Dose escalation of external beam radiotherapy for high-risk prostate cancer-Impact of multiple high-risk factor
Rei Umezw *, Koji In , Stoshi Nkmur ,Akihis Wkit , Hiroyuki Okmoto , Keisuke Tsuchid ,Tiro Kshihr , Kzum Koyshi , Ken Hrd ,Kn Tkhshi , Noy Murkmi , Yoshinori Ito ,Hiroshi Igki , Keiichi Jingu , Jun Itmi
a Department of Radiation Oncology, National Cancer Center Hospital, Chuo-ku, Tokyo, Japan
b Department of Radiation Oncology, Tohoku University Graduate School of Medicine, Sendai, Japan
KEYWORDS Prostate cancer;External beam radiotherapy;Dose escalation;Biochemical control
Abstract Objective: To retrospectively investigate the treatment outcomes of external beam radiotherapy with androgen deprivation therapy (ADT) in high-risk prostate cancer in three radiotherapy dose groups.Methods: Between 1998 and 2013, patients with high-risk prostate cancer underwent threedimensional conformal radiotherapy or intensity-modulated radiotherapy of 66 Gy, 72 Gy, or 78 Gy with ADT. Prostate-specific antigen (PSA) relapse was defined using the Phoenix definition. PSA relapse-free survival (PRFS) was evaluated in each radiotherapy dose group. Moreover, high-risk patients were divided into H-1 (patients with multiple high-risk factors) and H-2 (patients with a single high-risk factor) as risk subgroups.Results: Two hundred and eighty-nine patients with a median follow-up period of 77.3 months were analyzed in this study.The median duration of ADT was 10.1 months.Age,Gleason score,T stage, and radiotherapy dose influenced PRFS with statistical significance both in univariate and multivariate analyses.The 4-year PRFS rates in Group-66 Gy,Group-72 Gy and Group-78 Gy were 72.7%, 81.6% and 90.3%, respectively. PRFS rates in the H-1 subgroup differed with statistical significance with an increasing radiotherapy dose having a more favorable PRFS,while PRFS rates in H-2 subgroup did not differ with increase in radiotherapy dose.Conclusion: Dose escalation for high-risk prostate cancer in combination with ADT improved PRFS. PRFS for patients in the H-1 subgroup was poor, but dose escalation in those patients was beneficial, while dose escalation in the H-2 subgroup was not proven to be effective for improving PRFS.
1. Introduction
External beam radiotherapy (EBRT) is one of the mainstays of management of localized prostate cancer. Previous studies have shown that outcomes of treatment for prostate cancer are improved by a higher radiotherapy dose [1-6].With the introduction of intensity-modulated radiotherapy(IMRT) as a technique to reduce doses to organs at risk(OARs), it has become possible to perform dose-escalated EBRT for prostate cancer. Actually, there are some reports showing that high-dose radiotherapy improves the local control of prostate cancer and decreases the risk of distant metastases [7,8]. Additionally, some studies have shown a reduction in late toxicities by using IMRT compared with 3-dimensional conformal radiotherapy (3D-CRT) [9-11].
In our institution, dose escalation of EBRT for prostate cancer from 66 Gy to 78 Gy has also been performed with the introduction of IMRT. The purpose of this study was to evaluate retrospectively the safety and efficacy of dose escalation of EBRT for patients with high-risk prostate cancer undergoing definitive EBRT with androgen deprivation therapy (ADT). We also investigated whether there exist specific subgroups in patients with high-risk prostate cancer in which dose escalation might improve biochemical control. There have been few studies in which the efficacy of dose escalation in various subgroups of patients with high-risk prostate cancer was evaluated.
2. Materials and methods
2.1. Study population
Patients with high-risk prostate cancer who underwent definitive EBRT and ADT in the period between 1998 and 2013 were analyzed retrospectively.All of the patients had biopsy-confirmed prostate adenocarcinoma, and risk classification according to the National Comprehensive Cancer Network guidelines(www.nccn.org)was used in the present study. Patients with clinical stage T3a or higher, Gleason score (GS) of 8 or higher, or initial prostate-specific antigen (PSA) level of more than 20 ng/mL were classified as high-risk patients. In the present study, patients with a very high risk were included in the high-risk group. Patients with initial PSA level of more than 50 ng/mL and patients with lymph node or distant metastases were excluded. Patients in whom an obvious increase in PSA was detected during ADT before receiving radiotherapy were also excluded.
2.2. Radiotherapy
The methods used for radiotherapy in the early period of the present study were described previously [12]. Patients received 66 Gy, 72 Gy or 78 Gy to the prostate region in a daily fractional dose of 2 Gy. EBRT was performed by 3DCRT (66 Gy or 72 Gy) or IMRT (72 Gy or 78 Gy).
Some patients received whole pelvis EBRT of 40-46 Gy followed by localized irradiation.The others received EBRT only to the prostate and seminal vesicles. The radiation oncologists decided whether or not to irradiate the whole pelvis with consideration of the patient’s age,performance status, and number of high risk factors.
In radiation of the prostate with seminal vesicles, the prostate and bilateral seminal vesicles were defined as clinical target volume-local (CTV-Lo), with inclusion of extraprostatic tumor extension if present. Planning target volume-local (PTV-Lo) in IMRT was contoured as CTV-Lo with 5-8 mm margins except posteriorly, where 4-6 mm margins were added.PTV-Lo in 3D-CRT was defined as CTVLo plus 6-10 mm margins and posterior 4-10 mm margins.In whole pelvic irradiation, CTV-whole pelvis (CTV-WP)included the whole prostate, whole seminal vesicles, and pelvic lymph node regions including presacral, bilateral common iliac, internal iliac, external iliac and obturator lymph node regions. The PTV of the whole pelvis (PTV-WP)was defined as the CTV-WP with 10 mm margins except for a 7 mm margin posteriorly for the whole prostate and seminal vesicles and a 4 mm margin for pelvic lymph node regions.IMRT planning and 3D-CRT planning were performed by ECLIPSE (Varian Medical Systems, Palo Alto, CA, USA) and FOCUS (CMS, St. Louis, MO, USA), respectively. The dose constraints of the inverse planning of IMRT were PTV-Lo D95(dose irradiated more than 95% of PTV) of 90% or more of the prescribed dose and PTV-Lo V90(volume of the PTV receiving at least 90% of the prescribed dose) of 96% or more of the PTV-Lo. The maximal dose within the PTV-Lo should be less than 110% of the prescribed dose, and mean PTV-Lo dose should be 100%-102% of the prescribed dose. OAR constraints were rectal wall V40(volume of the OAR receiving more than or equal to 40 Gy):Less than 60%,V60: Less than 35%, V70: Less than 25%, V80: Less than 1%,and bladder wall V40:Less than 60%,and V70:Less than 35%.
2.3. ADT
All of the patients underwent ADT, but the length of ADT varied partly because some patients had already received ADT in other institutions at the time of referral. ADT was performed by maximum androgen blockade (MAB) consisting of luteinizing hormone-releasing hormone (LH-RH) plus an anti-androgen agent or LH-RH alone. Most of the patients received neoadjuvant hormonal therapy, and some patients continued to receive adjuvant hormonal therapy after EBRT.
2.4. Follow-up
Measurement of PSA was performed every 3-6 months after radiotherapy. PSA relapse was defined using the Phoenix definition(nadir plus 2 ng/mL)[13].Many patients in whom PSA had risen to more than 4 ng/mL after radiotherapy began to receive salvage hormonal therapy. Bone scintigraphy and CT were performed to detect distant metastases including lymph node metastasis as necessary.
Late toxicities were evaluated using the Common Terminology Criteria for Adverse Events version 4.03(http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40).Adverse events that occurred more than 90 days after radiotherapy were defined as late toxicities.
2.5. Statistical analysis
The characteristics of patients in Group-66 Gy,Group-72 Gy and Group-78 Gy were compared using the chi-square test for dichotomous variables or the Mann-Whitney test for continuous variables. PSA relapse-free survival (PRFS) was calculated from the start of radiotherapy with PSA relapse as an event and death without PSA relapse. We also evaluated distant metastases-free survival (DMFS), causespecific survival (CSS), and overall survival (OS). In DMFS calculation, death without distant metastasis was considered as censored.Lymph node metastases were included in distant metastases. In CSS calculation, patients who had PSA relapse or distant metastases and subsequently died were defined as patients who died due to prostate cancer.Patients lost to follow-up were censored at the time of the last follow-up observation in each survival. Survival curves were drawn by the Kaplan-Meier method. Differences between patient subgroups for prognostic factors were analyzed by the log-rank test as univariate analysis. Multivariate analysis using the Cox proportional hazards regression model was performed with the prognostic factors with p <0.05 in the univariate analysis. Moreover, the high-risk group was divided into H-1 (patients with multiple highrisk factors) and H-2 (patients with a single high-risk factor)as risk subgroups,and the efficacy of dose increment in each risk subgroup was investigated. We also evaluated treatment results in the H-1 subgroup in more detail by using further subgroups (an intermediate prognosis subgroup including both PSA >20 ng/mL and stage cT3-4, and a poor prognosis subgroup containing a combination of GS 8-10 with either PSA >20 ng/mL and/or stage cT3-4)identified by Joniau et al. [14]. All tests were two-sided,and statistical significance was set at the level of p <0.05. Statistical analysis was performed using JMP?10(SAS Institute Inc.,Cary,NC,USA).This study was approved by the institutional review board of National Cancer Center(2014-223).
3. Results
Two hundred and eighty-nine patients were analyzed in the present study. The patients’ characteristics in each radiotherapy dose group are shown in Table 1. The numbers of patients in Group-66 Gy,Group-72 Gy and Group-78 Gy were 73 (25%), 173 (60%) and 43 (15%), respectively. There were statistically significant differences between the radiotherapy dose groups in GS, T stage, length of ADT, and administration of IMRT and whole pelvis irradiation. The frequency of whole pelvis irradiation was higher and the duration of ADT was longer in Group-66 Gy than in the other groups. There were more patients with GS 8-10 in Group-72 Gy and there were more patients with T3-4 in Group-66 Gy.
The median follow-up period for all patients was 77.3 months (range, 3.4-168.0 months). The follow-up period for patients in Group-78 Gy was shorter than that for other patients because the patients were treated by IMRT,which was started in our institution in 2007 (Table 1). The PRFS,DMFS,CSS and OS rates in all patients was shown in Table 2.The 4-year and 7-year PRFS rates for all patients were 80.5%(95% confidence interval [CI]: 75.3%-84.8%) and 66.3%(95% CI: 59.7%-72.4%) (Fig. 1A).
Results of univariate and multivariate analyses of PRFS are shown in Table 3. In univariate analysis, age, GS, T stage,radiotherapy dose,and whole pelvis irradiation were statistically significant prognostic factors for PRFS. Multivariate analysis of PRFS showed statistical significance in age, GS, T stage, and radiotherapy dose. The 4-year PRFS rates in Group-66 Gy, Group-72 Gy and Group-78 Gy were 72.7% (95% CI: 61.1-81.9), 81.6% (95% CI: 74.8-86.8) and 90.3% (95% CI: 76.8-96.3), respectively. The 7-year PRFS rates in Group-66 Gy and Group-72 Gy were 54.3% (95% CI:42.0-66.1) and 69.5% (95% CI: 61.3-76.6), respectively.Because of the short follow-up period,the 7-year PRFS rate in Group-78 Gy could not be calculated (Fig. 1B).
The PRFS, DMFS, CSS and OS rates in the H-1 and H-2 subgroups was shown in Table 2. There was statistical significance between two subgroups in PRFS, DMFS, and CSS(p <0.001, p=0.012, and p=0.010). The 4-year PRFS rates in the H-1 and H-2 subgroups were 74.3% (95% CI:67.2%-80.3%) and 91.6% (95% CI: 84.0%-95.7%), respectively. The 7-year PRFS rates in the H-1 and H-2 subgroups were 57.4%(95%CI:48.9%-65.5%)and 83.7%(95%CI:73.3%-90.6%), respectively. In each risk subgroup, the prognostic significance of radiotherapy dose was examined(Table 4).In the H-1 subgroup with multiple high-risk factors, PRFS was significantly different according to radiotherapy dose groups with an increasing radiotherapy dose showing favorable PRFS (Fig. 2A). On the other hand, in H-2 subgroup with a single high-risk factor, the difference in PRFS rates did not reach statistical significance between radiotherapy dose groups (Fig. 2B). There were no statistically significant differences in DMFS, CSS, and OS according to radiotherapy dose groups in H-1 patients as well as in H-2 patients.
We also investigated details about H-1 subgroup. The PRFS, DMFS, CSS and OS rates in the intermediate and poor risk subgroups were shown in Table 2. There was statistical significance between two subgroups in PRFS(p=0.039).The 4-year PRFS rates in the intermediate risk and poor risk subgroups were 84.6% (95% CI: 73.7%-91.6%) and 69.1% (95%CI: 59.7%-77.1%), respectively. The 7-year PRFS rates in the intermediate risk and poor risk subgroups were 65.2%(95%CI:51.1%-77.1%)and 52.7%(95%CI:42.0%-63.1%),respectively.
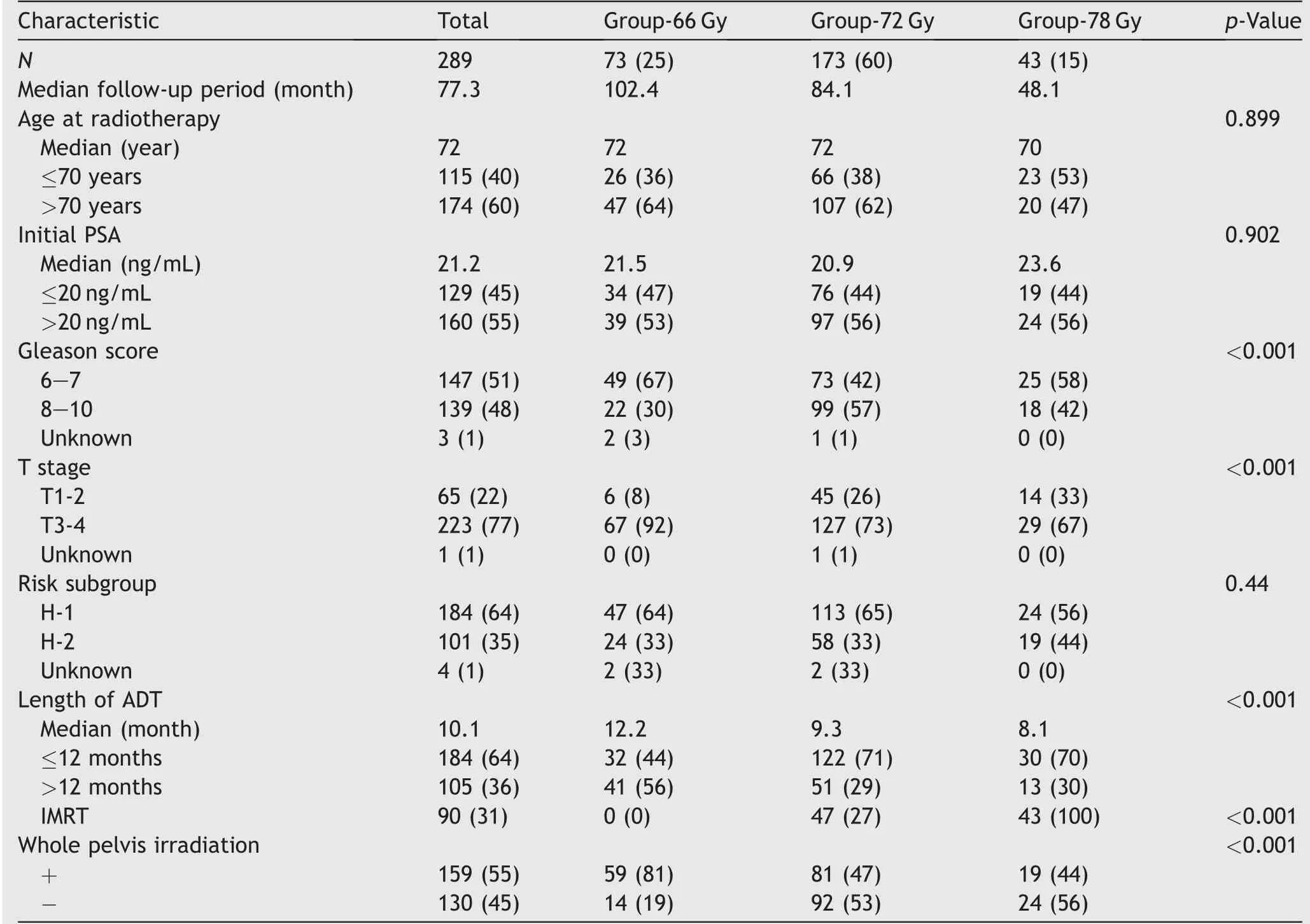
Table 1 Patients’ characteristics of each radiotherapy dose group.

Table 2 Prostate-specific antigen relapse-free survival(PRFS),distant metastases-free survival(DMFS),cause-specific survival(CSS) and overall survival (OS) of each subgroup.
While PRFS was significantly different according to radiotherapy dose groups in intermediate risk group (p=0.010),there were no significant differences in PRFS between three radiotherapy groups in poor risk group (p=0.200).
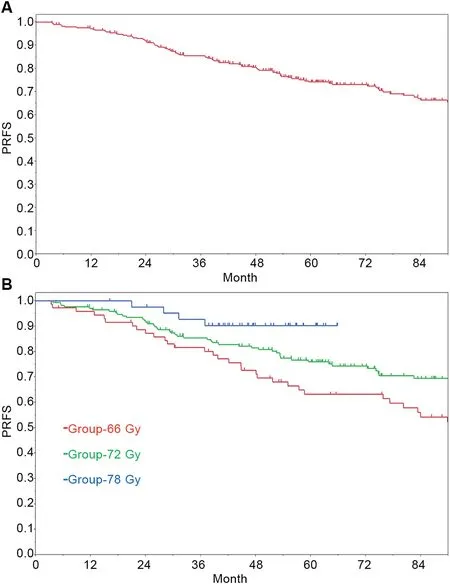
Figure 1 Prostate-specific antigen relapse-free survival(PRFS)in all patients(A)and in each radiotherapy dose group(B).
As for morbidities,the 5-year incidence rates of Grade 2 or greater genitourinary (GU) and gastrointestinal (GI)morbidities were 3.3% (95% CI: 1.6%-6.5%) and 3.0%(95% CI: 1.5%-5.1%), respectively. Late toxicities in each radiotherapy dose group are shown in Table 5.Although the follow-up period was comparatively short, there were no patients with late toxicities of Grade 2 or greater in Group-78 Gy.
4. Discussion
It has been shown that treatment outcomes were better for radiotherapy with ADT than for radiotherapy alone in intermediate-risk and high-risk prostate cancers[15-18].In addition, some studies showed that dose escalation in radiotherapy alone for prostate cancer led to an improvement of PRFS [1,6,19]. Thus, high-intensity treatment by dose escalation radiotherapy and ADT is deemed necessary for high-risk prostate cancer. Some studies demonstrated improvement of treatment outcomes by dose escalation in combinations with short-term ADT in high-risk prostate cancer[3,20].Dearnaley et al.[3]reported that the 5-year biochemical progression-free survival rates in patients with high-risk prostate cancer receiving 64 Gy and 74 Gy with hormonal therapy for 3-6 months were 43% and 57%,respectively. Zelefsky et al. [20] also reported that PRFS with 75.6 Gy or more was superior to that with 70.2 Gy or less in patients with T3 who received short-course ADT.Williams et al. [21] reported that patients treated with lower radiation doses had a significantly greater benefit for increasing ADT duration, and radiotherapy dose escalation in combination with long ADT has not been proven to be beneficial in high-risk prostate cancer. However, our study demonstrated improvement of PRFS by radiotherapy dose escalation in combination with ADT with a median duration of 10.1 months. Recent studies have shown treatment outcomes of high-dose radiotherapy with long-term ADT[22,23]. Zapatero et al. [23] reported that 2 years of adjuvant ADT combined with high-dose radiotherapy(76-82 Gy) improved biochemical control (5-year: 88% vs.76%) and OS (5-year: 96% vs. 82%) compared with shortterm ADT in high-risk cancer patients.Further investigation of the duration of ADT in patients receiving high dose irradiation is desirable.
There are differences in treatment outcomes among high-risk patients. Some studies showed worse treatment outcomes in patients with multiple high-risk factors than in patients with a single high-risk factor who received prostatectomy and brachytherapy-based treatment for highrisk prostate cancer [14,24,25]. Similarly, our study using EBRT also demonstrated a statistically significant difference in PRFS rates among risk subgroups with poor PRFS in patients with multiple high-risk factors (especially in the group having a combination of GS 8-10 with either PSA>20 ng/mL and/or stage cT3-4). Stenmark et al. [26] also revealed that an increasing number of high-risk factors was associated with worse outcomes. Detailed analysis in thepresent study showed that the improvement of PRFS by dose escalation was more remarkable in prostate cancer patients with multiple high-risk factors. Although Wattson et al. [25] reported that outcomes of brachytherapy with EBRT were better than outcomes of brachytherapy alone in patients with multiple high-risk factors, we reported here for the first time that PRFS was improved by radiotherapy dose escalation in those patients who received only EBRT.There were no significant differences according to dose escalation in the H-2 group in the present study.Bolla et al.[27] reported that no statistically significant interaction could be detected between treatment effect and radiotherapy dose in combination with 6 months of ADT in patients with intermediate-risk and high-risk prostate cancer.However,the characteristics of patients who were enrolled in their study seem to be similar to the characteristics of patients in the H-2 group in our study. We assume that the effect of dose escalation was weakened by using long-term ADT in those patients. Other studies also showed the treatment outcome about radiotherapy in favorable highrisk patients expecting good prognosis. A limited role of dose escalation was also suggested by Wattson et al. [25]and Muralidhar et al. [28], although they used mainly brachytherapy in their series.The role of radiotherapy dose escalation appears to be limited in prostate cancer patients with a single high-risk factor, provided that they undergo long-term ADT.
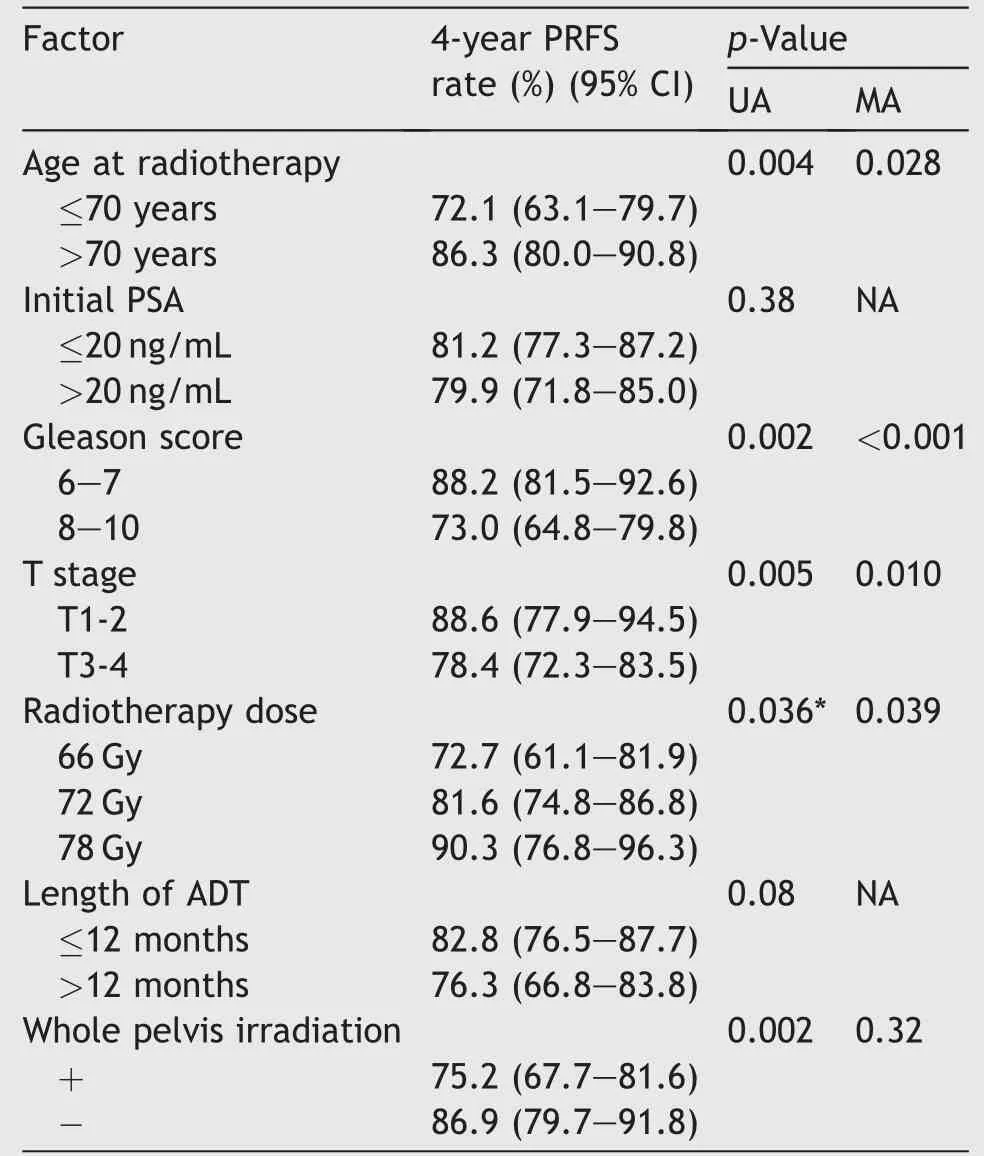
Table 3 Results of univariate and multivariate analyses of prostate-specific antigen relapse-free survival.
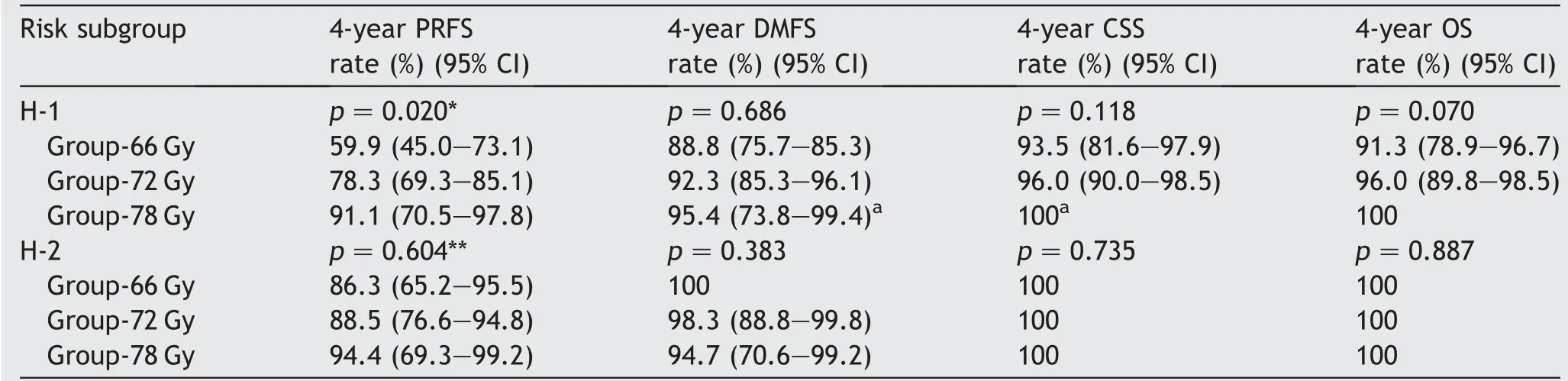
Table 4 PRFS, DMFS, CSS, and OS of risk subgroups according to radiotherapy dose group.
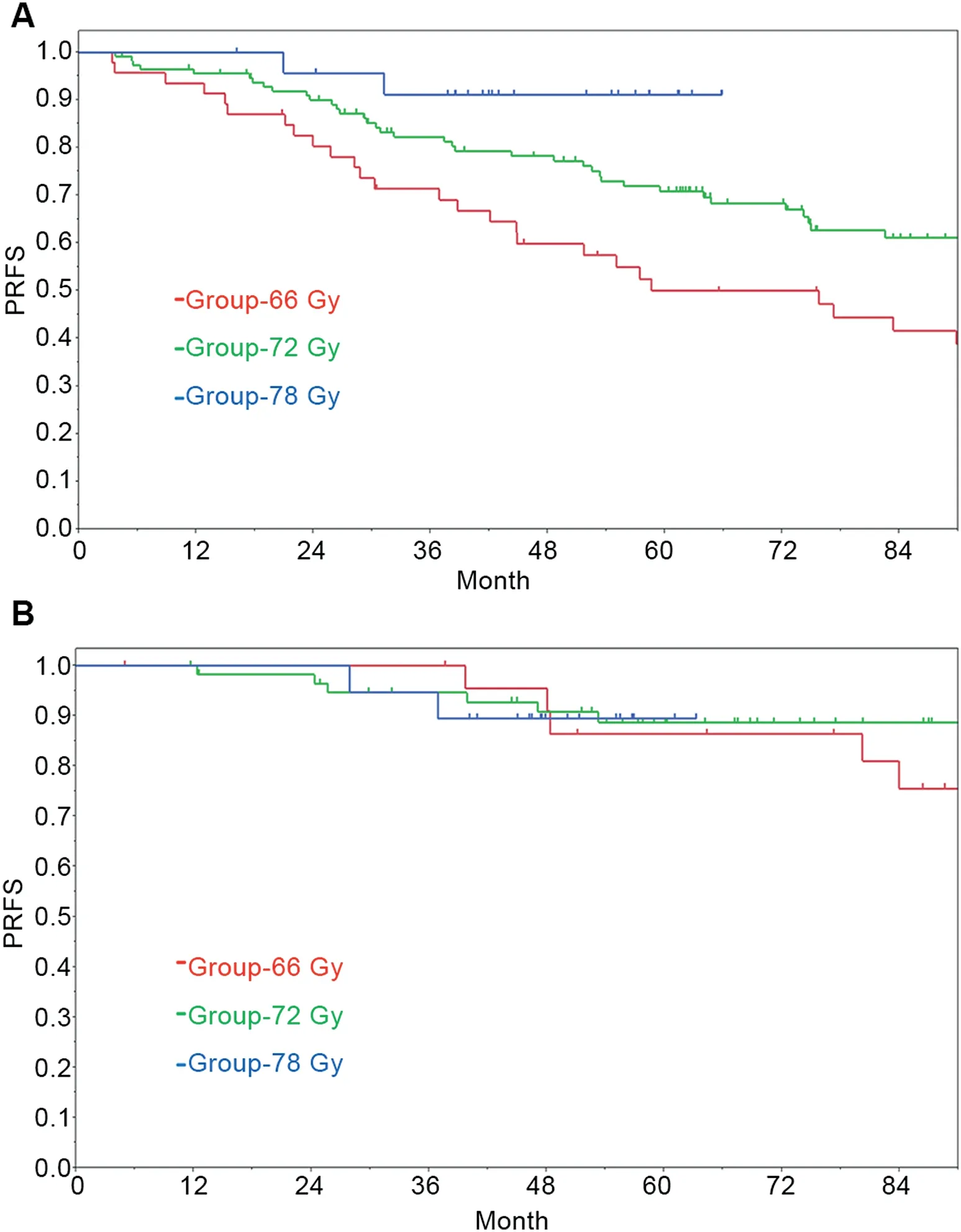
Figure 2 Prostate-specific antigen relapse-free survival(PRFS) in each radiotherapy dose group for H-1 patients (patients with multiple high-risk factors) (A) and H-2 patients(patients with a single high-risk factor) (B).
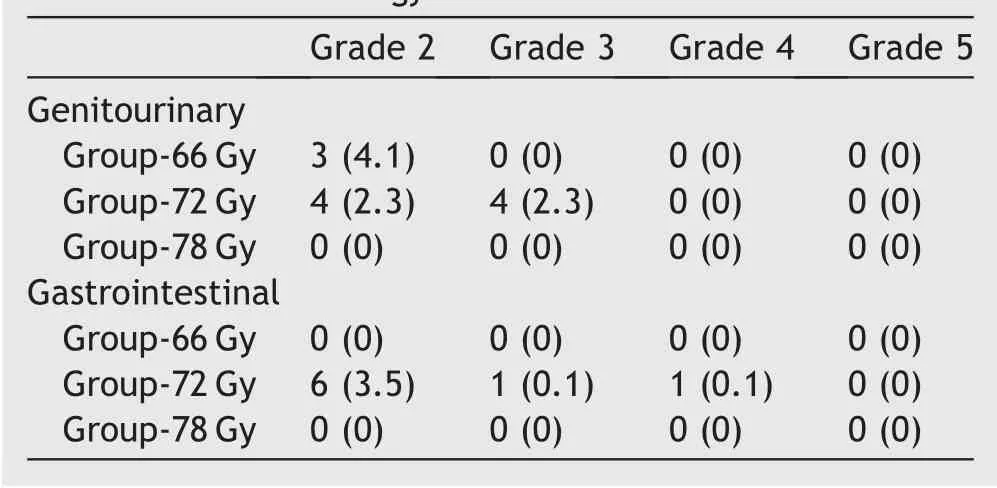
Table 5 Incidence of late toxicities (n [%]) according to the common terminology criteria for adverse events ver. 4.
There were some limitations in our retrospective study.First, there were imbalances in some prognostic factors between radiotherapy dose groups. However, the imbalances could be compensated by applying a multivariate analysis of possible prognostic factors of PRFS, and statistical significance of the impacts of radiotherapy dose on PRFS remained in multivariate analysis. Second, it was difficult to assess the efficacy of whole pelvis irradiation in the present study. Although it would be expected that those who have whole pelvis radiotherapy would have better treatment outcomes, PRFS in patients with whole pelvis irradiation was inferior to that in patients without whole pelvis irradiation in the present study. Those results might be affected by the higher frequency of whole pelvis irradiation in Group-66 Gy. Third, the follow-up period in Group-78 Gy was shorter than the follow-up periods in the other groups, and long-term results in Group-78 Gy could not be calculated. Finally, due to the retrospective nature of this study,the incidence of morbidities might have been underestimated. A prospective study on dose escalation in high-risk prostate cancer patients undergoing long-term ADT is warranted.
5. Conclusion
Dose escalation for high-risk prostate cancer in combination with ADT improved PRFS. The PRFS for patients in the H-1 subgroup was poor, but dose escalation in those patients appeared to be beneficial. Dose escalation in the H-2 subgroup was not proven to be effective for improving PRFS,and dose escalation has a limited role in those patients treated with long-term ADT.
Author contributions
Study concept and design:Rei Umezawa,Koji Inaba,Satoshi Nakamura,Akihisa Wakita,Hiroyuki Okamoto,Keiichi Jingu and Jun Itami.
Data acquisition: Rei Umezawa, Koji Inaba, Keisuke Tsuchida, Tairo Kashihara, Kazuma Kobayashi, Ken Harada,Kana Takahashi, Naoya Murakami, Yoshinori Ito, Hiroshi Igaki and Jun Itami.
Drafting of manuscript: Rei Umezawa and Jun Itami.
Critical revision of the manuscript: Keiichi Jingu and Jun Itami.
Final approval of manuscript: Rei Umezawa, Koji Inaba,Satoshi Nakamura, Akihisa Wakita, Hiroyuki Okamoto, Keisuke Tsuchida, Tairo Kashihara, Kazuma Kobayashi, Ken Harada, Kana Takahashi, Naoya Murakami, Yoshinori Ito,Hiroshi Igaki, Keiichi Jingu and Jun Itami.
Conflicts of interest
The authors declare no conflict of interest.
This work was supported in part by the Research and Development Fund of the National Cancer Center and by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development(AMED) (26-A-18 and 26-A-28).
 Asian Journal of Urology2019年2期
Asian Journal of Urology2019年2期
- Asian Journal of Urology的其它文章
- Asynchronous abdominal wall and sigmoid metastases in clear cell renal cell carcinoma:A case report and literature review
- Compliance in patients with dietary hyperoxaluria: A cohort study and systematic review
- Effect of warm bladder irrigation fluid for benign prostatic hyperplasia patients on perioperative hypothermia, blood loss and shiver: A meta-analysis
- Is Retzius-sparing robot-assisted radical prostatectomy associated with better functional and oncological outcomes?Literature review and meta-analysis
- Beyond prostate, beyond surgery and beyond urology: The “3Bs” of managing non-neurogenic male lower urinary tract symptoms
- Systemic treatment for metastatic prostate cancer
