Combination therapy with androgen deprivation for hormone sensitive prostate cancer:A new frontier
Tyler Etheridge,Shivshnkr Dmodrn,Adm Schultz,Kyle A.Richrds,Joseph Gwdzik,Bing Yng,Vincent Cryns,Dvid F.Jrrrd,c,*
aDepartment of Urology,University of Wisconsin-Madison,Madison,WI,USA
bDepartment of Medicine,University of Wisconsin-Madison,Madison,WI,USA
cUniversity of Wisconsin-Madison,Carbone Cancer Center,University of Wisconsin-Madison,Madison,WI,USA
KEYWORDS Prostate cancer;Cellular senescence;Androgen deprivation therapy;Combination therapy;Synthetic lethal targeting;Metformin;Statins
Abstract Androgen deprivation therapy(ADT)has been the standard of care for the last 75 years in metastatic hormone sensitive prostate cancer(PCa).However,this approach is rarely curative.Recent clinical trials have demonstrated that ADT combined with other agents,notably docetaxel and abiraterone,lead to improved survival.The mechanisms surrounding this improved cancer outcomes are incompletely defined.The response of cancer cells to ADT includes apoptosis and cell death,but a significant fraction remains viable.Our laboratory has demonstrated both in vitro and in vivo that cellular senescence occurs in a subset of these cells.Cellular senescence is a phenotype characterized by cell cycle arrest,senescenceassociated β-galactosidase(SA-β-gal),and a hypermetabolic state.Positive features of cellular senescence include growth arrest and immune stimulation,although persistence may release cytokines and growth factors that are detrimental.Senescent tumor cells generate a catabolic state with increased glycolysis,protein turnover and other metabolic changes that represent targets for drugs,like metformin,to be applied in a synthetic lethal approach.This review examines the response to ADT and the putative role of cellular senescence as a biomarker and therapeutic target in this context.
1.Introduction
Prostate cancer(PCa)is the most common male malignancy in the western world and is a leading cause of cancer death[1].For the last 75 years,advanced disease has been managed with androgen deprivation therapy(ADT),an approach that leads to disease regression,but rarely cure.Castration-resistant PCa generally results in death over 30-40 months depending on disease extent at diagnosis[2].Multiple phase III clinical trials(ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer[CHAARTED],A Randomized,Double-blind,Comparative Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy[ADT]Versus ADT Alone in Newly Diagnosed Subjects with High-Risk,Metastatic Hormone-na?ve Prostate Cancer[mHNPC][LATITUDE],Randomized Phase III Trial Comparing an Association of Hormonal Treatment and Docetaxel Versus the Hormonal Treatment Alone in Metastatic Prostate Cancers[GETUG-AFU 15],and Systemic Therapy in Advancing or Metastatic Prostate Cancer:Evaluation of Drug Ef ficacy[STAMPEDE])have recently demonstrated that in metastatic hormone-sensitive PCa,combining either docetaxel chemotherapy or abiraterone(an androgen signaling inhibitor)at the time of initiating ADT markedly improves overall survival in men[3-6].These clinical observations suggest that ADT induces susceptibilities in PCa cells that make them amenable to synergistic treatmentand improve cellkilling.This underexplored area has the potential to have a major impact on PCa survival by improving the cancer response to ADT.
One phenotype that arises with extended replication or after cell stress is cellular senescence,in which cells are arrested yet viable[7].Cellular senescence can also be induced after exposure to oxidative stress,DNA damaging agents,and ADT[8].In breast cancer,another hormonally dependent cancer,senescence results after tamoxifen exposure in estrogen receptor positive cells[9].Bene ficial aspects of cellular senescence include a therapeutic arrest andimmunestimulation.However,negativefeatures include reentry of a subset of these persistent cells into the cell cycle and a secretory phenotype that may induce the growth of surrounding tumor cells.Thus,removal of these cells may lead to improved cancer outcomes.
The late Dr.Donald Coffey was always a strong supporter of “thinking outside the box”.His work led to remarkable advances in their own right,but placed in the context of his mentees he had an even greater impact of the field.Dr.Coffey often marveled at nature and would often state“You are going to be surprised at the simplicity and beauty of the real answer”.This review,dedicated to him,focuses on a surprising and major shift in our approach to advanced hormone sensitive PCa,that of the role of ADT and combined therapy.It is an area that requires significant research effort to understand and refine recent clinical discoveries.We will review cancer cell responses to ADT,seek to understand the clinical responses and mechanisms underlying these new combinatorial therapies with ADT,and discuss putative targeting of senescent cells after ADT as a therapeutic niche to improve outcomes.
2.Cancer cell responses to ADT
One of the best studied responses to ADT is apoptosis or programmed cell death.Kyprianou and colleague[10,11]performed some of the early work that androgen deprivation leads to apoptosis in pre-clinical PCa models.Androgen withdrawal in murine xenografts and human PCa tissues is associated with a decrease in the proliferative index,but surprisingly low levels of apoptosis encompassing only 2%-3%of cells[12-14].Apoptosis typically occurs early after ADTwithin the first 72 h[15].In addition to apoptosis,other cell death mechanisms induced with ADT include autophagy,necrosis,and necroptosis[16].
Autophagyis an evolutionarily conserved catabolic pathway that targets cellular organelles and cytoplasmic constituents to the lysosomes for degradation.Autophagy,although a cell death pathway,can also function as a survival mechanism exploited by cancer cells under various physiological stresses[17,18].Androgen deprivation and tissue hypoxia,conditions which occur in prostate tissue after surgical or medical castration,lead to an increased adenosine monophosphate(AMP)-activated protein kinase(AMPK)activity in a threshold dependent manner in murine xenografts[19].Increased AMPK activity is associated with greater cell survival and the induction of autophagy.In vitro in hormone-sensitive PCa cells,autophagy has been reported to occur after bicalutamide,but this occurs at much higher toxic doses than those seen with the induction of cellular senescence[20].
Some tumor cells become quiescent after ADT and have the potential to reactivate.PCa stem-like cells are typically androgen receptor(AR)negative and may represent part of the normally quiescent cancer stem cell population that emerges and expands after ADT[21].These cells are rare,but express a specific surface antigen profile(CD44+/α2β1hi/CD133+)when isolated from primary PCa tissues and show high levels of clonogenic ability[22].
3.Cellular senescence as a response to ADT in PCa
The phenotype of these residual cells after ADT is complex,but cellular senescence represents an intriguing response that has potential for therapeutic exploitation.Replicative senescence was first described as a phenotype in primary cells after extensive culture and replicative exhaustion in vitro,which was linked to telomere shortening[23].More recently,DNA damage,increased oncogenic signaling,and oxidative stress have been found to result in induced or accelerated senescence[24].The propensity of tumor cells to undergo senescence induction in response to multiple anti-cancer therapies has been demonstrated[25].Notably,multiple solid tumor cell lines have shown dose-dependent cellular senescence induction in response to DNA damaging agents such as doxorubicin,diaziquone,cisplatin,and to a lesser degree ionizing radiation.Recently reported data suggest enzalutamide,an antiandrogen,potentiates DNA damage caused by radiation and significantly increases radiation-induced cellular senescence in LNCaP cells[26].
Senescent cells remain viable and metabolically active,but are permanently growth-arrested(Table 1)[24].They are persistent,in contrast to cells undergoing programmed responses including apoptosis,autophagy,and/or mitotic catastrophe using conventional cytotoxic agents.Growth arrest is achieved and maintained in either G1or G2/M phage,in part,by the increased expression of specific cyclin-dependent kinase inhibitors (CDKIs), including p16Ink4a,p21Waf1/Cip1and p27Kip1[27].Interestingly,transformed neoplastic cells that lack cellular senescenceassociated tumorsuppressorgenespresentin nontransformed cells(e.g.,p53 and Rb)retain the capacity to become senescent with exposure to doxorubicin,docetaxel,and other chemotherapy agents[28,29].
Cultured in vitro,senescent cells develop a distinct and recognizable flattened and enlarged morphology with a prominent nucleus and increased cytoplasmic granularity.Most notably,these cells can be visualized using a staining technique based on senescence-associated β-galactosidase(SA-β-gal)activity[30].This technique,which stains lysosomes in the perinuclear compartment blue at pH 6.0,is a widely accepted and utilized marker of cellular senescence,but has limitations in vivo,as it is not applicable to formalin- fixed archival tissues.
To resolve this issue,we validated one of the first antibodies to SA-β-gal protein(GLB1)in vitro,subsequently allowing the biology of cellular senescence in PCa in vivo to be interrogated[31].In hormonally intact prostate tissues,quantitative imaging detects increased GLB1 expression in high-gradeprostaticintraepithelialneoplasia(HGPIN)known to contain senescent cells compared to benign prostate tissues[31].This work also demonstrated that in intermediate grade PCa increased GLB1 predicts prostate-specific antigen(PSA)-free survival.Furthermore,senescent cells are found less commonly in high grade(Gleason score 8-10)versus intermediate grade(Gleason score 6-7)cancers.These findings support a tumor suppression aspect of cellular senescence seen in skin and many other aging organs.
These studies in our laboratory,and subsequently others,have demonstrated that cellular senescence is induced in androgen sensitive cells after ADT[32,33].Increased expression of the senescence-related proteins GLB1,the CDKI p27Kip1,and chromatin-regulating heterochromatin protein 1γ (HP1γ)are detected in 50%-80%of androgen sensitive LNCaP cells after being cultured in androgen-free media[8].In mice bearing LuCaP xenograft tumors in vivo,surgical castration similarly increases senescent markers[8].In another study,immunohistochemistry of human prostate tumors removed after ADT induced with goserelin acetate(Zoladex)showed a similar induction of GLB1,HP1γ,and decreased Ki-67[34].ADT induces a cellular growth arrest consistent with cellular senescence,including hypophosphorylation of Rb,reduction of cyclindependent kinase activity,and a G1/S block[27].More recently,we have found that in patients undergoing ADT prior to prostate removal for cancer,elevated cellular GLB1 levels measured by VECTRA automated immunoquantitation are noted in as many as 50%of cancer cells[35].GLB1 levels increase in tumors with longer ADT duration.
Despite permanent growth arrest,senescent cells are metabolically active and have increased energy demand,protein turnover,and glycolysis[36].The increased energy demand is marked by activation of AMPK,a cellular energy sensor[37].Increased glucose transport and amplified glycolytic enzyme and pyruvate kinase expression promote the utilization of glucose through non-oxidative glycolysis,similar to the Warburg phenomenon described in tumor cells[36].Additionally,higher amino acid transport and increased protein synthesis generate cytokines that characterize the proin flammatory secretory-associated senescent phenotype(SASP).These metabolic changes present an opportunity for improved therapeutic approaches when combined with ADT.
Interestingly,AR signaling may play an important role in the cellular senescence response.Blocking the AR,or paradoxically applying supraphysiologic levels of androgens,such as those used in bipolar androgen therapy,may induce cellular senescence[38].This may represent part of the biphasic growth response seen in AR expressing PCa cells,and induction of senescence in this context may function through the Src-P13K-Aktsignalingpathway.Pharmacologic induction of senescence has also been demonstrated in murine xenografts and human PCa tissues using the naturally occurring AR antagonist atraric acid[39].
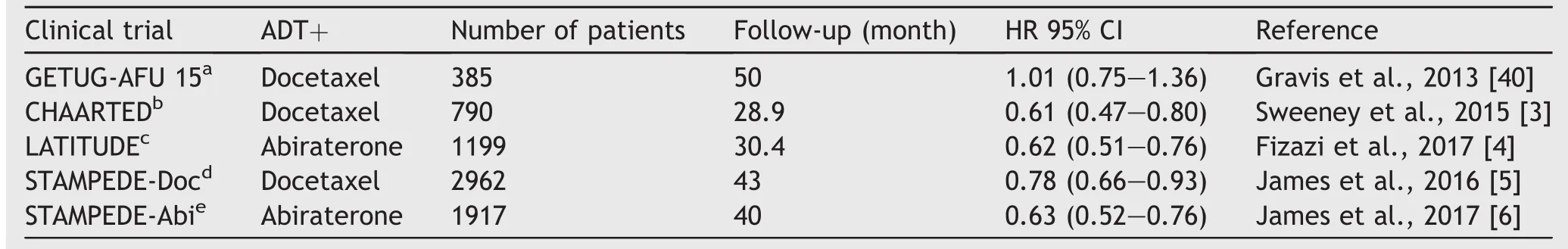
Table 1 Summary of clinical trial results using ADT in combination with other therapies for the treatment of metastatic hormone sensitive PCa.
4.Clinical trials suggest unique PCa susceptibilities after ADT
The application of ADT has always been approached as the sole systemic therapy for metastatic hormone sensitive PCa,traditionally using agents that target the substrate(testosterone)or block the AR.In a major paradigm shift,several recent trials using either docetaxel or androgen signaling inhibitors in combination with ADT for metastatic hormone sensitive PCa have demonstrated remarkable improvements in survival.This suggests ADT induces susceptibilities in cancer cells that may be exploited.The phase III CHAARTED trial(ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer),demonstrated upfront chemotherapy with concurrent docetaxel and ADT improves survival by 13.6 months versus ADT alone in hormone na?ve patients(Table 1)[3].The docetaxel group also experienced a significant delay in biochemical,symptomatic,or radiographic progression compared to controls(20.1 vs.11.7 months;hazard ratio [HR]= 0.61;95% confidence interval[CI]=0.51 to 0.72;p<0.001).These results were confirmed in the STAMPEDE(Systemic Therapy in Advancing or Metastatic Prostate Cancer:Evaluation of Drug Ef ficacy)[5]and GETUG-AFU 15 trials(Randomized Phase III Trial Comparing an Association of Hormonal Treatment and Docetaxel Versus the Hormonal Treatment Alone in Metastatic Prostate Cancers)[40].Docetaxel functions to inhibit the transport of the AR,potentiating ADT action[41].It is also a microtubule inhibitor having direct toxicity to cells.We anticipate this combination results in fewer persistent senescent cells than ADT alone post-treatment,but further mechanistic study is required regarding the synergistic activity of this combination.
The phase III LATITUDE trial(A Randomized,Doubleblind,Comparative Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy[ADT]Versus ADT Alone in Newly Diagnosed Subjects with High-Risk,Metastatic Hormone-na?ve Prostate Cancer[mHNPC])demonstrated upfront therapy with concurrent abiraterone and luteinizing hormone-releasing hormone agonists improves overall survival versus ADT alone(median not reached versus 34.7 months,HR=0.62;95%CI:0.51-0.76;p<0.001)in hormone na?ve patients[4].The abiraterone group also experienced a significant delay in radiographic progression-free survival compared to control(33.0 months vs.14.8 months;HR=0.47;95%CI:0.39-0.55;p<0.001).The abiraterone and ADT arm of the STAMPEDE trial also showed improved outcomes[6].Abiraterone irreversibly inhibits the Cytochrome P450 17α-hydroxylase/17,20-lyase(CYP17)enzyme expressed in adrenal tissues,reducing androgen synthesis from all sources[42].Although the combination of CYP17 inhibition and ADT demonstrates more effective androgen depletion than either agent alone [43],resistance to abiraterone develops,likely through increase CYP17 expression[44],enzymatic alterations[45],and/or gain of function 3β-hydroxysteroid dehydrogenase type 1 mutation [46]. These clinical observations suggest that the initiation of ADT induces susceptibilities in PCa cells that make them amenable to synergistic treatment and improved cell killing.Further study is required to delineate the synergistic activity of abiraterone and ADT,along with clearly defining the mechanisms of resistance in castration-resistant PCa.
5.Exploiting cellular senescence as synthetic therapy
Cellularsenescence may have detrimentalfeatures.Oncogene-induced cellular senescence imposes selective pressures that promote the outgrowth of senescenceresistant aggressive tumor cellsubpopulations [47].Although cellular senescence is cytostatic and offers a potential survival advantage in some models,long-term exposure of surrounding cells to the SASP,and resulting proin flammatory cytokines and growth factors,may have deleterious effects on surrounding cells[7].In a recent Nature paper,it was reported that lymphoma cells released from chemotherapy-induced senescence results in a population of cells exhibiting a stem cell phenotype that exhibits highly aggressive growth potential upon escape from cellcycle blockade[48].This population is enriched in relapsing hematologic tumors.It has been proposed that ADT-induced cellular senescence might play a role in the chemoresistance that arises with intermittent ADT[32].
The cellular senescence phenotype presents unique opportunities that have the potential to be exploited for therapeutic cure(Fig.1)[49].D?rr and colleagues[36]have induced senescence in lymphoma cells and used compounds that target the inhibition of glucose transporters,glycolytic enzymes,and adenosine triphosphate(ATP)depletion to generate synthetic lethality in cancer cells(Table 2).These combinations lead to improved survival and elimination of cancer cells through caspase-12-and caspase-3-mediated endoplasmic-reticulum-related apoptosis[36].Additionally,higher amino acid transport and increased protein synthesis generate cytokines that characterize the proinflammatory SASP.These findings highlight the hypercatabolic nature of senescent cells after induction with ADT and other agents that is therapeutically exploitable by synthetic lethal metabolic targeting.These approaches have been largely unexplored to date in PCa,but as outlined below,interesting supportive data exist.
The therapeutic induction of cellular senescence from chemotherapeutics,such as cyclophosphamide,results in augmented protein translation with the resultant accumulation of misfolded proteins,which activates the conserved proteotoxic stress response(PSR)[36].The substantial proteotoxic stress in cellular senescence evokes energyconsuming countermeasures for cell survival.The PSR is orchestrated by the transcription factorheatshock factor 1(Hsf1),which regulates the gene expression of several families of molecular chaperones(heat shock protein(Hsp)27,Hsp70,Hsp90 and others)that attenuate proteotoxic stress and promote cell survival by preventing protein aggregation,refolding non-native proteins,or delivering them to the proteasome[54].Anabolic tumor cells are more dependent on the Hsf1-mediated PSR than normal cells for cell survival[55].The PSR may represent an additional novel targetable vulnerability of senescent transformed cells that can be exploited therapeutically,a hypothesis that has yet to be tested.
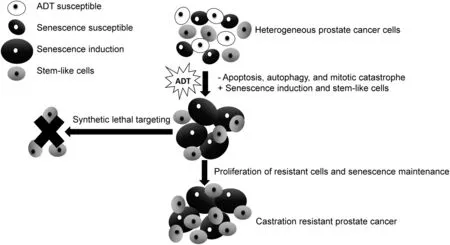
Figure 1 Synthetic lethal targeting of ADT induced cellular senescence for improved prostate cancer cell killing.ADT,androgen deprivation therapy.
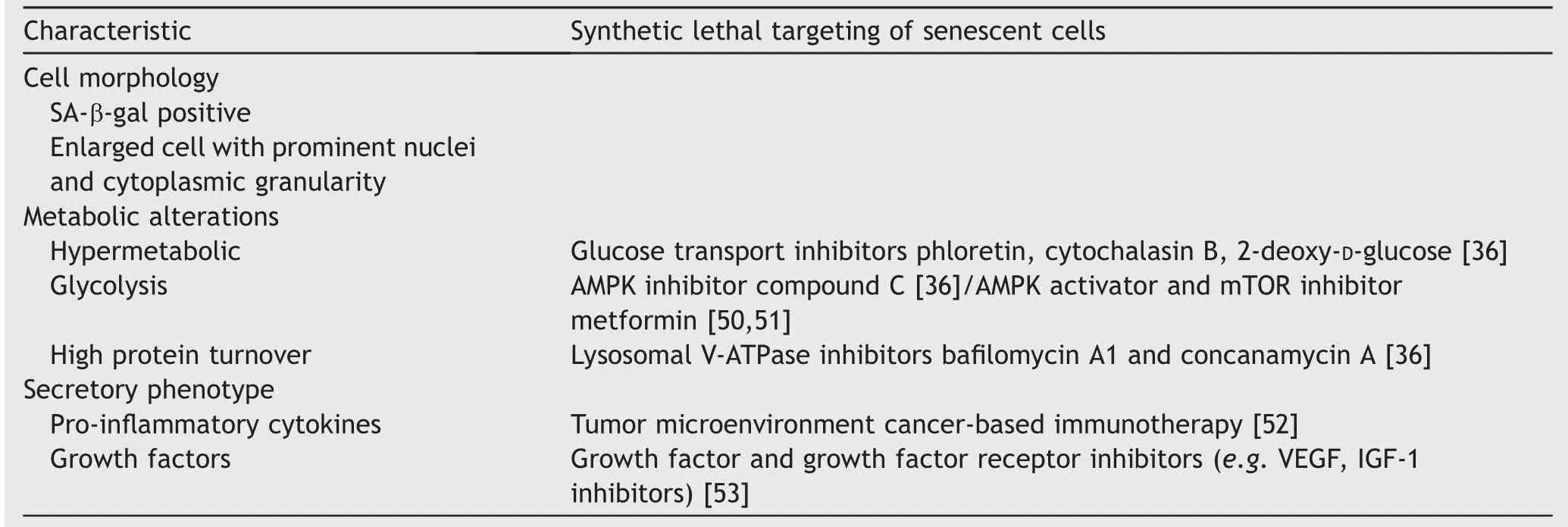
Table 2 Presence of the cellular senescence phenotype offers therapeutic opportunities.
Metformin is an oral anti-diabetic agent in the biguanide class that has generated interest as a cancer therapy.Metformin is an intriguing drug that may not only enhance chemotherapy response for established tumors,but also demonstrates anticancer activity as a single agent[56].Metformin inhibits the mammalian target of rapamycin(mTOR),a central regulator of cell growth and survival,in part by activating AMPK[51].Additionally,metformin inhibits Hsf1 by activating AMPK,which in turns phosphorylates Hsf1 on Ser 121 to inhibit its activity[57].This disables the protective effects of Hsf1 against proteotoxic stress.Synergistic activity after neoadjuvant chemotherapywassuggested in an analysisshowing improved clinical response in diabetic patients with breast cancerreceivingmetforminandneoadjuvantchemotherapy[58].These patients received doxorubicin,which is well known for its senescence-inducing properties in prostate and breast cancer[28,29].
Given the metabolic susceptibilities that ADT induces,metformin leads to increased cell kill when combined with ADT.Metformin has been combined with the antiandrogen bicalutamide in vitro and in animal models[59].This combination significantly reduced clonogenicity(p<0.005)and tumor growth with greater effects in AR-positive cells.In unpublished data,we have found LNCaP,LaPC4,and CWR22 PCa lines all demonstrate synthetic lethal responses as calculated by Calcusyn(Biosoft,Cambridge,UK)to low dose bicalutamide(1-5 umol/L)to induce senescence initially, followed by metformin at low dose(0.1-1 mmol/L)[60].Increased apoptosis peaks 2 days after metformin application.
In a large observational study of 87 344 veterans,we demonstrated that metformin combined with ADT improved overall survival(HR=0.82,95%CI:0.78-0.86),reduced the risk of skeletal related events(HR=0.84,95%CI:0.74-0.96), and improved cancer specific survival(HR=0.70,95%CI:0.64-0.77)[61].Skeletal related events, defined as pathologic fracture, spinalcord compression,and/or necessity for bone radiation or surgery due to pain or impending fracture,was used as one surrogate fordisease progression.Although retrospective observational studies do not prove causality,these data merit further clinical trial investigation.Notably,metformin is an inexpensive,widely used drug associated with minimal side effects even when used in the non-diabetic population.Therefore,few barriers exist to prevent its clinical translation in PCa.
Statins are another widely used and inexpensive drug that may provide synergistic lethality with ADT given the metabolic profile of senescent cells.Statins are a class of oralanti-hypercholesterolemiaagentsthatinhibit3-hydroxy-3-methylglutaryl-CoA (HMG CoA) reductase.Although they are associated with myopathy and elevated transaminases,the side effect profile is favorable[62].The use of statins reduces PSA in vivo[63],through downregulation of AR expression and activity due to enhanced proteolysis of the AR protein[64].In LNCaP cells,the combination of simvastatin and ADT demonstrates increased growth inhibition in AR positive lines [65]. Statins also compete with the dihydrotestosterone precursor for Solute Carrier Organic Anion Transporter Family Member 2B1 mediated transport,thus decreasing androgen availability in PCa cells[66].Men with hormone sensitive PCa undergoing ADT and taking statin drugs experienced a delayed time to disease progression compared to men not taking statins(median 27.5 months;95%CI:21.1-37.7 vs.17.4 months;95%CI:14.9-21.1).Simvastatin has also been shown to decrease the secretory phenotype of senescent human fibroblasts,including interleukin-6,by inhibiting protein prenylation[67].Whether statins directly induce cell death in senescent cells is unknown as of yet.
Other characteristics of senescent cells include a hypermetabolic phenotype comprised of enhanced glycolysis and protein turnover,providing a critical cellular senescence associated metabolic liability that may be targeted therapeutically. Senescent cells are selectively susceptible to inhibition of glucose transporters(e.g.cytochalasin B),or to the pharmacological competitor(2-deoxy-D-glucose)[36].Senescent cells also rely on an intact lysosomal protein degradation machinery to buffer proteotoxic stress.Exposure of senescent cells to ba filomycin A1 or concanamycin A,specific inhibitors of lysosomal V-ATPases,or to a cocktail of lysosomal protease inhibitors all generate increased death in therapy induced senescent cells[36].With use of these inhibitor in cell culture,senescent cell death occurred at significantly higher rates.They provide an avenue for therapeutic exploitation that should be examined further.
6.Conclusion and future directions
ADT has been used as the primary approach to advanced hormone sensitive PCa for over 75 years.Recent clinical trials have demonstrated combining ADT with other agents improves survival.ADT and docetaxel(or abiraterone)should be considered standard of care for patients with metastatic hormone sensitive PCa given recent trial results.Research is needed to define and understand these observations to further improve our progress in this area.Cellular senescence is a distinctive phenotype characterized by metabolic alterations and growth arrest.ADT induced cellular senescence in PCa may be deleterious;however,it may offer a unique opportunity for synthetic lethal targeting of residual PCa cells.Several recent retrospective hypothesis-generating studies suggest combining ADT with agents that target metabolism,including metformin,may improve patient survival.Future work should focus on further delineating the response of tumors to ADT and cellular senescence biology in this context.
Conflicts of interest
The study was supported by DOD Prostate Cancer Research Program PC150221,R.Stephenson Family Fund.
Author contributions
Study concept and design:Kyle A.Richards,Bing Yang,Vincent Cryns,David F.Jarrard.
Drafting of manuscript:Tyler Etheridge,Shivashankar Damodaran,Adam Schultz,KyleA.Richards,Joseph Gawdzik,Bing Yang,Vincent Cryns,David F.Jarrard.
 Asian Journal of Urology2019年1期
Asian Journal of Urology2019年1期
- Asian Journal of Urology的其它文章
- Application of fluorescence in situ hybridization in the detection of bladder transitional-cell carcinoma:A multi-center clinical study based on Chinese population☆
- Detection of androgen receptor(AR)and AR-V7 in small cell prostate carcinoma:Diagnostic and therapeutic implications
- Albumin-linked prostate-specific antigen-activated thapsigargin-and niclosamide-based molecular grenades targeting the microenvironment in metastatic castration-resistant prostate cancer
- Prostate tumor neuroendocrine differentiation via EMT:The road less traveled
- Regulatory signaling network in the tumor microenvironment of prostate cancer bone and visceral organ metastases and the development of novel therapeutics
- Potential impact of combined inhibition of 3α-oxidoreductases and 5α-reductases on prostate cancer
