Synthesis plasmonic Bi/BiVO4photocatalysts with enhanced photocatalytic activity for degradation of tetracycline(TC)☆
Nianjun Kang,Dongbo Xu*,Weidong Shi*
School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang 212013,China
Keywords:Bi/BiVO4Plasmonic Photocatalysts Tetracycline(TC)Visible light
ABSTRACT In recent years,the excessive use of antibiotics has become a serious problem for human health.BiVO4regarded as one of the most promising visible-light-driven photocatalysts was used to degrade the antibiotics.In this paper,we fabricated Bi/BiVO4plasmonic photocatalysts which enhanced the photocatalytic activity of BiVO4for degradation of tetracycline(TC)antibiotic.The Bi/BiVO4photocatalysts were characterized by X-ray diffraction,X-ray photoelectron spectroscopy,scanning electron microscopy,transmission electron microscopy and high-resolution transmission electron microscopy.In addition,the photocatalytic experiment results show that the 0.04-Bi/BiVO4sample has the best photocatalytic activity for 2 times than the pure BiVO4photocatalyst.The cycle experiments,after four repetitions of the experiments,showed the sample still maintained a high photocatalytic activity.Finally,the photocatalytic reaction mechanism was also studied by free radical capture experiments and electron paramagnetic resonance spectroscopy.
1.Introduction
The problem of environmental pollution and energy shortage is very serious,and it has become a core strategic issue that countries around the world need to solve.At the same time,it also aroused the interest of researchers in developing new high-efficiency photocatalysts.Therefore,many researchers on the photocatalyst investment in energy conversion and environmental governance put a lot of energy and achieved gratifying results such as photocatalytic pollution degradation and hydrogen energy conversion[1-5].In the past few decades,various metal oxide semiconductors such as Bi2MoO6(2.7 eV)[6],Bi4V2O11(2.2 eV)[7],BiVO4(2.4 eV)[8],Bi2WO6(2.7 eV)[9],Bi2O3(2.8 eV)[10],FeVO4(2.2 eV)[11]and Cu2O(2.2 eV)[12]as narrow bandgap semiconductor materials,have the advantage of responding to visible light(approximately 45% of solar spectrum)and were identified as visible-light-driven photocatalyst.Among them,BiVO4is regarded as one of the most promising visible-light-driven photocatalysts due to its good optical and electrical properties such as visible light excitation,controllable morphology and suitable bandgap structure[13-15].It has been reported that the photocatalytic activity is closely related to the photocatalytic material morphology and its photogenerated charge separation efficiency[16-18].However,the separation efficiency of photogenerated carriers of BiVO4is very low and does not meet the practical needs in many applications.Therefore,it has very important practical significance for BiVO4material to overcome the above defects.
Recently,the construction of noble metal-semiconductor photocatalysts has been considered as one of the effective means to improve photocatalytic performance[19-21].Theoretically,the noble metal nanoparticles on the semiconductor photocatalyst composite as“electronic groove”attract the photogenerated electrons,so that the charge transfer between the interfaces accelerated in the metal-semiconductor nanocomposites.Up to now,there have been a large number of reports on deposition of precious metals such as Au,Ag or Pt nanoparticles on other semiconductor materials to enhance their photocatalytic activity[22-26].Wang et al.[27]successfully fabricated a metal-semiconductor heterojunction photocatalyst by depositing ultrafine Ag nanoparticles on Bi2MoO6microspheres.The photocatalytic experiments indicate that the activity of Ag/Bi2MoO6photocatalyst is significantly improved.Di et al.[28]assembled Ag QDs on the surface of BiOBr by combining ion exchange method and solvothermal method,and successfully prepared the Ag QDs/BiOBr heterojunction photocatalysts.Moreover,the activity of Ag QDs/BiOBr photocatalysts was explored by degrading organic contaminants(CIP,TC,etc.).The experimental results show that the plasma resonance effect(SPR)of Ag QDs plays a key role in the improvement of photocatalytic activity.However,precious metals are very expensive,and limit their practical application to a large extent.Fortunately,many studies have shown that the lower-cost metallic bismuth(Bi)has a plasmonic resonance effect(SPR effect)similar to that of precious metals,and has a great potential to replace the application potential of precious metals in the field of photocatalysis[29-31].According to reports,Bi metals can be combined with a variety of semiconductor materials to construct metal-semiconductor photocatalysts,such as Bi/BiOCl,Bi/Bi2MoO6and Bi/g-C3N4,which exhibited high photocatalytic activity[32-34].The experimental results show that all of Bi-based nanocomposites have significantly improved photocatalytic activity compared to single components.Therefore,the SPR effect of Bi metal and the special electronic structure of BiVO4semiconductor materials can be combined to design and synthesize Bi/BiVO4heterojunction photocatalyst so as to improve the photocatalytic activity.Up to now,there has been no report by Bi metalmodified BiVO4material to improve its photocatalytic activity.
In this paper,the Bi/BiVO4photocatalyst was successfully prepared by a NaBH4reduction method at room temperature.On this basis,the morphology and composition of the samples were investigated by XRD,XPS,TEM and HRTEM analyses.In addition,the photocatalytic activity of the Bi/BiVO4photocatalyst was evaluated by degrading the TC organic contaminant solution,and the optimum ratio was determined.Finally,the photocatalytic reaction mechanism of Bi/BiVO4heterojunction photocatalyst was also proposed by exploring the relationship between free radical capture experiments and ESR analysis.
2.Experimental
2.1.Materials and reagents
Ammonium vanadate(NH4VO3),bismuth nitrate(Bi(NO3)3.5H2O),nitric acid(HNO3),sodium borohydride(NaBH4),triethanolamine(TEOA),isopropanol(IPA),1,4-benzoquinone(BQ),DMPO and tetracycline hydrochloride(TC)were used.All used chemicals were purchased from Aladdin's Chemical Reagent Co.,Ltd.,and without further purification.The experimental water is deionized water.
2.2.Preparation of photocatalysts
2.2.1.Preparation of BiVO4
BiVO4was obtained by a coprecipitation method[15].The amount of 32ml HNO3(1mol?L-1)was added to the 50ml beaker,and then 5 mmol Bi(NO3)3·5H2O was added under sonication.After the ultrasonic dispersion,5 mmol NH4VO3and 3.0 g urea were added,stirring until the solution was orange.Finally,the suspension was transferred into a water bath at 80°C,keeping the reaction for 24 h.After the reaction is complete,the mixture solution changed into bright yellow,and is cooled to room temperature.The sample is recovered and washed three times with deionized water and absolute ethyl alcohol,then dried in an oven at 60°C to give the final samples.
2.2.2.Preparation of Bi/BiVO4heterojunction photocatalysts
The Bi/BiVO4photocatalysts were prepared by using a NaBH4reduction method[34].Firstly,0.2 g of BiVO4sample was added to a beaker containing 30 ml H2O,and dispersed by sonication.Then,10 ml of NaBH4solution(concentrations of 0.01,0.02,0.04,0.08 and 0.16 g per100 ml of H2O,respectively)was added dropwise into the suspension under vigorous agitation.Observably,the bright yellow suspension first turned gray,and then turned black.After reaction for 1 h,the blackyellow precipitate was filtered,washed twice with deionized water and ethanol,and then dried in an oven at 60°C for 12 h to obtain the final samples.To distinguish the photocatalysts,the samples were labeled as 0.01-Bi/BiVO4,0.02-Bi/BiVO4,0.04-Bi/BiVO4,0.08-Bi/BiVO4,and 0.16-Bi/BiVO4,respectively.
2.3.Sample characterization
The crystal structure of the samples was analyzed by X-ray diffraction(XRD)spectroscopy(Cu Kαradiation,λ=0.154178 nm).The morphologies of the samples were determined by SEM images(S-4800 field emission).The transmission electron microscopy(TEM)and highresolution TEM(HRTEM)pictures of the samples were used to characterize the lattice structure(Tecnai G2 F30 S-Twin,an accelerating voltage of 200 kV).The UV-vis diffuse reflectance(DRS)spectra were measured by a spectrophotometer(UV-2450,BaSO4as a reflectance standard).An electrochemical workstation was used to measure the photocurrent intensity of the sample(CHI 660B,three-electrode system as a standard battery).X-ray photoelectron spectroscopy(XPS)was obtained by an electronic spectrometer using a 150 Al KαX-ray source.The spectrometer was used to detect the active species during the reactionand?OH free radical trapping agent).
2.4.Photocatalytic activity evaluation
The photocatalytic activity of BiVO4and Bi/BiVO4samples with different contents of Bi was evaluated by degradation of TC solution(10 mg·L-1)under visible light irradiation.Specifically,an aqueous TC solution of 100 mL was added into a Pyrex photocatalytic reactor.Then,50 mg of samples was dispersed in the solution,stirring for 60 min in darkness.After the solution reaches the adsorption-desorption equilibrium,the reactor was illuminated by a 250 W Xe lamp with a 400 nm cut-off filter.During the photocatalytic reaction,water circulation control should be introduced to keep the reactor at room temperature.At 10 min for irradiation intervals,5 mL reactor solution was extracted by the sampling tube from the reactor,and centrifuged to remove the precipitate.Finally,the clear liquid was measured by a UV-vis spectrophotometer(TU-1810)to corroborate the absorbance of TC at 357 nm,which is corresponding with the photocatalytic ability of the samples.
2.5.Photocatalytic recycle experiments
The photocatalytic stability of the photocatalysts was measured by degrading the TC solution under visible light irradiation.The 0.04-Bi/BiVO4sample was chosen as the target sample for the best photocatalytic activity to evaluate the recycle experiments.In detail,the 0.04-Bi/BiVO4sample was collected by centrifugal,washing and drying processes after completing a cycle test.The recycle experiments were carried out four times.
3.Results and Discussion
3.1.XRD analysis
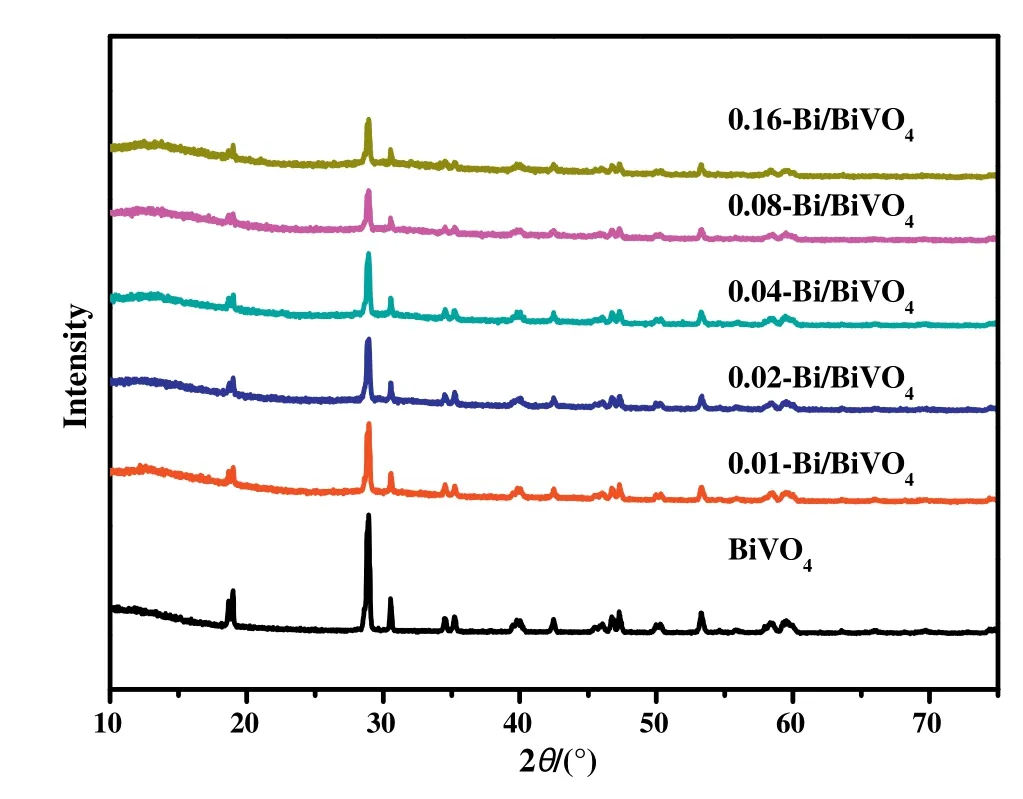
Fig.1.X-ray diffraction(XRD)diagram of BiVO4and Bi/BiVO4samples.
The XRD analysis was used to explore the material composition and crystal structure of the samples.Fig.1 shows the XRD patterns of pure BiVO4and Bi/BiVO4samples,which can be indexed as the standard BiVO4(JCPDS card No.14-0688)[15].Apparently,the diffraction peaks of pure BiVO4indicate its good crystallinity.After increasing the NaBH4concentration,the intensity of the diffraction peak gradually decreases.In addition,we can observe that the[110]and[040]crystal planes of BiVO4exposed at the 18.5°and 30.6°positions in the spectrum are relatively prominent,and there is no diffraction peak of any impurity in the graph.After research and verification,these two crystal faces are more conducive to charge carrier movement.Studies have confirmed that due to the different energy levels of the two interfaces in the conduction band and valence band,photogenerated electrons and holes are more concentrated on the two crystal faces[35].This shows that the prepared BiVO4samples have highly exposed[110]and[040]crystal planes,which contribute to the full utilization of photogenerated electrons and holes.
3.2.SEM and TEM analysis
The morphology features of the samples can be characterized by SEM analysis.As shown in Fig.2(a,d),BiVO4is formed by a stack of cubes of size 1 to 1.5 μm(Fig.2(a)).After NaBH4reduction,there was no significant change in the surface of the BiVO4material(Fig.2(d)).In order to investigate the lattice characteristics between the samples,BiVO4and 0.04-Bi/BiVO4samples were characterized by TEM and HRTEM analysis.Among them,Fig.2(b,c)and(e,f)are the TEM and HRTEM images of BiVO4and 0.04-Bi/BiVO4materials,respectively.Compared with BiVO4,many small particles were produced on the surface of 0.04-Bi/BiVO4photocatalyst.The particles were characterized by HRTEM and found to be Bi metal nanoparticles.In addition,HRTEM image shows the characteristic lattice fringes of Bi nanoparticles,which can be indexed to the[012]crystal surface of Bi metal(d(012)=0.328 nm)[14,33,34].It can be seen that the surface of the BiVO4material produces Bi simple substance.
3.3.XPS analysis
The surface chemical composition of pure BiVO4and 0.04-Bi/BiVO4was studied by using X-ray photoelectron spectroscopy(XPS)analysis.Fig.3(a)shows the XPS spectra of BiVO4and 0.04-Bi/BiVO4samples,indicating that all samples consist of Bi,V,O,and C elements.In this spectrum,the peak of C 1 comes from unstable carbon.As shown in Fig.3(b),the 158.9 and 164.2 eV positions of Bi 4f for BiVO4in the XPS spectrum has two distinct intense peaks,which attributed to the Bi3+species of original BiVO4[15].Obviously,compared to BiVO4,the two weak peaks of 0.04-Bi/BiVO4at 156.9 and 162.3 eV can be observed due to the presence of metallic Bi nanoparticles,which is corresponding with the HRTEM images as shown in Fig.2(c,f).Fig.3(c)is an XPS spectrum of V 2p of BiVO4and 0.04-Bi/BiVO4samples.After 0.04-Bi/BiVO4was treated with NaBH4,the binding energy of VO4-in BiVO4was changed.Why does the color of the suspension change from the bright yellow to black during the experiment?This provides an appropriate explanation:With the addition of a solution of NaBH4,Bi3+ions of BiVO4can be reduced to elemental Bi nanoparticles,and the color of Bi metal is black[34].As shown in Fig.3(d),there is a strong peak at 529.5 eV in the O 1s spectrum,which is consistent with the binding energies of the Bi--O bonds of BiVO4and 0.04-Bi/BiVO4.The changes of V 2p and O 1s reflect the binding energy of metal Bi and BiVO4interaction.
3.4.DRS analysis
The UV-vis diffuse reflection spectra(UV-vis DRS)was used to investigated the light absorption properties of BiVO4and Bi/BiVO4samples in the wavelength coverage of 300-700 nm.As shown in Fig.4,BiVO4has strong absorption of ultraviolet and visible light.Due to the SPR effect of metal Bi nanoparticles,the light absorption effect of the Bi/BiVO4samples in the visible light region is slightly enhanced.Moreover,Bi/BiVO4samples can observe a light red shift compared with BiVO4.It indicates that the SPR effect of Bi metal similar to noble metals can significantly improve the ability of light absorption,which is essential for improving photocatalytic performance.
3.5.Photocurrent analysis
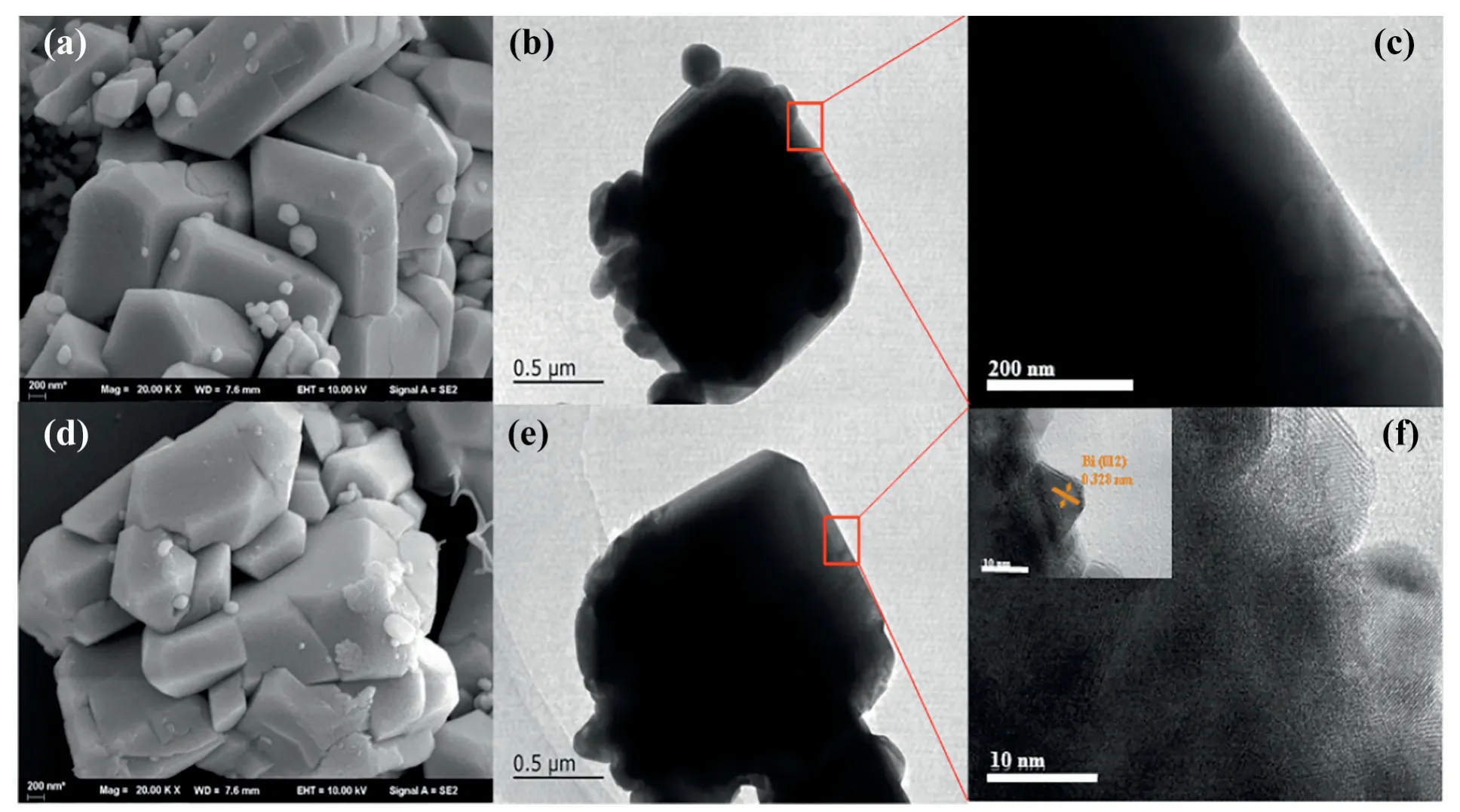
Fig.2.(a)SEM diagram of BiVO4sample,(b)(c)TEM diagram;(d)SEM,(e)(f)TEM of 0.04-Bi/BiVO4sample.
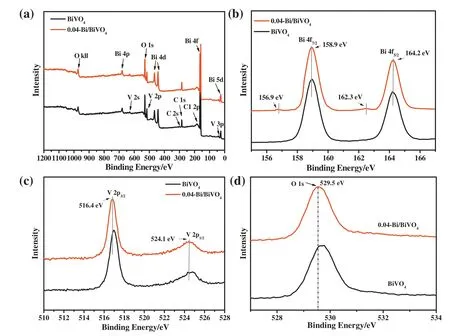
Fig.3.X-ray photoelectron spectroscopy(XPS)of BiVO4and 0.04-Bi/BiVO4samples.
Fig.5 shows the transient photocurrent curves for BiVO4and Bi/BiVO4samples.The curve records the photocurrent intensity of different samples during a switch light of 300 s.As shown in Fig.5,pure BiVO4shows a lower photocurrent density than other heterogeneous structures.In addition,the photocurrent density of 0.04-Bi/BiVO4is the highest.In general,the strong photocurrent intensity indicates that the photocatalysts have higher photogenic electron-hole separation efficiency.The experimental results show that compared with BiVO4,the introduction of Bi metal nanoparticles can enhance the photocurrent intensity of Bi/BiVO4samples,which indicates that Bi metal can be used as a typical semi-metal material to promote the charge transfer between the interfaces.
3.6.Photocatalytic activity
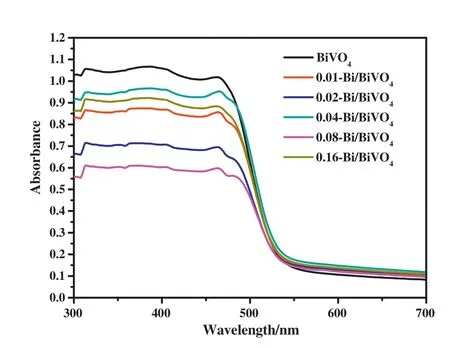
Fig.4.The UV-vis DRS curve in the UV and visible region of BiVO4and Bi/BiVO4samples.
The photocatalytic activity of BiVO4and Bi/BiVO4samples was investigated by degrading TC solution(10 mg·L-1)under visible light irradiation.Fig.6(a)is a plot of the photocatalytic activity of 0.04-Bi/BiVO4sample for TC degradation under visible light.The figure shows the concentration changes of TC solution degraded by 0.04-Bi/BiVO4sample at different time periods.Among them,the 0.04-Bi/BiVO4photocatalyst showed the highest photocatalytic activity to the contaminants at the beginning,and the photocatalytic activity of the sample became stable after turning on the Xe lamp for 20 min.Fig.6(b)shows the reaction curves of different samples to TC solution under dark and visible light conditions.In particular,the adsorption-desorption equilibrium was reached between the substances after darkness for 40 min.Compared to BiVO4,the photocatalytic activity of Bi/BiVO4has been greatly improved for degradation of TC solution under visible light.Moreover,0.04-Bi/BiVO4sample has the highest photocatalytic activity for TC solution,and reached to 74.7%.This is consistent with the transient photocurrent intensity detected by the earlier.Furthermore,the cycle experiments were used to examine the stability of the photocatalysts,0.04-Bi/BiVO4sample is selected as the target sample,and TC solution(10 mg·L-1)is the target contaminant.The results of the experiment are shown in Fig.6(c).After four repetitions of the experiment,0.04-Bi/BiVO4sample still maintained a high photocatalytic activity,and its activity was reduced from the original 74.7%to 70.8%.The results show that the Bi/BiVO4heterojunction photocatalyst has higher photocatalytic activity and good stability.
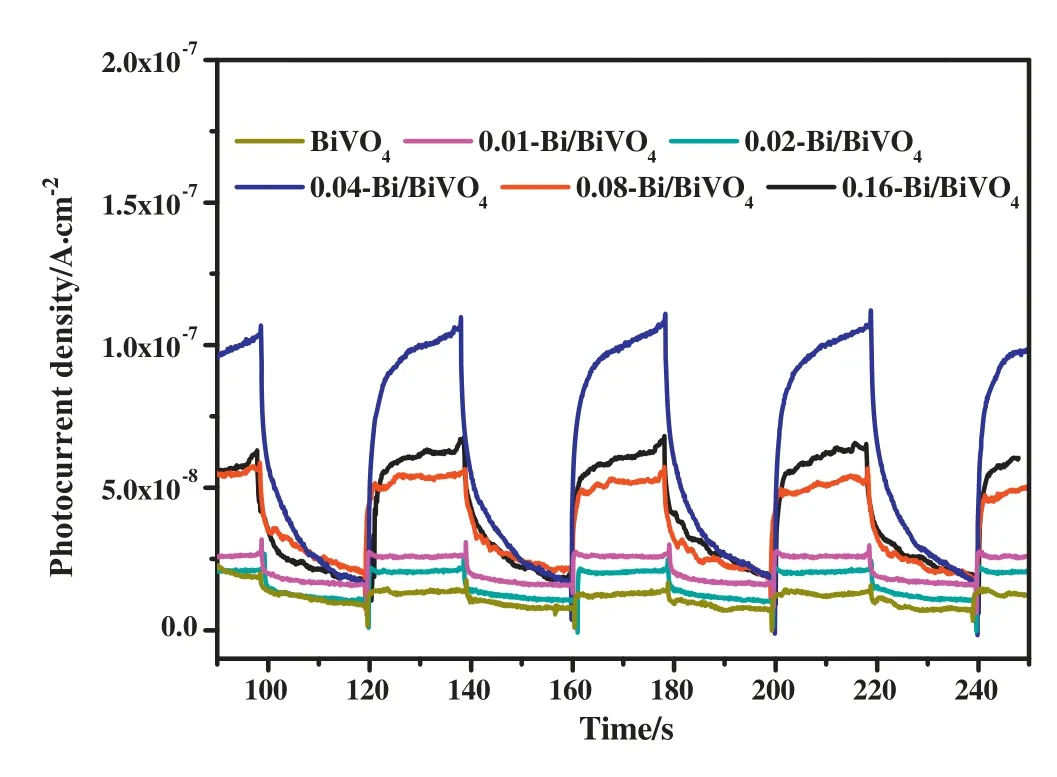
Fig.5.The transient light current response curve of BiVO4and other Bi/BiVO4samples in the visible light.
3.7.Photocatalytic reaction mechanism
The active species of this photocatalytic reaction system were studied by free radical capture experiments.Among them,0.04-Bi/BiVO4photocatalyst was selected as the target sample,TC solution was the target contaminant,and TEOA,BQ and IPA were used as h+,and?OH capture agents.The illumination time was 60 min.Fig.7 shows the effect of different capture agents on the activity of the 0.04-Bi/BiVO4sample.When TEOA was added to the solution,the degradation rate of the photocatalyst was reduced to a minimum(about 19.8%).When BQ was added into the reaction solution,the photocatalytic activity for the sample by TC degradation was 60.3%.When IPA is added to the reaction solution,the degradation rate for TC solution was reduced to 31.7%,and much lower than the photocatalytic efficiency without capture agent(74.7%).The results indicate that h+and?OH free radicals are the active species of the Bi/BiVO4photocatalytic system.
In order to further explore the active species of the Bi/BiVO4photocatalytic reaction system,the free radicals of the system were detected by electron paramagnetic resonance spectroscopy(ESR).DMPO was acted as a trapping agent.Fig.8 shows the ESR spectra of BiVO4and 0.04-Bi/BiVO4samples under different conditions.In the visible light,the intensity of characteristic peaks for?OH radicals over BiVO4is significantly weaker than that of the 0.04-Bi/BiVO4sample.In addition,the signal intensity of?OH radicals for 0.04-Bi/BiVO4samples in the spectra is obviously stronger than that of the dark conditions,which indicates that illumination can promote the generation of a large number of?OH radicals in the photocatalytic system.Notably,BiVO4and 0.04-Bi/BiVO4samples also have a small amount offree radical production in the light.The experiment results show that the signal intensity of the detected active species is consistent with the capture experimental date.Therefore,the corresponding photocatalytic reaction process[36-38]can be proposed:
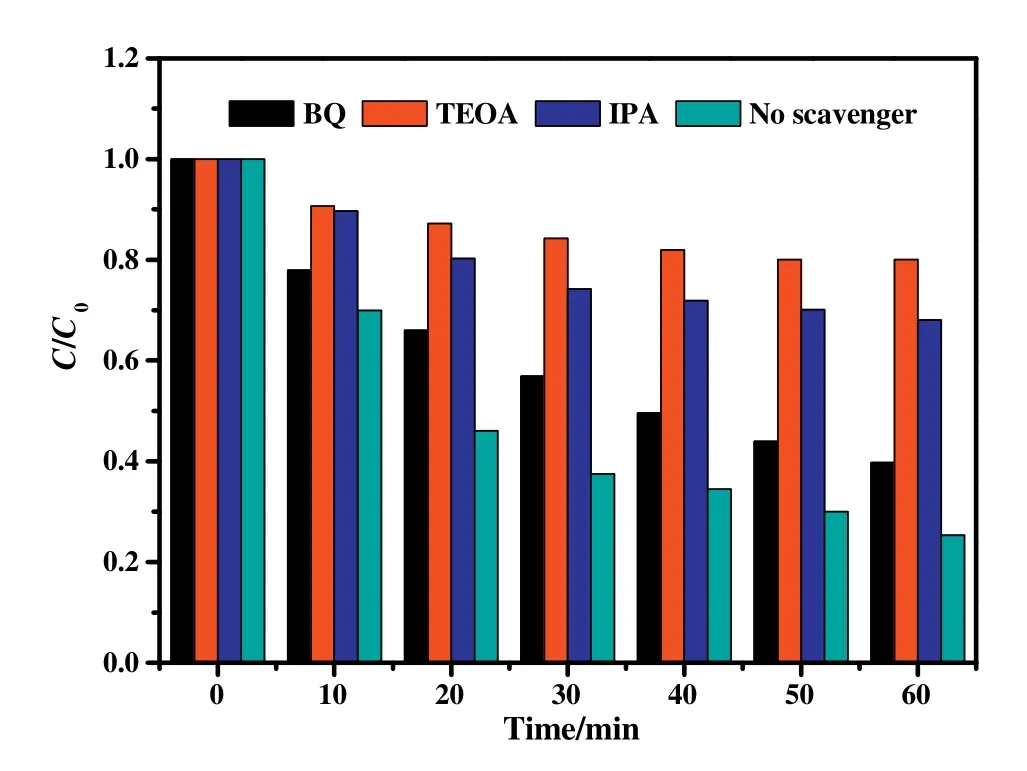
Fig.7.0.04-Bi/BiVO4samples for degrading TC solution curve in the visible light,BQ,TEOA and IPA respectively as?,h+and?OH radical scavenger.
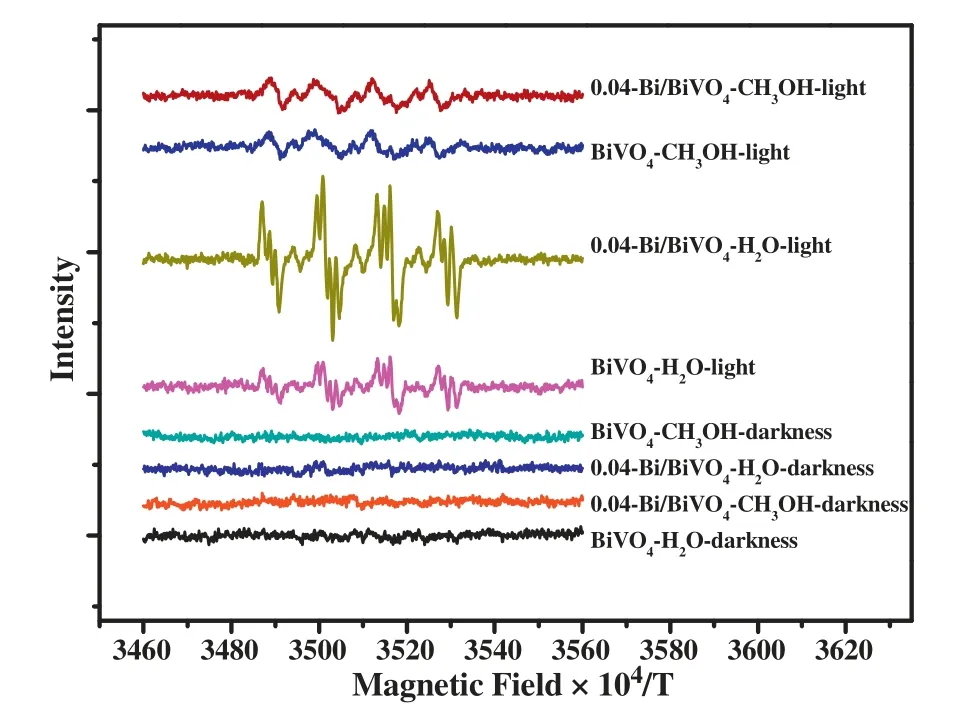
Fig.8.ESR spectra of active species captured by DMPO.In the dispersed samples of methanol,the superoxide free radicals(?)were detected,and hydroxyl radical(?OH)was detected in the dispersed samples in the water.
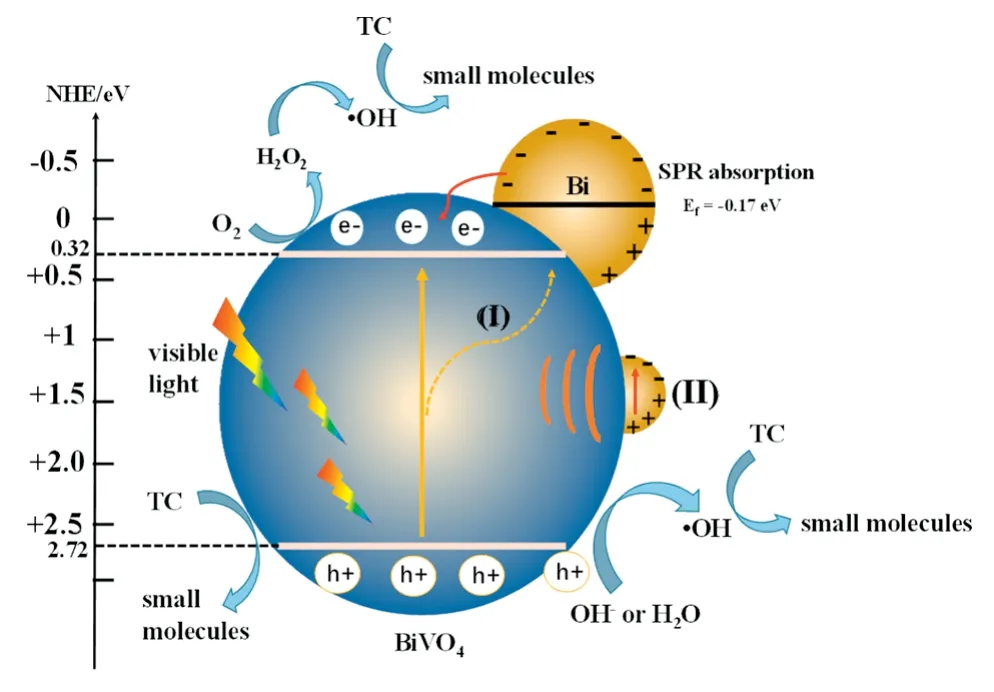
Fig.9.The photocatalytic reaction mechanism of Bi/BiVO4.
On this basis,the reactive mechanism of enhanced photocatalytic performance for this Bi/BiVO4system can be proposed.As shown in Fig.9,it can be summarized as the synergistic effect of the following factors.Firstly,the SPR effect of Bi nanoparticles on the BiVO4surface contributes to the absorption of visible light,as shown in Fig.4 by the UVvis DRS analysis.Secondly,the Bi nanoparticles were excited by visible light due to the EPR effect,the electron transfer rates of the surface and interface become faster(Eq.(1)).Thirdly,the photogenerated electrons of Bi particles will transfer to BiVO4(Eq.(3))because the Fermi level of metal Bi(-0.17 eV)is higher than the conduction band position of BiVO4(0.20 eV)[15,39,40].After the electrons are released,Bi metal particles will have more positive potentials to generate positive charges,which is similar to the role of noble metal nanoparticles in the field of photocatalysis.The photogenerated electrons in the valence band of BiVO4will be accepted by Bi nanoparticles,and return to its original state.Therefore,the photoelectrons in the valence band from BiVO4transfer to the Bi nanoparticles(I)effectively suppressing the recombination of photogenerated carriers.
4.Conclusions
In this paper,Bi/BiVO4photocatalyst was successfully prepared by a NaBH4reduction method.Firstly,the BiVO4precursor was synthesized by co-precipitation process.Then,the final samples were obtained by in-situ reduction using NaBH4solution,and dried at 80°C in an oven.On this basis,the composition and structure of BiVO4and Bi/BiVO4samples were studied by XRD,XPS,SEM and TEM analysis.The optical properties of Bi/BiVO4photocatalysts and active groups involved in the photocatalytic reaction were measured by DRS and ESR techniques.In addition,the photocatalytic performance of BiVO4and Bi/BiVO4photocatalysts was evaluated by photocatalytic experiments(including degradation,capture,and repeated experiments)for TC solution under visible light irradiation.The experimental results show that the Bi/BiVO4photocatalyst has excellent photocatalytic activity and good stability.Finally,the reactive mechanism of photocatalytic system was used to explain the reason why the Bi nanoparticles can improve the electronhole separation and excellent photocatalytic activity of Bi/BiVO4photocatalyst.
 Chinese Journal of Chemical Engineering2019年12期
Chinese Journal of Chemical Engineering2019年12期
- Chinese Journal of Chemical Engineering的其它文章
- Preparation and characterization of a novel antibacterial acrylate polymer composite modified with capsaicin☆
- Controlling pore structures of Pd-doped organosilica membranes by calcination atmosphere for gas separation☆
- Relationship between equilibrium potential and radius of lanthanides electrolyzed on the zinc cathode
- Effect of gas composition on nitric oxide removal from simulated flue gas with DBD-NPC method☆
- Optimization of the N2generation selectivity in aqueous nitrate reduction using internal circulation micro-electrolysis☆
- Solubility and mass transfer of H2,CH4,and their mixtures in vacuum gas oil:An experimental and modeling study☆
