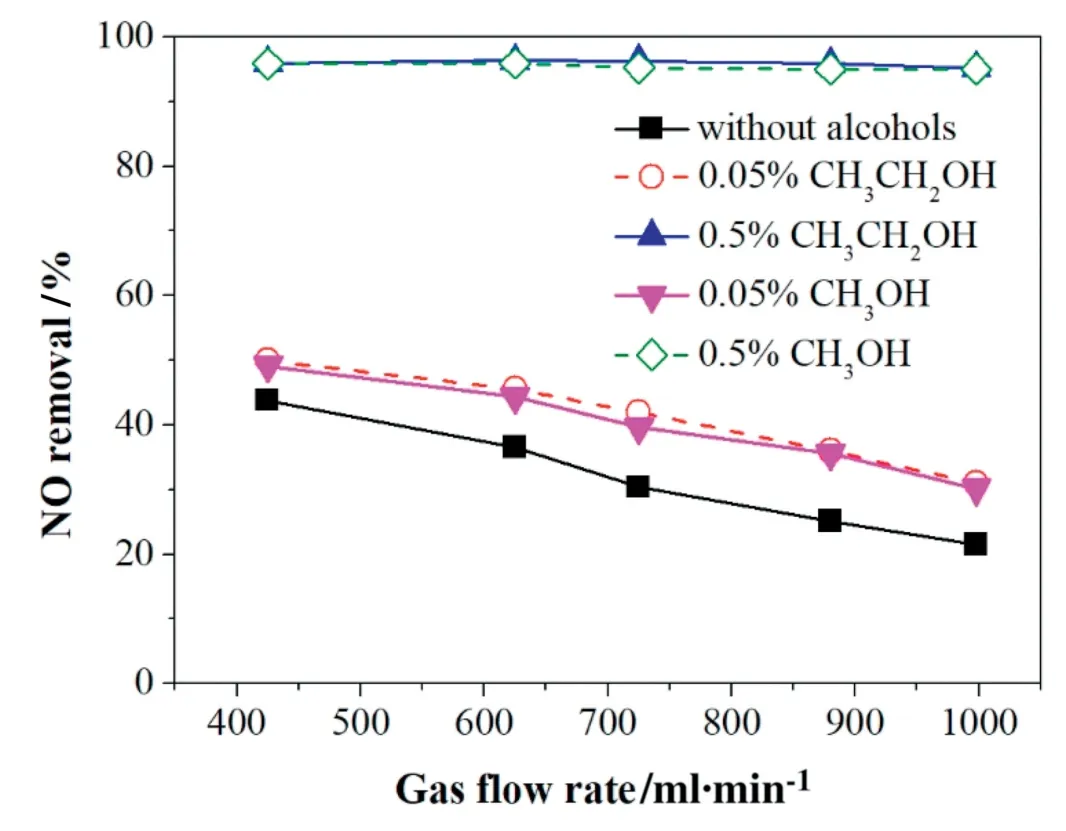
Fig.14.Effect of gas flow rate on NO removal with the addition of alcohols.The initial NO,H2O,O2,CO2concentration,and applied current were 500 ppm,10%,3%,10%,and 1.67 A,respectively.
Moreover,the figure also indicated that due to 0.5%alcohols addition,the negative effects of higher NO concentration,10% H2O,10%CO2and 3%O2on NO removal were almost eliminated.
3.5.6.2.Effect of gas flow rate with alcohols added on NO removal.As Fig.14 shows,the NO removal efficiency decreased with the gas flow without adding alcohols,due to that a fast flow shortens the residence time of NO in the reactor,increases the rate of NO supply and decreases the availability of the reactive species to NO molecules.However,the alcohols addition enhanced the NO removal efficiency obviously.With the addition of 0.5% alcohols,the NO removal efficiencies were close to 100%as the gas flow increases from 400 to 1000 ml/min.Here,the alcohol addition increased the number of radicals and intermediates produced from alcohols during the discharge process that promoted NO removal.
Moreover,the figure also indicated that the addition of 0.5%alcohols could obviously inhibit the negative effects of higher gas flow,10%H2O,10%CO2and 3%O2on NO removal.With the addition of 0.5%alcohols,the NO removal efficiency reached approximately 95% at 1000 ml·min-1compared to that of 21.5%without alcohols addition.
4.Conclusions
The new NO removal method of DBD-NPC was investigated with the different gas compositions in this study.The NO removal efficiency decreased with the increase of O2,CO2,and H2O concentrations,due to that they consume N species and electrons,and produce NO and NO2.The negative effects of O2,CO2,and H2O on NO removal were in descending order.
The addition of alcohols(methanol and ethanol)mitigated the negative effects of O2,CO2and H2O,and the effect of alcohols was greater with more alcohols added,due to that the free radicals produced from alcohols can not only consume O2,but also oxidize NO into NO2,and the NOX(NO,NO2)can be converted into N2by alcohol molecules further.Moreover,the effect of ethanol on NO removal was greater than that of methanol,due to the fact that ethanol generates more free radicals to consume more O2.Finally,the effect of alcohols was also verified in the simulated flue gas.
Based on the above effects of alcohols,the addition of 0.5 vol%alcohol enhanced the NO removal efficiency from 21.5%to 95%under the conditions of 1000 ml·min-1gas flow,500 ppm NO,10%H2O,3%O2,10% CO2at 1.67 A.It suggests that this new and simple NO removal method(without catalyst,additional heating,and pressure)should be effective and valuable for flue gas denitrification application.
 Chinese Journal of Chemical Engineering2019年12期
Chinese Journal of Chemical Engineering2019年12期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
- Preparation and characterization of a novel antibacterial acrylate polymer composite modified with capsaicin☆
- Controlling pore structures of Pd-doped organosilica membranes by calcination atmosphere for gas separation☆
- Relationship between equilibrium potential and radius of lanthanides electrolyzed on the zinc cathode
- Optimization of the N2generation selectivity in aqueous nitrate reduction using internal circulation micro-electrolysis☆
- Solubility and mass transfer of H2,CH4,and their mixtures in vacuum gas oil:An experimental and modeling study☆
